Oncoviral Infections and Small Extracellular Vesicles
Abstract
1. Introduction
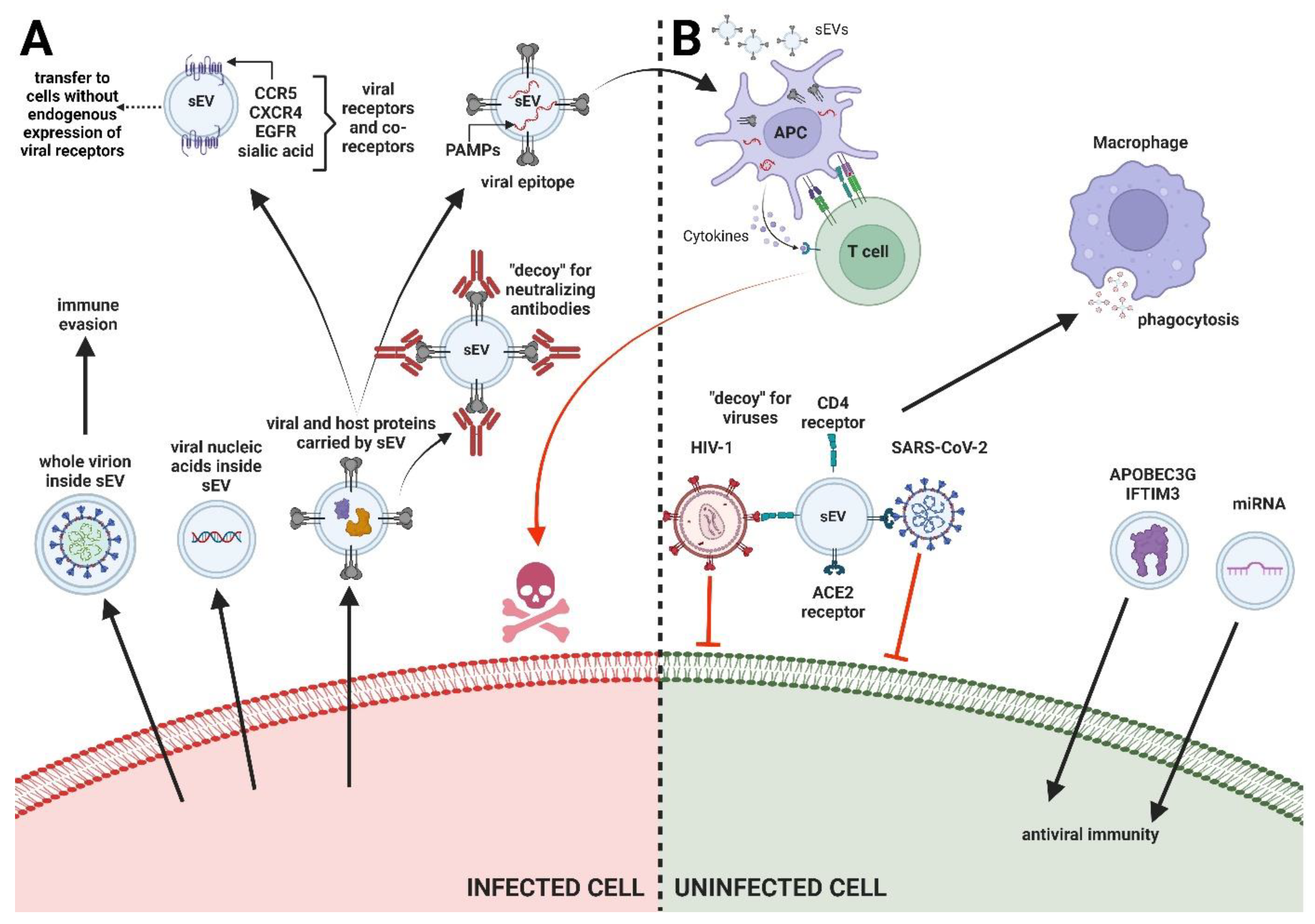
2. Types of Interactions between sEV and Viruses
2.1. sEV and Inhibition of Viral Infections
2.1.1. Transfer of Host Restrictive Factors
2.1.2. Binding and Neutralization of Virions by sEV Containing Entry Receptors
2.1.3. miRNA Transfer for Enhancing Immunity to Infection
2.1.4. Stimulation of Antiviral Immune Responses
2.2. sEV Effects on Viral Propagation
2.2.1. Transport of Viral Cargo via sEV
En Bloc Transmission of Virions
Transfer of Nucleic Acids
Transfer of Viral Proteins
2.3. Hijacking of sEV Biogenesis Pathways by Viruses
3. sEV Significance in Oncovirus Infections
3.1. RNA Oncoviruses
3.1.1. Retroviruses
HTLV
3.1.2. Flaviviruses
HCV
| Virus | Active Molecule in sEV | Mechanism | Effect | Refs. | |
|---|---|---|---|---|---|
| Viral Cargo | Cellular Cargo | ||||
| RNA oncoviruses | |||||
| HTLV-1 | Tax, mRNAs (tax, hbz, env), genomic RNA | Proinflammatory cytokines, miR-21, miR-155,VEGF, CD45,CD43, ICAM-1, LFA-1 | Provide protection for virions against neutralization and environmental factors | Promote infection | [119] |
| Prevent Fas-mediated apoptosis by inducing the cFLIP and NFkB signaling pathways | Increase survival of target cells, inhibit apoptosis | [119] | |||
| Transfer adhesion factors to the target cell | Increase cell-to-cell contact and agglutination potential | [121,128] | |||
| Carry angiogenic factors and promote the proliferation of target cells | Promote tumor progression | [123] | |||
| HCV | intact virions, E2 protein, genomic RNA, viral RNAs | miR-122, miR-21, miR-34a | Transfer of intact virions; enable the infection of target cells by defective virions (e.g., without surface glycoproteins) | Increase infection efficiency | [126] |
| Provide physical protection against the neutralization of virions | Impart resistance to neutralization by anti-HCV neutralizing antibodies | [81] | |||
| Modify gene expression in target cells by transferring different miRNAs | Create favorable tumor environment | [129] | |||
| Transport miR-122 to target cells, as well as HCV-RNA in a complex with miR-122-Ago2-HSP90 | Promotes HCV replication | [125] | |||
| Transport miR-21 to target cells | High levels of miR-21 expression in plasma sEV are correlated with clinical-pathological features of HCC | [130] | |||
| Transport of miR-34a | Induction of apoptosis | [131] | |||
| DNA oncoviruses | |||||
| HBV | intact virions, viral DNA, viral RNA, proteins (HBsAg, HBcAg, HBeAg, HBx, P pro-tein), HBV-miR-3 | miR-122, miR-21, miR-29, miR-135a-5p | Transfer of intact virions | Facilitate virus propagation | [75] |
| Transport of miR-135a-5p that alters gene expression in the target cell | Exhibit antiapoptotic activity towards cancer cells | [132] | |||
| Decrease of RIG-I expression in NK cells and reduction of NFkB pathway activation | Inhibition of proliferation, cytotoxic activity and interferon ɣ production of NK cells | [133] | |||
| Transport of HBV-miR-3 | Promotion of angiogenesis | [134] | |||
| Transport viral HBx protein that lowers the level of expression of antitumorigenic miR-122 | Promote tumorigenesis | [135] | |||
| HBV infection increases the expression of miR-21 and miR-29 in sEV, which alters gene expression in target cells | Inhibition of IL-12 release by macrophages and dendritic cells | [132] | |||
| HPV | viral DNA, viral proteins (E6, E7), E6/E7 mRNA | apoptosis inhibitors (surviving, XIAP, c-IAP1, c-IAP-2, ML-IAP), immunoregulatory molecules (CD276, CD47), calmodulin, MUC16, SIRPA | Transfer of viral oncoproteins E6/E7 | Promotion of tumorigenesis | [86] |
| Transport of MUC16 and SIRPA proteins | Induce epithelial-mesenchymal transition | [36] | |||
| Generate endoplasmic reticulum stress in target cells and decrease expression of proteins associated with tight junctions (ZO-1, claudin-5) | Promotion of metastasis and tumor progression | [36] | |||
| Transport of immunomodulatory factors | Stimulate dendritic cell (DC) maturation and sustain their function | [136] | |||
| Transport of apoptosis inhibitors (surviving, XIAP, c-IAP-1, c-IAP-2, ML-IAP) | Inhibition of apoptosis of cancer cells | [137] | |||
| EBV | LMP-1, LMP-2A, gp350, viral RNA, viral miRNA, EBERs | HIF1α, Galectin-9, EGFR | Transfer oncogenic viral proteins LMP-1 and LMP-2A | Induction of tumorigenesis | [93,138] |
| Transport of HIF1α factor | Induction of angiogenesis | [139,140] | |||
| Increasing level of EGFR expression | Promotion of metastasis | [141] | |||
| Viral protein LMP-1 modify content of cargo of sEV to increase expression proteins involved in EBV infection | Increase infection efficiency | [142] | |||
| miRNAs modulate viral and host gene expression | Facilitate infection and immune evasion | [142,143] | |||
| KSHV | viral RNA, viral miRNA | IL-1β, IFI16 | Transported viral miRNAs affect cell metabolism and tumorigenesis | Creation of microenvironment favorable to tumor development | [144,145] |
| Removal of immune-inducing factors from the cell) | Facilitate immune response evasion | [146,147] | |||
| Complement system activation | Promotion of long-term latency by activating NFκB pathway | [148] | |||
| MCPyV | viral oncoproteins, ALTO | miR-375 | Transport of viral oncoproteins and circular RNA (ALTO) | Increase expression of host genes associated with pathogenesis of MCPyV infection | [149,150] |
3.2. DNA Oncoviruses
3.2.1. Hepadnaviruses
HBV
3.2.2. Papillomaviruses
HPV
3.2.3. Herpesviruses
EBV
KSHV
3.2.4. Polyomaviruses
MCPyV
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Couch, Y.; Buzàs, E.I.; Vizio, D.D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lötvall, J.; Raposo, G.; Stahl, P.D.; Théry, C.; et al. A Brief History of Nearly EV-erything—The Rise and Rise of Extracellular Vesicles. J. Extracell. Vesicles 2021, 10, e12144. [Google Scholar] [CrossRef]
- Chargaff, E.; West, R. The Biological Significance of the Thromboplastic Protein of Blood. J. Biol. Chem. 1946, 166, 189–197. [Google Scholar] [CrossRef]
- Wolf, P. The Nature and Significance of Platelet Products in Human Plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular Vesicles in Physiological and Pathological Conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yu, L.; Ma, T.; Xu, W.; Qian, H.; Sun, Y.; Shi, H. Small Extracellular Vesicles Isolation and Separation: Current Techniques, Pending Questions and Clinical Applications. Theranostics 2022, 12, 6548–6575. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-J.; Jung, G.N.; Park, W.-T.; Seo, M.-S.; Lee, G.W. Therapeutic Potential of Small Extracellular Vesicles Derived from Mesenchymal Stem Cells for Spinal Cord and Nerve Injury. Front. Cell Dev. Biol. 2023, 11, 1151357. [Google Scholar] [CrossRef] [PubMed]
