Practical Suggestions for Assessing Rabbit Haemorrhagic Disease Virus 2 Risk to Endangered Native Lagomorphs in North America and Southern Africa
Abstract
:1. Introduction
2. Review of Key Factors Influencing RHD Epidemiology and Rabbit Survival
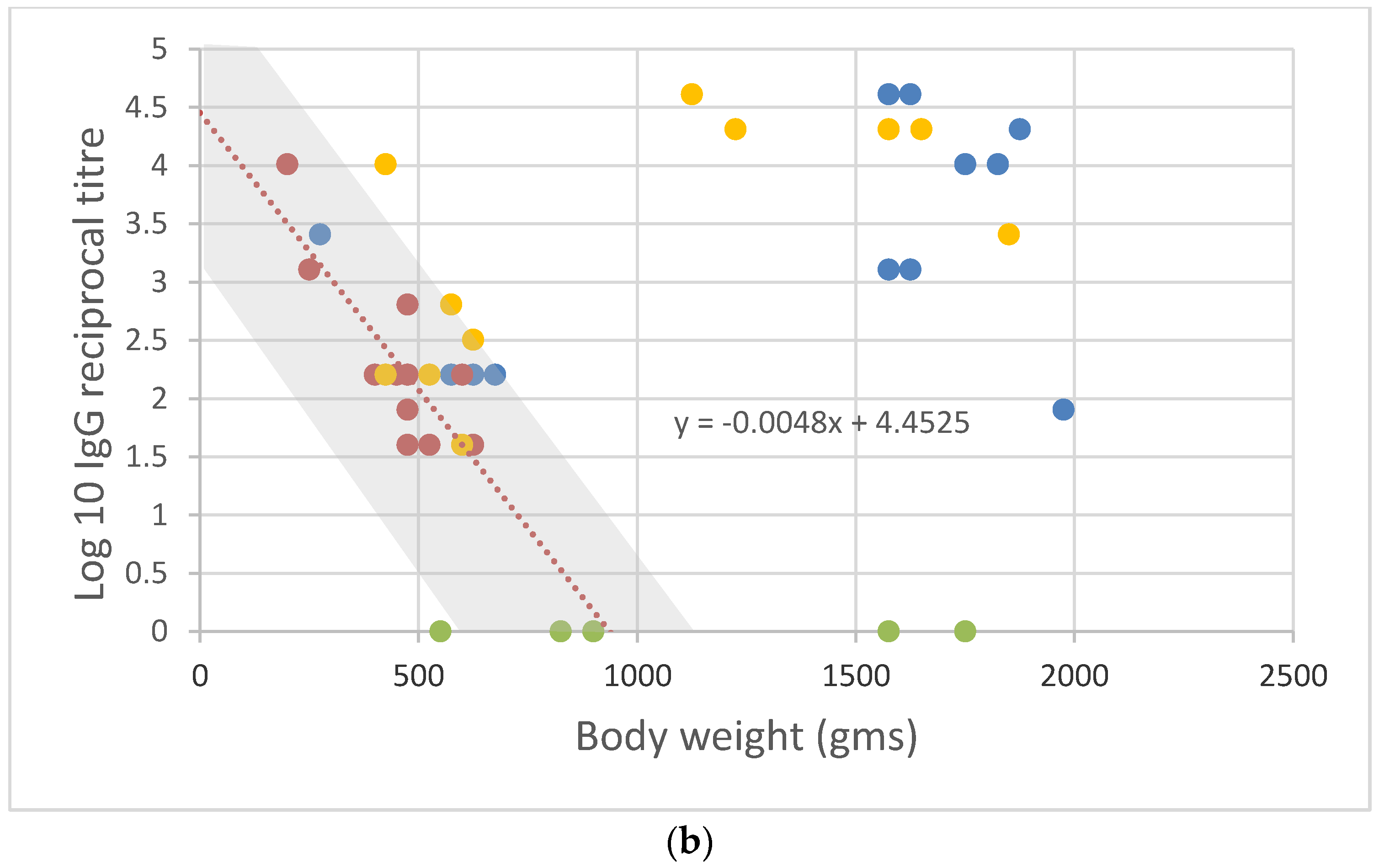
2.1. Rabbit Susceptibility
2.2. Rate of Virus Spread and Survival from Disease
2.3. Rabbit and Virus Coevolution
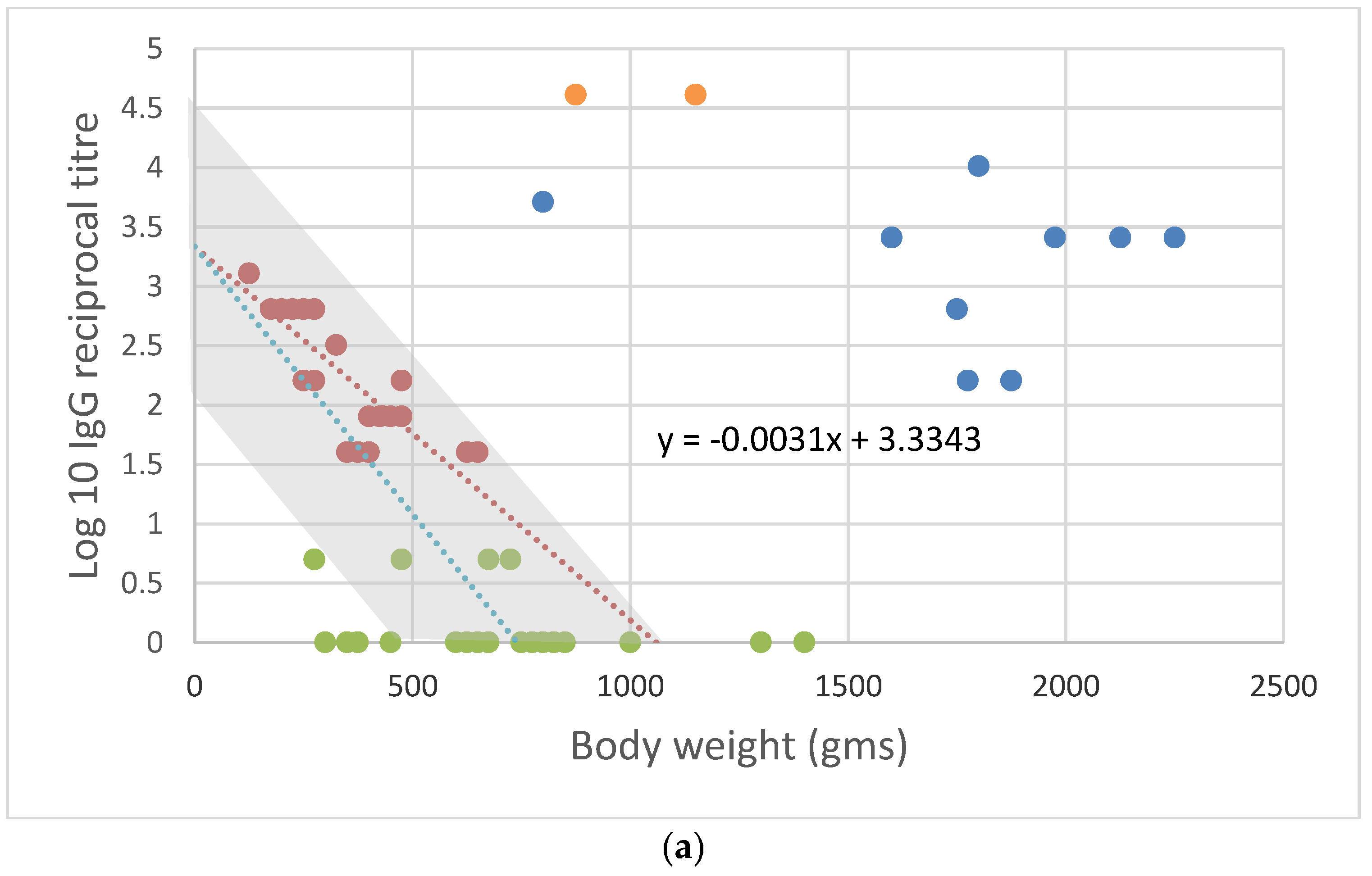
3. Assessing Risk to Other Lagomorphs
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.J.; Xue, H.P.; Pu, B.Q.; Qian, N.H. A new viral disease in rabbits. Anim. Husb. Vet. Med. (Xumu Yu Shouyi) 1984, 16, 253–255. [Google Scholar]
- Marcato, P.S.; Benazzi, C.; Vecchi, G.; Galeotti, M.; Della Salda, L.; Sarli, G.; Lucidi, P. Clinical and pathological features of viral haemorrhagic disease of rabbits and the European brown hare syndrome. Rev. Sci. Tech. 1991, 10, 371–392. [Google Scholar] [CrossRef]
- Morisse, J.P.; Le Gall, G.; Boilletot, E. Hepatitis of viral origin in Leporidae: Introduction and aetiological hypotheses. Rev. Sci. Tech. Off. Int. Epiz. 1991, 10, 269–310. [Google Scholar] [CrossRef]
- Fenner, F.; Fantini, B. Biological Control of Vertebrate Pests: The History of Myxomatosis—An Experiment in Evolution; CABI Publishing: Wallingford, UK, 1999; pp. 19–23. [Google Scholar]
- O’Hara, P. The illegal introduction of rabbit haemorrhagic disease virus in New Zealand. Rev. Sci. Tech. Off. Int. Epiz. 2006, 25, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Ohlinger, R.F.; Haas, B.; Meyers, G.; Weiland, F.; Thiel, H.J. Identification and characterization of the virus causing rabbit haemorrhagic disease. J. Virol. 1990, 64, 3331–3336. [Google Scholar] [CrossRef]
- Lavazza, A.; Vecchi, G. Osservazioni su alcuni episodi di mortalità nelle lepri. Evidenziazione al microscopio elettronico di una particella virale. Nota preliminare. Sel. Vet. 1989, 30, 461–467. [Google Scholar]