- Nieuwland, R.; Siljander, P.R. A Beginner’s Guide to Study Extracellular Vesicles in Human Blood Plasma and Serum. J. Extracell. Vesicles 2024, 13, e12400. [Google Scholar] [CrossRef] [PubMed]
- Małys, M.S.; Köller, M.C.; Papp, K.; Aigner, C.; Dioso, D.; Mucher, P.; Schachner, H.; Bonelli, M.; Haslacher, H.; Rees, A.J.; et al. Small Extracellular Vesicles Are Released Ex Vivo from Platelets into Serum and from Residual Blood Cells into Stored Plasma. J. Extracell. Biol. 2023, 2, e88. [Google Scholar] [CrossRef] [PubMed]
- Hadpech, S.; Chaiyarit, S.; Phuangkham, S.; Sukphan, S.; Thongboonkerd, V. The Modulatory Effects of Large and Small Extracellular Vesicles from Normal Human Urine on Calcium Oxalate Crystallization, Growth, Aggregation, Adhesion on Renal Cells, and Invasion through Extracellular Matrix: An in Vitro Study. Biomed. Pharmacother. 2024, 173, 116393. [Google Scholar] [CrossRef] [PubMed]
- Tengler, L.; Tiedtke, M.; Schütz, J.; Bieback, K.; Uhlig, S.; Theodoraki, M.-N.; Nitschke, K.; Worst, T.S.; Seiz, E.; Scherl, C.; et al. Optimization of Extracellular Vesicles Preparation from Saliva of Head and Neck Cancer Patients. Sci. Rep. 2024, 14, 946. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Bartold, P.M.; Ivanovski, S. The Emerging Role of Small Extracellular Vesicles in Saliva and Gingival Crevicular Fluid as Diagnostics for Periodontitis. J. Periodontal. Res. 2022, 57, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Cross, T.; Øvstebø, R.; Brusletto, B.S.; Trøseid, A.-M.S.; Olstad, O.K.; Aspelin, T.; Jackson, C.J.; Chen, X.; Utheim, T.P.; Haug, K.B.F. RNA Profiles of Tear Fluid Extracellular Vesicles in Patients with Dry Eye-Related Symptoms. Int. J. Mol. Sci. 2023, 24, 15390. [Google Scholar] [CrossRef] [PubMed]
- Höög, J.L.; Lötvall, J. Diversity of Extracellular Vesicles in Human Ejaculates Revealed by Cryo-Electron Microscopy. J. Extracell. Vesicles 2015, 4, 28680. [Google Scholar] [CrossRef] [PubMed]
- Jonak, S.T.; Liu, Z.; Liu, J.; Li, T.; D’Souza, B.V.; Schiaffino, J.A.; Oh, S.; Xie, Y.-H. Analyzing Bronchoalveolar Fluid Derived Small Extracellular Vesicles Using Single-Vesicle SERS for Non-Small Cell Lung Cancer Detection. Sens. Diagn. 2023, 2, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Krušić Alić, V.; Malenica, M.; Biberić, M.; Zrna, S.; Valenčić, L.; Šuput, A.; Kalagac Fabris, L.; Wechtersbach, K.; Kojc, N.; Kurtjak, M.; et al. Extracellular Vesicles from Human Cerebrospinal Fluid Are Effectively Separated by Sepharose CL-6B—Comparison of Four Gravity-Flow Size Exclusion Chromatography Methods. Biomedicines 2022, 10, 785. [Google Scholar] [CrossRef]
- Quiralte, M.; Barquín, A.; Yagüe Fernández, M.; Navarro, P.; Grazioso, T.P.; Sevillano, E.; Rodriguez Moreno, J.F.; Balarezo-Saldivar, A.; Peinado, H.; Izquierdo, E.; et al. Proteomic Profiles of Peritoneal-Derived Small Extracellular Vesicles Correlate with Outcome in Ovarian Cancer Patients. J. Clin. Investig. 2024, 134, e176161. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Quarto, R.; Bollini, S. Small Extracellular Vesicles from Human Amniotic Fluid Samples as Promising Theranostics. Int. J. Mol. Sci. 2022, 23, 590. [Google Scholar] [CrossRef] [PubMed]
- Vahkal, B.; Kraft, J.; Ferretti, E.; Chung, M.; Beaulieu, J.-F.; Altosaar, I. Review of Methodological Approaches to Human Milk Small Extracellular Vesicle Proteomics. Biomolecules 2021, 11, 833. [Google Scholar] [CrossRef] [PubMed]
- Muraki, R.; Morita, Y.; Ida, S.; Kitajima, R.; Furuhashi, S.; Takeda, M.; Kikuchi, H.; Hiramatsu, Y.; Takanashi, Y.; Hamaya, Y.; et al. Phosphatidylcholine in Bile-derived Small Extracellular Vesicles as a Novel Biomarker of Cholangiocarcinoma. Cancer Med. 2023, 12, 13007–13018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Kenific, C.M.; Zhang, H.; Lyden, D. An Exosome Pathway without an ESCRT. Cell Res. 2021, 31, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, J.; Ajezi, S.; Avci, Ç.B.; Karimipour, M.; Geranmayeh, M.H.; Nourazarian, A.; Sokullu, E.; Rezabakhsh, A.; Rahbarghazi, R. Exosomes and Their Application in Biomedical Field: Difficulties and Advantages. Mol. Neurobiol. 2018, 55, 3372–3393. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Blanco, L.A.; Bou-Habib, D.C. The Role of Extracellular Vesicles from Human Macrophages on Host-Pathogen Interaction. Int. J. Mol. Sci. 2021, 22, 10262. [Google Scholar] [CrossRef] [PubMed]
- Hazrati, A.; Soudi, S.; Malekpour, K.; Mahmoudi, M.; Rahimi, A.; Hashemi, S.M.; Varma, R.S. Immune Cells-Derived Exosomes Function as a Double-Edged Sword: Role in Disease Progression and Their Therapeutic Applications. Biomark. Res. 2022, 10, 30. [Google Scholar] [CrossRef]
- Emerson, L.E.; Barker, H.; Tran, T.; Barker, S.; Enslow, S.; Ou, M.; Hoffman, C.; Jones, M.; Pascual, D.W.; Edelmann, M.J. Extracellular Vesicles Elicit Protective Immune Responses against Salmonella Infection. J. Extracell. Vesicles 2022, 11, e12267. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhou, Y.; Zhang, J.; Zhang, Y.; Long, S.; Lin, X.; Yang, A.; Duan, J.; Yang, N.; Yang, Z.; et al. NK Cell-Derived Exosomes Enhance the Anti-Tumor Effects against Ovarian Cancer by Delivering Cisplatin and Reactivating NK Cell Functions. Front. Immunol. 2023, 13, 1087689. [Google Scholar] [CrossRef] [PubMed]
- Razizadeh, M.H.; Zafarani, A.; Taghavi-Farahabadi, M.; Khorramdelazad, H.; Minaeian, S.; Mahmoudi, M. Natural Killer Cells and Their Exosomes in Viral Infections and Related Therapeutic Approaches: Where Are We? Cell Commun. Signal. 2023, 21, 261. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Li, J.; Li, L.; Sun, J.; Fabbri, M.; Wayne, A.S.; Seeger, R.C.; Jong, A.Y. Extracellular Vesicles Derived from Natural Killer Cells Use Multiple Cytotoxic Proteins and Killing Mechanisms to Target Cancer Cells. J. Extracell. Vesicles 2019, 8, 1588538. [Google Scholar] [CrossRef] [PubMed]
- Iraci, N.; Leonardi, T.; Gessler, F.; Vega, B.; Pluchino, S. Focus on Extracellular Vesicles: Physiological Role and Signalling Properties of Extracellular Membrane Vesicles. Int. J. Mol. Sci. 2016, 17, 171. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Huang, D.; Li, Z.; Wang, X.; Yang, T.; Zhao, M.; Wu, J.; Zhong, T. The Role and Application of Small Extracellular Vesicles in Breast Cancer. Front. Oncol. 2022, 12, 980404. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xia, Y.; Smollar, J.; Mao, W.; Wan, Y. The Roles of Small Extracellular Vesicles in Lung Cancer: Molecular Pathology, Mechanisms, Diagnostics, and Therapeutics. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic BMSC-Derived Exosomal miRNAs Promote Metastasis of Lung Cancer Cells via STAT3-Induced EMT. Mol. Cancer 2019, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Benito-Martín, A.; Jasiulionis, M.G.; García-Silva, S. Extracellular Vesicles and Melanoma: New Perspectives on Tumor Microenvironment and Metastasis. Front. Cell Dev. Biol. 2022, 10, 1061982. [Google Scholar] [CrossRef]
- Schneider, N.; Hermann, P.C.; Eiseler, T.; Seufferlein, T. Emerging Roles of Small Extracellular Vesicles in Gastrointestinal Cancer Research and Therapy. Cancers 2024, 16, 567. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Sánchez, V.; Rodríguez-Hernández, R.M.; Aguilar-Ruíz, S.R.; Torres-Aguilar, H.; Romero-Tlalolini, M.d.l.A. Extracellular Vesicles in Cervical Cancer and HPV Infection. Membranes 2021, 11, 453. [Google Scholar] [CrossRef] [PubMed]
- Moulin, C.; Crupi, M.J.F.; Ilkow, C.S.; Bell, J.C.; Boulton, S. Extracellular Vesicles and Viruses: Two Intertwined Entities. Int. J. Mol. Sci. 2023, 24, 1036. [Google Scholar] [CrossRef] [PubMed]
- Mui, U.N.; Haley, C.T.; Tyring, S.K. Viral Oncology: Molecular Biology and Pathogenesis. J. Clin. Med. 2017, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Nolte-‘t Hoen, E.; Cremer, T.; Gallo, R.C.; Margolis, L.B. Extracellular Vesicles and Viruses: Are They Close Relatives? Proc. Natl. Acad. Sci. USA 2016, 113, 9155–9161. [Google Scholar] [CrossRef] [PubMed]
- Alenquer, M.; Amorim, M.J. Exosome Biogenesis, Regulation, and Function in Viral Infection. Viruses 2015, 17, 5066–5083. [Google Scholar] [CrossRef]
- Bou, J.-V.; Taguwa, S.; Matsuura, Y. Trick-or-Trap: Extracellular Vesicles and Viral Transmission. Vaccines 2023, 11, 1532. [Google Scholar] [CrossRef] [PubMed]
- Khatua, A.K.; Taylor, H.E.; Hildreth, J.E.K.; Popik, W. Exosomes Packaging APOBEC3G Confer Human Immunodeficiency Virus Resistance to Recipient Cells. J. Virol. 2009, 83, 512–521. [Google Scholar] [CrossRef]
- Warren, C.J.; Santiago, M.L.; Pyeon, D. APOBEC3: Friend or Foe in Human Papillomavirus Infection and Oncogenesis? Annu. Rev. Virol. 2022, 9, 375–395. [Google Scholar] [CrossRef] [PubMed]
- Lovšin, N.; Gangupam, B.; Bergant Marušič, M. The Intricate Interplay between APOBEC3 Proteins and DNA Tumour Viruses. Pathogens 2024, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Kwasnik, M.; Socha, W.; Czech, B.; Wasiak, M.; Rola, J.; Rozek, W. Protein-Coding Region Derived Small RNA in Exosomes from Influenza A Virus–Infected Cells. Int. J. Mol. Sci. 2023, 24, 867. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hinson, E.R.; Cresswell, P. The Interferon-Inducible Protein Viperin Inhibits Influenza Virus Release by Perturbing Lipid Rafts. Cell Host Microbe 2007, 2, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Nasr, N.; Maddocks, S.; Turville, S.G.; Harman, A.N.; Woolger, N.; Helbig, K.J.; Wilkinson, J.; Bye, C.R.; Wright, T.K.; Rambukwelle, D.; et al. HIV-1 Infection of Human Macrophages Directly Induces Viperin Which Inhibits Viral Production. Blood 2012, 120, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Guo, H.; Xu, C.; Chang, J.; Gu, B.; Wang, L.; Block, T.M.; Guo, J.-T. Identification of Three Interferon-Inducible Cellular Enzymes That Inhibit the Replication of Hepatitis C Virus. J. Virol. 2008, 82, 1665–1678. [Google Scholar] [CrossRef]
- Fernbach, S.; Hale, B.G. SARS-CoV-2 Takes the Bait: Exosomes as Endogenous Decoys. PLoS Biol. 2022, 20, e3001787. [Google Scholar] [CrossRef] [PubMed]
- El-Shennawy, L.; Hoffmann, A.D.; Dashzeveg, N.K.; McAndrews, K.M.; Mehl, P.J.; Cornish, D.; Yu, Z.; Tokars, V.L.; Nicolaescu, V.; Tomatsidou, A.; et al. Circulating ACE2-Expressing Extracellular Vesicles Block Broad Strains of SARS-CoV-2. Nat. Commun. 2022, 13, 405. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H. The Complex Role of Extracellular Vesicles in HIV Infection. BMB Rep. 2023, 56, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.V.d.; Castro, R.O.d.; Silva, E.Z.M.d.; Silveira, P.P.; Silva-Januário, M.E.d.; Arruda, E.; Jamur, M.C.; Oliver, C.; Aguiar, R.S.; daSilva, L.L.P. Nef Neutralizes the Ability of Exosomes from CD4+ T Cells to Act as Decoys during HIV-1 Infection. PLoS ONE 2014, 9, e113691. [Google Scholar] [CrossRef] [PubMed]
- Suptawiwat, O.; Ruangrung, K.; Boonarkart, C.; Puthavathana, P.; Maneechotesuwan, K.; Charngkaew, K.; Chomanee, N.; Auewarakul, P. Microparticle and Anti-Influenza Activity in Human Respiratory Secretion. PLoS ONE 2017, 12, e0183717. [Google Scholar] [CrossRef] [PubMed]
- Bedford, J.G.; Infusini, G.; Dagley, L.F.; Villalon-Letelier, F.; Zheng, M.Z.M.; Bennett-Wood, V.; Reading, P.C.; Wakim, L.M. Airway Exosomes Released During Influenza Virus Infection Serve as a Key Component of the Antiviral Innate Immune Response. Front. Immunol. 2020, 11, 887. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.N.; Majumdar, N.; Williams, F.; Rajput, S.; Pokhrel, L.R.; Cook, P.P.; Akula, S.M. MicroRNAs: Small but Key Players in Viral Infections and Immune Responses to Viral Pathogens. Biology 2023, 12, 1334. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Xu, C.; Fang, S.; Zhao, P.; Wang, Y.; Liu, H.; Yuan, W.; Qi, Z. Exosomal MicroRNAs Derived From Umbilical Mesenchymal Stem Cells Inhibit Hepatitis C Virus Infection. Stem Cells Transl. Med. 2016, 5, 1190–1203. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-J.; Lee, E.-N.; Park, J.-H.; Lee, J.K.; Cho, G.J.; Park, I.-H.; Shin, O.S. Anti-Viral Activities of Umbilical Cord Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Against Human Respiratory Viruses. Front. Cell. Infect. Microbiol. 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Heidari, F.; Seyedebrahimi, R.; Yang, P.; Farsani, M.; Ababzadeh, S.; Kalhor, N.; Manoochehri, H.; Sheykhhasan, M.; Azimzadeh, M. Exosomes in Viral Infection: Effects for Pathogenesis and Treatment Strategies. Biocell 2023, 47, 2597–2608. [Google Scholar] [CrossRef]