- Parra, F.; Prieto, M. Purification and characterization of a calicivirus as the causative agent of a lethal hemorrhagic disease in rabbits. J. Virol. 1990, 64, 4013–4015. [Google Scholar] [CrossRef]
- Wirblich, C.; Meyers, G.; Ohlinger, V.F.; Capucci, L.; Eskens, U.; Haas, B.; Thiel, H.J. European brown hare syndrome virus: Relationship to rabbit hemorrhagic disease virus and other caliciviruses. J. Virol. 1994, 68, 5164–5173. [Google Scholar] [CrossRef]
- Lavazza, A.; Scicluna, M.T.; Capucci, L. Susceptibility of hares and rabbits to the European brown hare syndrome virus (EBHSV) and rabbit haemorrhagic disease virus (RHDV) under experimental conditions. J. Vet. Med. Ser. B 1996, 43, 401–410. [Google Scholar] [CrossRef]
- Gregg, D.A.; House, C.; Meyer, R.; Berninger, M. Viral haemorrhagic disease of rabbits in Mexico: Epidemiology and viral characterization. Rev. Sci. Tech. Off. Int. Epiz. 1991, 10, 435–451. [Google Scholar] [CrossRef]
- Le Pendu, J.; Abrantes, J.; Bertagnoli, S.; Guitton, J.; Le Gall-Reculé, G.; Lopes, A.; Marchandeau, S.; Alda, F.; Almeida, T.; Alves, P.; et al. Proposal for a unified classification system and nomenclature of lagoviruses. J. Gen. Virol. 2017, 98, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Capucci, L.; Fusi, P.; Lavazza, A.; Pacciarini, M.L.; Rossi, C. Detection and preliminary characterization of a new rabbit calicivirus related to rabbit hemorrhagic disease virus but nonpathogenic. J. Virol. 1996, 70, 8614–8623. [Google Scholar] [CrossRef]
- Strive, T.; Wright, J.; Kovaliski, J.; Botti, G.; Capucci, L. The non-pathogenic Australian lagovirus RCV-A1 causes a prolonged infection and elicits partial cross-protection to rabbit haemorrhagic disease virus. Virology 2010, 398, 125–134. [Google Scholar] [CrossRef]