- Souza, A.G.; Colli, L.M. Extracellular Vesicles and Interleukins: Novel Frontiers in Diagnostic and Therapeutic for Cancer. Front. Immunol. 2022, 13, 836922. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, W.; Freeman, M.L.; Lederman, M.M.; Vasilieva, E.; Romero, R.; Margolis, L. A System of Cytokines Encapsulated in ExtraCellular Vesicles. Sci. Rep. 2018, 8, 8973. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.S.; Diergaarde, B.; Whiteside, T.L. Small Extracellular Vesicles in Plasma Carry Luminal Cytokines That Remain Undetectable by Antibody-Based Assays in Cancer Patients and Healthy Donors. BJC Rep. 2024, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Velandia-Romero, M.L.; Calderón-Peláez, M.A.; Balbás-Tepedino, A.; Márquez-Ortiz, R.A.; Madroñero, L.J.; Barreto Prieto, A.; Castellanos, J.E. Extracellular Vesicles of U937 Macrophage Cell Line Infected with DENV-2 Induce Activation in Endothelial Cells EA.Hy926. PLoS ONE 2020, 15, e0227030. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.A.; Zhao, H.; Yue, S.C.; Anandaiah, A.; Koziel, H.; Tachado, S.D. Novel HIV-1 MiRNAs Stimulate TNFα Release in Human Macrophages via TLR8 Signaling Pathway. PLoS ONE 2014, 9, e106006. [Google Scholar] [CrossRef] [PubMed]
- Assil, S.; Webster, B.; Dreux, M. Regulation of the Host Antiviral State by Intercellular Communications. Viruses 2015, 7, 4707–4733. [Google Scholar] [CrossRef] [PubMed]
- El Safadi, D.; Mokhtari, A.; Krejbich, M.; Lagrave, A.; Hirigoyen, U.; Lebeau, G.; Viranaicken, W.; Krejbich-Trotot, P. Exosome-Mediated Antigen Delivery: Unveiling Novel Strategies in Viral Infection Control and Vaccine Design. Vaccines 2024, 12, 280. [Google Scholar] [CrossRef] [PubMed]
- Lindenbergh, M.F.S.; Stoorvogel, W. Antigen Presentation by Extracellular Vesicles from Professional Antigen-Presenting Cells. Annu. Rev. Immunol. 2018, 36, 435–459. [Google Scholar] [CrossRef] [PubMed]
- Rey-Cadilhac, F.; Rachenne, F.; Missé, D.; Pompon, J. Viral Components Trafficking with(in) Extracellular Vesicles. Viruses 2023, 15, 2333. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Hensley, L.; McKnight, K.L.; Hu, F.; Madden, V.; Ping, L.; Jeong, S.-H.; Walker, C.; Lanford, R.E.; Lemon, S.M. A Pathogenic Picornavirus Acquires an Envelope by Hijacking Cellular Membranes. Nature 2013, 496, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.M.; Tsueng, G.; Sin, J.; Mangale, V.; Rahawi, S.; McIntyre, L.L.; Williams, W.; Kha, N.; Cruz, C.; Hancock, B.M.; et al. Coxsackievirus B Exits the Host Cell in Shed Microvesicles Displaying Autophagosomal Markers. PLoS Pathog. 2014, 10, e1004045. [Google Scholar] [CrossRef] [PubMed]
- Altan-Bonnet, N. Extracellular Vesicles Are the Trojan Horses of Viral Infection. Curr. Opin. Microbiol. 2016, 32, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.E.; Rossignol, E.D.; Chang, D.; Zaia, J.; Forrester, I.; Raja, K.; Winbigler, H.; Nicastro, D.; Jackson, W.T.; Bullitt, E. Complexity and Ultrastructure of Infectious Extracellular Vesicles from Cells Infected by Non-Enveloped Virus. Sci. Rep. 2020, 10, 7939. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Xiong, S. Exosomes Mediate Coxsackievirus B3 Transmission and Expand the Viral Tropism. PLoS Pathog. 2023, 19, e1011090. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Jirintai, S.; Takahashi, M.; Kobayashi, T.; Tanggis; Nishizawa, T.; Kouki, T.; Yashiro, T.; Okamoto, H. Hepatitis E Virus Egress Depends on the Exosomal Pathway, with Secretory Exosomes Derived from Multivesicular Bodies. J. Gen. Virol. 2014, 95 Pt 10, 2166–2175. [Google Scholar] [CrossRef] [PubMed]
- Iša, P.; Pérez-Delgado, A.; Quevedo, I.R.; López, S.; Arias, C.F. Rotaviruses Associate with Distinct Types of Extracellular Vesicles. Viruses 2020, 12, 763. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Glitscher, M.; Tonnemacher, S.; Schollmeier, A.; Raupach, J.; Zahn, T.; Eberle, R.; Krijnse-Locker, J.; Basic, M.; Hildt, E. Presence of Intact Hepatitis B Virions in Exosomes. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, X.; Yu, Q.; He, J.J. Exosome-Associated Hepatitis C Virus in Cell Cultures and Patient Plasma. Biochem. Biophys. Res. Commun. 2014, 455, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Kordbacheh, R.; Sin, J. Extracellular Vesicles: A Novel Mode of Viral Propagation Exploited by Enveloped and Non-Enveloped Viruses. Microorganisms 2024, 12, 274. [Google Scholar] [CrossRef] [PubMed]
- Columba Cabezas, S.; Federico, M. Sequences within RNA Coding for HIV-1 Gag P17 Are Efficiently Targeted to Exosomes. Cell. Microbiol. 2013, 15, 412–429. [Google Scholar] [CrossRef]
- Sukriti, S.; Choudhary, M.C.; Maras, J.S.; Sharma, S.; Thangariyal, S.; Singh, A.; Das, S.; Islam, M.; Sharma, S.; Trehanpati, N.; et al. Extracellular Vesicles from Hepatitis B Patients Serve as Reservoir of Hepatitis B Virus DNA. J. Viral. Hepat. 2019, 26, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Sanada, T.; Hirata, Y.; Naito, Y.; Yamamoto, N.; Kikkawa, Y.; Ishida, Y.; Yamasaki, C.; Tateno, C.; Ochiya, T.; Kohara, M. Transmission of HBV DNA Mediated by Ceramide-Triggered Extracellular Vesicles. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnaiah, V.; Thumann, C.; Fofana, I.; Habersetzer, F.; Pan, Q.; de Ruiter, P.E.; Willemsen, R.; Demmers, J.A.A.; Stalin Raj, V.; Jenster, G.; et al. Exosome-Mediated Transmission of Hepatitis C Virus between Human Hepatoma Huh7.5 Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 13109–13113. [Google Scholar] [CrossRef] [PubMed]