- Cavadini, P.; Molinari, S.; Merzoni, F.; Vismarra, A.; Posautz, A.; Alzaga Gil, V.; Chiari, M.; Giannini, F.; Capucci, L.; Lavazza, A. Widespread occurrence of the non-pathogenic hare calicivirus (HaCV Lagovirus GII.2) in captive-reared and free-living wild hares in Europe. Transbound. Emerg. Dis. 2021, 68, 509–518. [Google Scholar] [CrossRef]
- Le Gall-Reculé, G.; Zwingelstein, F.; Boucher, S.; Le Normand, B.; Plassiart, G.; Portejoie, Y.; Decors, A.; Bertagnoli, S.; Guérin, J.-L.; Marchandeau, S. Detection of a new variant of rabbit haemorrhagic disease virus in France. Vet. Rec. 2011, 168, 137–138. [Google Scholar] [CrossRef]
- Le Gall-Reculé, G.; Lavazza, A.; Marchandeau, S.; Bertagnoli, S.; Zwingelstein, F.; Cavadini, P.; Martinelli, N.; Lombardi, G.; Guérin, J.L.; Lemaitre, E.; et al. Emergence of a new lagovirus related to Rabbit Haemorrhagic Disease Virus. Vet. Res. 2013, 44, 81. [Google Scholar] [CrossRef] [PubMed]
- Rouco, C.; Aguayo-Adán, J.A.; Santoro, S.; Abrantes, J.; Delibes-Mateos, M. Worldwide rapid spread of the novel rabbit haemorrhagic disease virus (GI.2/RHDV2/b). Transbound. Emerg. Dis. 2019, 66, 1762–1764. [Google Scholar]
- Capucci, L.; Cavadini, P.; Schiavitto, M.; Lombardi, G.; Lavazza, A. Increased pathogenicity in rabbit haemorrhagic disease virus type 2 (RHDV2). Vet. Rec. 2017, 180, 426. [Google Scholar] [CrossRef] [PubMed]
- Puggioni, G.; Cavadini, P.; Maestrale, C.; Scivoli, R.; Botti, G.; Ligios, C.; Le Gall-Reculé, G.; Lavazza, A.; Capucci, L. The new French 2010 Rabbit Hemorrhagic Disease Virus causes an RHD-like disease in the Sardinian Cape hare (Lepus capensis mediterraneus). Vet. Res. 2013, 44, 96. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.M.; Marques, S.; Silva, E.; Scivoli, R.; Botti, G.; Ligios, C.; Le Gall-Reculé, G.; Lavazza, A.; Capucci, L. Detection of RHDV strains in the Iberian hare (Lepus granatensis): Earliest evidence of rabbit lagovirus cross-species infection. Vet. Res. 2014, 45, 94. [Google Scholar] [CrossRef] [PubMed]
- Velarde, R.; Cavadini, P.; Neimanis, A.; Cabezón, O.; Chiari, M.; Gaffuri, A.; Lavín, S.; Grilli, G.; Gavier-Widén, D.; Lavazza, A.; et al. Spillover events of infection of Brown Hares (Lepus europaeus) with Rabbit Haemorrhagic Disease Type 2 Virus (RHDV2) caused sporadic cases of a European Brown Hare Syndrome-Like Disease in Italy and Spain. Transbound. Emerg. Dis. 2017, 64, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, M.; Maley, M.; Dagleish, M.; Boag, B. Rabbit haemorrhagic disease virus type 2 in hares in Scotland. Vet. Rec. 2019, 185, 23. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.W.; Marnell, F.; Barrett, D.; Reid, N.; Hanna, R.E.B.; McElroy, M.C.; Casey, M. Rabbit Haemorrhagic Disease Virus 2 (RHDV2; GI.2) in Ireland focusing on wild Irish Hares (Lepus timidus hibernicus): An overview of the first outbreaks and contextual review. Pathogens 2022, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Asin, J.; Rejmanek, D.; Clifford, D.L.; Mikolon, A.B.; Henderson, E.E.; Nyaoke, A.C.; Macías-Rioseco, M.; Streitenberger, N.; Beingesser, J.; Woods, L.W.; et al. Early circulation of rabbit haemorrhagic disease virus type 2 in domestic and wild lagomorphs in southern California, USA (2020–2021). Transbound. Emerg. Dis. 2022, 69, e394–e405. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F.; Kelly, P.A.; Hamilton, L.P.; Lloyd, M.R.; Willians, E.A.; Youngblom, J.J. Recovering the endangered Riparian Brush Rabbit (Sylvilagus bachmani riparius): Reproduction and growth in confinement and survival after translocation. In Lagomorph Biology; Alves, P.C., Ferrand, N., Hackländer, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 349–361. [Google Scholar]
- U.S. Fish and Wildlife Service. Draft Recovery Plan for the Columbia Basin Distinct Population Segment of the Pygmy Rabbit (Brachylagus idahoensis); U.S. Fish and Wildlife Service: Portland, OR, USA, 2007; 118p.