- Mata-Rocha, M.; Rodríguez-Hernández, R.M.; Chávez-Olmos, P.; Garrido, E.; Robles-Vázquez, C.; Aguilar-Ruiz, S.; Torres-Aguilar, H.; González-Torres, C.; Gaytan-Cervantes, J.; Mejía-Aranguré, J.M.; et al. Presence of HPV DNA in Extracellular Vesicles from HeLa Cells and Cervical Samples. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2020, 38, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Canitano, A.; Venturi, G.; Borghi, M.; Ammendolia, M.G.; Fais, S. Exosomes Released in Vitro from Epstein-Barr Virus (EBV)-Infected Cells Contain EBV-Encoded Latent Phase mRNAs. Cancer Lett. 2013, 337, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, N.R.; Chadha, R.; Kumar, S.; Choedon, T.; Reddy, V.S.; Kumar, V. The HBx Gene of Hepatitis B Virus Can Influence Hepatic Microenvironment via Exosomes by Transferring Its mRNA and Protein. Virus Res. 2017, 240, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Otaguiri, K.K.; Dos Santos, D.F.; Slavov, S.N.; Depieri, L.V.; Palma, P.V.B.; Meirelles, F.V.; Covas, D.T.; da Silveira, J.C.; Kashima, S. TAX-mRNA-Carrying Exosomes from Human T Cell Lymphotropic Virus Type 1-Infected Cells Can Induce Interferon-Gamma Production In Vitro. AIDS Res. Hum. Retroviruses 2018, 34, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Chiantore, M.V.; Mangino, G.; Iuliano, M.; Zangrillo, M.S.; De Lillis, I.; Vaccari, G.; Accardi, R.; Tommasino, M.; Columba Cabezas, S.; Federico, M.; et al. Human Papillomavirus E6 and E7 Oncoproteins Affect the Expression of Cancer-Related microRNAs: Additional Evidence in HPV-Induced Tumorigenesis. J. Cancer Res. Clin. Oncol. 2016, 142, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Wower, I.K.; Brandebourg, T.D.; Wower, J. New Insights on the Mobility of Viral and Host Non-Coding RNAs Reveal Extracellular Vesicles as Intriguing Candidate Antiviral Targets. Pathogens 2020, 9, 876. [Google Scholar] [CrossRef] [PubMed]
- Troyer, Z.; Alhusaini, N.; Tabler, C.O.; Sweet, T.; de Carvalho, K.I.L.; Schlatzer, D.M.; Carias, L.; King, C.L.; Matreyek, K.; Tilton, J.C. Extracellular Vesicles Carry SARS-CoV-2 Spike Protein and Serve as Decoys for Neutralizing Antibodies. J. Extracell. Vesicles 2021, 10, e12112. [Google Scholar] [CrossRef] [PubMed]
- Masciopinto, F.; Giovani, C.; Campagnoli, S.; Galli-Stampino, L.; Colombatto, P.; Brunetto, M.; Yen, T.S.B.; Houghton, M.; Pileri, P.; Abrignani, S. Association of Hepatitis C Virus Envelope Proteins with Exosomes. Eur. J. Immunol. 2004, 34, 2834–2842. [Google Scholar] [CrossRef] [PubMed]
- Arakelyan, A.; Fitzgerald, W.; Zicari, S.; Vanpouille, C.; Margolis, L. Extracellular Vesicles Carry HIV Env and Facilitate Hiv Infection of Human Lymphoid Tissue. Sci. Rep. 2017, 7, 1695. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Xu, Y.; Liu, N.; Lv, D.; Chen, Y.; Liu, Z.; Jin, X.; Xiao, M.; Lavillette, D.; Zhong, J.; et al. Extracellular Vesicles from Zika Virus-Infected Cells Display Viral E Protein That Binds ZIKV-Neutralizing Antibodies to Prevent Infection Enhancement. EMBO J. 2023, 42, e112096. [Google Scholar] [CrossRef] [PubMed]
- Sami Saribas, A.; Cicalese, S.; Ahooyi, T.M.; Khalili, K.; Amini, S.; Sariyer, I.K. HIV-1 Nef Is Released in Extracellular Vesicles Derived from Astrocytes: Evidence for Nef-Mediated Neurotoxicity. Cell Death Dis. 2018, 8, e2542. [Google Scholar] [CrossRef] [PubMed]
- Nkosi, D.; Sun, L.; Duke, L.C.; Meckes, D.G., Jr. Epstein-Barr Virus LMP1 Manipulates the Content and Functions of Extracellular Vesicles to Enhance Metastatic Potential of Recipient Cells. PLOS Pathog. 2020, 16, e1009023. [Google Scholar] [CrossRef]
- Narayanan, A.; Jaworski, E.; Van Duyne, R.; Iordanskiy, S.; Guendel, I.; Das, R.; Currer, R.; Sampey, G.; Chung, M.; Kehn-Hall, K.; et al. Exosomes Derived from HTLV-1 Infected Cells Contain the Viral Protein Tax. Retrovirology 2014, 11 (Suppl. 1), O46. [Google Scholar] [CrossRef]
- Mack, M.; Kleinschmidt, A.; Brühl, H.; Klier, C.; Nelson, P.J.; Cihak, J.; Plachý, J.; Stangassinger, M.; Erfle, V.; Schlöndorff, D. Transfer of the Chemokine Receptor CCR5 between Cells by Membrane-Derived Microparticles: A Mechanism for Cellular Human Immunodeficiency Virus 1 Infection. Nat. Med. 2000, 6, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Thaker, S.K.; Ch’ng, J.; Christofk, H.R. Viral Hijacking of Cellular Metabolism. BMC Biol. 2019, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Dolja, V.V.; Krupovic, M. The Logic of Virus Evolution. Cell Host Microbe 2022, 30, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, M.; Li, S.; Bu, Y.; Xu, Z.; Zhu, G.; Wu, C.; Zhao, K.; Li, A.; Chen, Q.; et al. Hepatitis B Virus Hijacks TSG101 to Facilitate Egress via Multiple Vesicle Bodies. PLoS Pathog. 2023, 19, e1011382. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Lata, S.; Ali, A.; Banerjea, A.C. Dengue Haemorrhagic Fever: A Job Done via Exosomes? Emerg. Microbes Infect. 2019, 8, 1626–1635. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Kim, J.-H. Diverse Effects of Exosomes on COVID-19: A Perspective of Progress From Transmission to Therapeutic Developments. Front. Immunol. 2021, 12, 716407. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Koike, M.; Moriishi, E.; Kawabata, A.; Tang, H.; Oyaizu, H.; Uchiyama, Y.; Yamanishi, K. Human Herpesvirus-6 Induces MVB Formation, and Virus Egress Occurs by an Exosomal Release Pathway. Traffic 2008, 9, 1728–1742. [Google Scholar] [CrossRef] [PubMed]
- Eliassen, E.; Lum, E.; Pritchett, J.; Ongradi, J.; Krueger, G.; Crawford, J.R.; Phan, T.L.; Ablashi, D.; Hudnall, S.D. Human Herpesvirus 6 and Malignancy: A Review. Front. Oncol. 2018, 8, 512. [Google Scholar] [CrossRef] [PubMed]
- Flomm, F.J.; Soh, T.K.; Schneider, C.; Wedemann, L.; Britt, H.M.; Thalassinos, K.; Pfitzner, S.; Reimer, R.; Grünewald, K.; Bosse, J.B. Intermittent Bulk Release of Human Cytomegalovirus. PLoS Pathog. 2022, 18, e1010575. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, O.; Akbarzadeh, S.; Ghazanfari Hashemi, M.; Gholami, M.; Amini, P.; Yekanipour, Z.; Tabatabaie, R.; Yasamineh, S.; Hosseini, P.; Poortahmasebi, V. Hepatitis A: Viral Structure, Classification, Life Cycle, Clinical Symptoms, Diagnosis Error, and Vaccination. Can. J. Infect. Dis. Med. Microbiol. 2023, 2023, 4263309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, J.; Zi, R.; Ji, L.; Hu, J.; Wu, Z.; Fu, Y. Enterovirus 71-Induced Autophagosome Fusion with Multivesicular Bodies Facilitates Viral RNA Packaging into Exosomes. Microb. Pathog. 2022, 173 Pt A, 105875. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Celma, C.C.; Roy, P. Influence of Cellular Trafficking Pathway on Bluetongue Virus Infection in Ovine Cells. Viruses 2015, 7, 2378–2403. [Google Scholar] [CrossRef] [PubMed]
- Roy, P. Bluetongue Virus Assembly and Exit Pathways. Adv. Virus. Res. 2020, 108, 249–273. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.H.; Cada, A.K. Inside Job: How the ESCRTs release HIV-1 from infected cells. Biochem. Soc. Trans. 2018, 46, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Ip, N.C.Y.; Abbink, T.E.M.; Kenyon, J.C.; Lever, A.M.L. ESCRT-II Functions by Linking to ESCRT-I in Human Immunodeficiency Virus-1 Budding. Cell. Microbiol. 2020, 22, e13161. [Google Scholar] [CrossRef] [PubMed]
- Hudait, A.; Hurley, J.H.; Voth, G.A. Dynamics of Upstream ESCRT Organization at the HIV-1 Budding Site. Biophys. J. 2023, 122, 2655–2674. [Google Scholar] [CrossRef] [PubMed]
- Arii, J.; Watanabe, M.; Maeda, F.; Tokai-Nishizumi, N.; Chihara, T.; Miura, M.; Maruzuru, Y.; Koyanagi, N.; Kato, A.; Kawaguchi, Y. ESCRT-III Mediates Budding across the Inner Nuclear Membrane and Regulates Its Integrity. Nat. Commun. 2018, 9, 3379. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Wilson, D.W. Seeking Closure: How Do Herpesviruses Recruit the Cellular ESCRT Apparatus? J. Virol. 2019, 93, e00392-19. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.S.; Chang, Y. Why Do Viruses Cause Cancer? Highlights of the First Century of Human Tumour Virology. Nat. Rev. Cancer 2010, 10, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Moore, P.S.; Weiss, R.A. Human Oncogenic Viruses: Nature and Discovery. Philosophical. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160264. [Google Scholar] [CrossRef] [PubMed]
- Green, V.A.; Munshi, S.U.; Marakalala, M.J.; Mourão, M.M. Molecular Mechanisms of Viral Infection and Propagation: An Overview of the Second Advanced Summer School in Africa. IUBMB Life 2010, 62, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Owliaee, I.; Khaledian, M.; Boroujeni, A.K.; Shojaeian, A. Engineered Small Extracellular Vesicles as a Novel Platform to Suppress Human Oncovirus-Associated Cancers. Infect. Agents Cancer 2023, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, D.; Yan, W.; Wang, Y.; You, J.; Wan, X.; Xi, D.; Luo, X.; Han, M.; Ning, Q. Interferon-Induced Macrophage-Derived Exosomes Mediate Antiviral Activity Against Hepatitis B Virus Through miR-574-5p. J. Infect Dis. 2021, 223, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Chinnici, C.M.; Russelli, G.; Bulati, M.; Miceli, V.; Gallo, A.; Busà, R.; Tinnirello, R.; Conaldi, P.G.; Iannolo, G. Mesenchymal Stromal Cell Secretome in Liver Failure: Perspectives on COVID-19 Infection Treatment. World J. Gastroenterol. 2021, 27, 1905–1919. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, E.; Narayanan, A.; Van Duyne, R.; Shabbeer-Meyering, S.; Iordanskiy, S.; Saifuddin, M.; Das, R.; Afonso, P.V.; Sampey, G.C.; Chung, M.; et al. Human T-Lymphotropic Virus Type 1-Infected Cells Secrete Exosomes That Contain Tax Protein. J. Biol. Chem. 2014, 289, 22284–22305. [Google Scholar] [CrossRef] [PubMed]
- Kawano, K.; Hashikura, Y.; Umekita, K. Purification Method of Extracellular Vesicles Derived from Human T-Cell Leukemia Virus Type 1-Infected Cells without Virions. Viruses 2024, 16, 249. [Google Scholar] [CrossRef]
- Pinto, D.O.; Al Sharif, S.; Mensah, G.; Cowen, M.; Khatkar, P.; Erickson, J.; Branscome, H.; Lattanze, T.; DeMarino, C.; Alem, F.; et al. Extracellular Vesicles from HTLV-1 Infected Cells Modulate Target Cells and Viral Spread. Retrovirology 2021, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Kalinichenko, S.; Komkov, D.; Mazurov, D. HIV-1 and HTLV-1 Transmission Modes: Mechanisms and Importance for Virus Spread. Viruses 2022, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- El-Saghir, J.; Nassar, F.; Tawil, N.; El-Sabban, M. ATL-Derived Exosomes Modulate Mesenchymal Stem Cells: Potential Role in Leukemia Progression. Retrovirology 2016, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Dreux, M.; Garaigorta, U.; Boyd, B.; Décembre, E.; Chung, J.; Whitten-Bauer, C.; Wieland, S.; Chisari, F.V. Short-Range Exosomal Transfer of Viral RNA from Infected Cells to Plasmacytoid Dendritic Cells Triggers Innate Immunity. Cell Host Microbe 2012, 12, 558–570. [Google Scholar] [CrossRef]
- Bukong, T.; Momen-Heravi, F.; Kodys, K.; Bala, S.; Szabo, G. Exosomes from Hepatitis C Infected Patients Transmit HCV Infection and Contain Replication Competent Viral RNA in Complex with Ago2-miR122-HSP90. PLoS Pathog. 2014, 10, e1004424. [Google Scholar] [CrossRef] [PubMed]
- Karamichali, E.; Chihab, H.; Kakkanas, A.; Marchio, A.; Karamitros, T.; Pogka, V.; Varaklioti, A.; Kalliaropoulos, A.; Martinez-Gonzales, B.; Foka, P.; et al. HCV Defective Genomes Promote Persistent Infection by Modulating the Viral Life Cycle. Front. Microbiol. 2018, 9, 2942. [Google Scholar] [CrossRef] [PubMed]
- Giannessi, F.; Aiello, A.; Franchi, F.; Percario, Z.A.; Affabris, E. The Role of Extracellular Vesicles as Allies of HIV, HCV and SARS Viruses. Viruses 2020, 12, 571. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.O.; DeMarino, C.; Pleet, M.L.; Cowen, M.; Branscome, H.; Al Sharif, S.; Jones, J.; Dutartre, H.; Lepene, B.; Liotta, L.A.; et al. HTLV-1 Extracellular Vesicles Promote Cell-to-Cell Contact. Front. Microbiol. 2019, 10, 2147. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Kanda, T.; Yokosuka, O.; Kato, N.; Matsuoka, S.; Moriyama, M. Exosomes and Hepatocellular Carcinoma: From Bench to Bedside. Int. J. Mol. Sci. 2019, 20, 1406. [Google Scholar] [CrossRef]
- Wang, H.; Hou, L.; Li, A.; Duan, Y.; Gao, H.; Song, X. Expression of Serum Exosomal MicroRNA-21 in Human Hepatocellular Carcinoma. Biomed Res. Int. 2014, 2014, 864894. [Google Scholar] [CrossRef] [PubMed]
- Badami, E.; Carcione, C.; Chinnici, C.M.; Tinnirello, R.; Conaldi, P.G.; Iannolo, G. HCV Interplay With Mir34a: Implications in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 803278. [Google Scholar] [CrossRef] [PubMed]
- Kouwaki, T.; Fukushima, Y.; Daito, T.; Sanada, T.; Yamamoto, N.; Mifsud, E.J.; Leong, C.R.; Tsukiyama-Kohara, K.; Kohara, M.; Matsumoto, M.; et al. Extracellular Vesicles Including Exosomes Regulate Innate Immune Responses to Hepatitis B Virus Infection. Front. Immunol. 2016, 7, 335. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Han, Q.; Hou, Z.; Zhang, C.; Tian, Z.; Zhang, J. Exosomes Mediate Hepatitis B Virus (HBV) Transmission and NK-Cell Dysfunction. Cell. Mol. Immunol. 2017, 14, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cao, D.; Han, N.; Zhang, M.; Jiang, W.; Wang, X.; Zeng, Q.; Tang, H. Hepatitis B Virus-Encoded MicroRNA (HBV-miR-3) Inhibits FIH-1 Expression to Promote Tumor Angiogenesis in HBV-Related Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2023, 10, 2337–2353. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhou, R.; Li, N.; Huang, Y.; Fan, X.-G. Hepatitis B Virus X Protein in Liver Tumor Microenvironment. Tumor. Biol. 2016, 37, 15371–15381. [Google Scholar] [CrossRef] [PubMed]
- Nagel, R.; Martens-de Kemp, S.R.; Buijze, M.; Jacobs, G.; Braakhuis, B.J.M.; Brakenhoff, R.H. Treatment Response of HPV-Positive and HPV-Negative Head and Neck Squamous Cell Carcinoma Cell Lines. Oral Oncol. 2013, 49, 560–566. [Google Scholar] [CrossRef]
- Honegger, A.; Leitz, J.; Bulkescher, J.; Hoppe-Seyler, K.; Hoppe-Seyler, F. Silencing of Human Papillomavirus (HPV) E6/E7 Oncogene Expression Affects Both the Contents and the Amounts of Extracellular Microvesicles Released from HPV-Positive Cancer Cells. Int. J. Cancer 2013, 133, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.K.-F.; Dawson, C.W.; Lung, H.L.; Wong, K.-L.; Young, L.S. The Role of EBV-Encoded LMP1 in the NPC Tumor Microenvironment: From Function to Therapy. Front. Oncol. 2021, 11, 640207. [Google Scholar] [CrossRef] [PubMed]
- Aga, M.; Bentz, G.L.; Raffa, S.; Torrisi, M.R.; Kondo, S.; Wakisaka, N.; Yoshizaki, T.; Pagano, J.S.; Shackelford, J. Exosomal HIF1α Supports Invasive Potential of Nasopharyngeal Carcinoma-Associated LMP1-Positive Exosomes. Oncogene 2014, 33, 4613–4622. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Jang, S.-I.; Ong, H.L.; Perez, P.; Tandon, M.; Ambudkar, I.; Illei, G.; Alevizos, I. Targeting the Ca2+ Sensor STIM1 by Exosomal Transfer of Ebv-miR-BART13-3p Is Associated with Sjögren’s Syndrome. EBioMedicine 2016, 10, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Meckes, D.G.; Shair, K.H.Y.; Marquitz, A.R.; Kung, C.-P.; Edwards, R.H.; Raab-Traub, N. Human Tumor Virus Utilizes Exosomes for Intercellular Communication. Proc. Natl. Acad. Sci. USA 2010, 107, 20370–20375. [Google Scholar] [CrossRef] [PubMed]
- Mrizak, D.; Martin, N.; Barjon, C.; Jimenez-Pailhes, A.-S.; Mustapha, R.; Niki, T.; Guigay, J.; Pancré, V.; de Launoit, Y.; Busson, P.; et al. Effect of Nasopharyngeal Carcinoma-Derived Exosomes on Human Regulatory T Cells. J. Natl. Cancer Inst. 2015, 107, 363. [Google Scholar] [CrossRef] [PubMed]
- Klibi, J.; Niki, T.; Riedel, A.; Pioche-Durieu, C.; Souquere, S.; Rubinstein, E.; Le Moulec, S.; Guigay, J.; Hirashima, M.; Guemira, F.; et al. Blood Diffusion and Th1-Suppressive Effects of Galectin-9-Containing Exosomes Released by Epstein-Barr Virus-Infected Nasopharyngeal Carcinoma Cells. Blood 2009, 113, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Chugh, P.E.; Sin, S.-H.; Ozgur, S.; Henry, D.H.; Menezes, P.; Griffith, J.; Eron, J.J.; Damania, B.; Dittmer, D.P. Systemically Circulating Viral and Tumor-Derived microRNAs in KSHV-Associated Malignancies. PLoS Pathog. 2013, 9, e1003484. [Google Scholar] [CrossRef] [PubMed]
- Hoshina, S.; Sekizuka, T.; Kataoka, M.; Hasegawa, H.; Hamada, H.; Kuroda, M.; Katano, H. Profile of Exosomal and Intracellular microRNA in Gamma-Herpesvirus-Infected Lymphoma Cell Lines. PLoS ONE 2016, 11, e0162574. [Google Scholar] [CrossRef] [PubMed]
- Barrett, L.; Dai, L.; Wang, S.; Qin, Z. Kaposi’s Sarcoma-Associated Herpesvirus and Extracellular Vesicles. J. Med. Virol. 2021, 93, 3294–3299. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.V.; Kerur, N.; Bottero, V.; Dutta, S.; Chakraborty, S.; Ansari, M.A.; Paudel, N.; Chikoti, L.; Chandran, B. Kaposi’s Sarcoma-Associated Herpesvirus Latency in Endothelial and B Cells Activates Gamma Interferon-Inducible Protein 16-Mediated Inflammasomes. J. Virol. 2013, 87, 4417–4431. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Lee, J.; Lee, S.; Kang, S.-K.; Park, S.J.; Yoo, S.-M.; Lee, M.-S. Extracellular Vesicles From KSHV-Infected Cells Stimulate Antiviral Immune Response Through Mitochondrial DNA. Front. Immunol. 2019, 10, 876. [Google Scholar] [CrossRef]
- Yang, R.; Lee, E.E.; Kim, J.; Choi, J.H.; Kolitz, E.; Chen, Y.; Crewe, C.; Salisbury, N.J.H.; Scherer, P.E.; Cockerell, C.; et al. Characterization of ALTO-Encoding Circular RNAs Expressed by Merkel Cell Polyomavirus and Trichodysplasia Spinulosa Polyomavirus. PLoS Pathog. 2021, 17, e1009582. [Google Scholar] [CrossRef]
- Renwick, N.; Cekan, P.; Masry, P.A.; McGeary, S.E.; Miller, J.B.; Hafner, M.; Li, Z.; Mihailovic, A.; Morozov, P.; Brown, M.; et al. Multicolor microRNA FISH Effectively Differentiates Tumor Types. J. Clin. Investig. 2013, 123, 2694–2702. [Google Scholar] [CrossRef] [PubMed]
- Emanuelson, C.; Ankenbruck, N.; Kumbhare, R.; Thomas, M.; Connelly, C.; Baktash, Y.; Randall, G.; Deiters, A. Transcriptional Inhibition of MicroRNA miR-122 by Small Molecules Reduces Hepatitis C Virus Replication in Liver Cells. J. Med. Chem. 2022, 65, 16338–16352. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, M.; Palmer, M.A.; Wilson, J.A. MicroRNA-122 Regulation of HCV Infections: Insights from Studies of miR-122-Independent Replication. Pathogens 2022, 11, 1005. [Google Scholar] [CrossRef] [PubMed]
- Cabral, B.C.A.; Hoffmann, L.; Bottaro, T.; Costa, P.F.; Ramos, A.L.A.; Coelho, H.S.M.; Villela-Nogueira, C.A.; Ürményi, T.P.; Faffe, D.S.; Silva, R. Circulating microRNAs Associated with Liver Fibrosis in Chronic Hepatitis C Patients. Biochem. Biophys. Rep. 2020, 24, 100814. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Wang, Y.; Bai, X.; Zhang, Y. Exosomes in HBV Infection. Clin. Chim. Acta 2023, 538, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Chen, J.; Megger, D.A.; Zhang, X.; Kozlowski, M.; Zhang, L.; Fang, Z.; Li, J.; Chu, Q.; Wu, M.; et al. Label-Free Proteomic Analysis of Exosomes Derived from Inducible Hepatitis B Virus-Replicating HepAD38 Cell Line. Mol. Cell. Proteom. 2017, 16 (Suppl. 1), S144–S160. [Google Scholar] [CrossRef] [PubMed]
- Todorova, V.K.; Byrum, S.D.; Mackintosh, S.G.; Jamshidi-Parsian, A.; Gies, A.J.; Washam, C.L.; Jenkins, S.V.; Spiva, T.; Bowman, E.; Reyna, N.S.; et al. Exosomal MicroRNA and Protein Profiles of Hepatitis B Virus-Related Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2023, 24, 13098. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, M.; Yamamoto, Y.; Otsuka, M.; Kitamura, K.; Ito, M.; Kawai, H.D.; Muramatsu, M.; Kagawa, T.; Kotani, A. Extracellular Vesicles Secreted by HBV-Infected Cells Modulate HBV Persistence in Hydrodynamic HBV Transfection Mouse Model. J. Biol. Chem. 2020, 295, 12449–12460. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-Y.; Xu, P.; Li, J.-H.; Zeng, C.-H.; Song, H.-F.; Chen, H.; Zhu, Y.-B.; Song, Y.-Y.; Lu, H.-L.; Shen, C.-P.; et al. Clinical Significance of Dynamics of Programmed Death Ligand-1 Expression on Circulating CD14+ Monocytes and CD19+ B Cells with the Progression of Hepatitis B Virus Infection. Viral. Immunol. 2017, 30, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.; Mackensen, A. Contribution of the PD-L1/PD-1 Pathway to T-Cell Exhaustion: An Update on Implications for Chronic Infections and Tumor Evasion. Cancer Immunol. Immunother. 2007, 56, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, Y.; Duan, J.; Ma, Y.; Shen, Z.; Wei, L.; Cui, X.; Zhang, J.; Xie, Y.; Liu, J. Quantitative Proteomic Analysis of Exosome Protein Content Changes Induced by Hepatitis B Virus in Huh-7 Cells Using SILAC Labeling and LC-MS/MS. J. Proteome Res. 2014, 13, 5391–5402. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-C.; Xia, Y.-R.; Zhou, P.; Xue, X.; Ding, S.; Liu, L.-J.; Zhu, F. Hepatitis B Core Antigen Modulates Exosomal miR-135a to Target Vesicle-Associated Membrane Protein 2 Promoting Chemoresistance in Hepatocellular Carcinoma. World J. Gastroenterol. 2021, 27, 8302–8322. [Google Scholar] [CrossRef] [PubMed]
- Guenat, D.; Hermetet, F.; Prétet, J.-L.; Mougin, C. Exosomes and Other Extracellular Vesicles in HPV Transmission and Carcinogenesis. Viruses 2017, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Jutzy, J.M.S.; Aspe, J.R.; McGregor, D.W.; Neidigh, J.W.; Wall, N.R. Survivin Is Released from Cancer Cells via Exosomes. Apoptosis 2011, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, M.M.A.; Ferguson Bennit, H.R.; Gonda, A.; Diaz Osterman, C.J.; Hibma, A.; Khan, S.; Wall, N.R. Exosomes Secreted from Human Cancer Cell Lines Contain Inhibitors of Apoptosis (IAP). Cancer Microenviron. 2015, 8, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Honegger, A.; Schilling, D.; Bastian, S.; Sponagel, J.; Kuryshev, V.; Sültmann, H.; Scheffner, M.; Hoppe-Seyler, K.; Hoppe-Seyler, F. Dependence of Intracellular and Exosomal microRNAs on Viral E6/E7 Oncogene Expression in HPV-Positive Tumor Cells. PLoS Pathog. 2015, 11, e1004712. [Google Scholar] [CrossRef] [PubMed]
- Kannan, A.; Hertweck, K.L.; Philley, J.V.; Wells, R.B.; Dasgupta, S. Genetic Mutation and Exosome Signature of Human Papilloma Virus Associated Oropharyngeal Cancer. Sci. Rep. 2017, 7, 46102. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.-K.; Park, J.-S. The Role of HPV E6 and E7 Oncoproteins in HPV-Associated Cervical Carcinogenesis. Cancer Res. Treat. 2005, 37, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Dyson, N.; Howley, P.M.; Münger, K.; Harlow, E. The Human Papilloma Virus-16 E7 Oncoprotein Is Able to Bind to the Retinoblastoma Gene Product. Science 1989, 243, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, S.; Sharma, P.; Theodoraki, M.-N.; Pietrowska, M.; Yerneni, S.S.; Lang, S.; Ferrone, S.; Whiteside, T.L. Molecular and Functional Profiles of Exosomes From HPV(+) and HPV(−) Head and Neck Cancer Cell Lines. Front. Oncol. 2018, 8, 445. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, R.; Trischitta, P.; Costa, M.; Venuti, A.; Tamburello, M.P.; Sciortino, M.T. Update of Natural Products and Their Derivatives Targeting Epstein–Barr Infection. Viruses 2024, 16, 124. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Cosmopoulos, K.; Thorley-Lawson, D.A.; van Eijndhoven, M.A.J.; Hopmans, E.S.; Lindenberg, J.L.; de Gruijl, T.D.; Würdinger, T.; Middeldorp, J.M. Functional Delivery of Viral miRNAs via Exosomes. Proc. Natl. Acad. Sci. USA 2010, 107, 6328–6333. [Google Scholar] [CrossRef] [PubMed]
- Giehler, F.; Ostertag, M.S.; Sommermann, T.; Weidl, D.; Sterz, K.R.; Kutz, H.; Moosmann, A.; Feller, S.M.; Geerlof, A.; Biesinger, B.; et al. Epstein-Barr Virus-Driven B Cell Lymphoma Mediated by a Direct LMP1-TRAF6 Complex. Nat. Commun. 2024, 15, 414. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, M.; Zhang, Z. The Role of LMP1 in Epstein-Barr Virus-Associated Gastric Cancer. Curr. Cancer Drug Targets 2024, 24, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Chen, Y.; Jia, X.; Liu, Y. The Anti-Apoptotic Role of EBV-LMP1 in Lymphoma Cells. Cancer Manag. Res. 2020, 12, 8801–8811. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Yoo, S.-M.; Choi, H.S.; Mun, J.Y.; Kang, H.-G.; Lee, J.; Park, J.; Gao, S.-J.; Lee, M.-S. Extracellular Vesicles from KSHV-Infected Endothelial Cells Activate the Complement System. Oncotarget 2017, 8, 99841–99860. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.A.M.; Alfhili, M.A.; Pakala, P.; Simon, S.; Hussain, J.; McCubrey, J.A.; Akula, S.M. miRNAs and Their Roles in KSHV Pathogenesis. Virus Res. 2019, 266, 15–24. [Google Scholar] [CrossRef]
- Yogev, O.; Lagos, D.; Enver, T.; Boshoff, C. Kaposi’s Sarcoma Herpesvirus microRNAs Induce Metabolic Transformation of Infected Cells. PLoS Pathog. 2014, 10, e1004400. [Google Scholar] [CrossRef] [PubMed]
- Yogev, O.; Henderson, S.; Hayes, M.J.; Marelli, S.S.; Ofir-Birin, Y.; Regev-Rudzki, N.; Herrero, J.; Enver, T. Herpesviruses Shape Tumour Microenvironment through Exosomal Transfer of Viral microRNAs. PLoS Pathog. 2017, 13, e1006524. [Google Scholar] [CrossRef] [PubMed]
- Dass, D.; Dhotre, K.; Chakraborty, M.; Nath, A.; Banerjee, A.; Bagchi, P.; Mukherjee, A. miRNAs in Herpesvirus Infection: Powerful Regulators in Small Packages. Viruses 2023, 15, 429. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal Integration of a Polyomavirus in Human Merkel Cell Carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Konstantinell, A.; Bruun, J.-A.; Olsen, R.; Aspar, A.; Škalko-Basnet, N.; Sveinbjørnsson, B.; Moens, U. Secretomic Analysis of Extracellular Vesicles Originating from Polyomavirus-Negative and Polyomavirus-Positive Merkel Cell Carcinoma Cell Lines. Proteomics 2016, 16, 2587–2591. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Spassova, I.; Gravemeyer, J.; Ritter, C.; Horny, K.; Lange, A.; Gambichler, T.; Ødum, N.; Schrama, D.; Schadendorf, D.; et al. Merkel Cell Carcinoma-Derived Exosome-Shuttle miR-375 Induces Fibroblast Polarization by Inhibition of RBPJ and P53. Oncogene 2021, 40, 980–996. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ważny, Ł.; Whiteside, T.L.; Pietrowska, M. Oncoviral Infections and Small Extracellular Vesicles. Viruses 2024, 16, 1291. https://doi.org/10.3390/v16081291
Ważny Ł, Whiteside TL, Pietrowska M. Oncoviral Infections and Small Extracellular Vesicles. Viruses. 2024; 16(8):1291. https://doi.org/10.3390/v16081291
Chicago/Turabian StyleWażny, Łukasz, Theresa L. Whiteside, and Monika Pietrowska. 2024. "Oncoviral Infections and Small Extracellular Vesicles" Viruses 16, no. 8: 1291. https://doi.org/10.3390/v16081291
APA StyleWażny, Ł., Whiteside, T. L., & Pietrowska, M. (2024). Oncoviral Infections and Small Extracellular Vesicles. Viruses, 16(8), 1291. https://doi.org/10.3390/v16081291