- Lederhouse, C. Rabbit Hemorrhagic Disease’s Spread Appears to Be Slowing. JAVMA News, 13 July 2023. Available online: https://www.avma.org/news/rabbit-hemorrhagic-diseases-spread-appears-be-slowing (accessed on 14 August 2024).
- Cima, G. Rabbit, Hare Populations Recovering from Viral Disease. JAVMA News, 1 August 2021. Available online: https://www.avma.org/javma-news/2021-08-01/rabbit-hare-populations-recovering-viral-disease (accessed on 14 August 2024).
- Emerging Risk Notice. Rabbit Hemorrhagic Disease Virus, Type 2 US Department of Agriculture. 2020. Available online: https://www.aphis.usda.gov/animal_health/downloads/rhdv2.pdf (accessed on 14 August 2024).
- Bosco-Lauth, A.M.; Cominsky, B.; Porter, S.; Root, J.J.; Schueler, A.; Anderson, G.; Vander Wal, S.; Benson, A. A novel vaccine candidate against rabbit hemorrhagic disease virus 2 (RHDV2) confers protection in domestic rabbits. Am. J. Vet. Res. 2022, 83, ajvr.22.05.0095. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.G.; Pienaar, E.F.; Kohl, M.T. Barriers to management of a foreign animal disease at the wildlife-domestic animal interface: The case of rabbit hemorrhagic disease in the United States. Front. Conserv. Sci. 2022, 3. Available online: https://www.frontiersin.org/articles/10.3389/fcosc.2022.857678 (accessed on 12 August 2024). [CrossRef]
- Mohamed, F.; Gidlewski, T.; Berninger, M.L.; Petrowski, H.M.; Bracht, A.J.; de Rueda, C.B.; Barrette, R.W.; Grady, M.; O’Hearn, E.S.; Lewis, C.E.; et al. Comparative susceptibility of eastern cottontails and New Zealand white rabbits to classical rabbit haemorrhagic disease virus (RHDV) and RHDV2. Transbound. Emerg. Dis. 2022, 69, e968–e978. [Google Scholar] [CrossRef]
- Tefft, B.C.; Chapman, J.A. Social behavior of the New England cottontail, Sylvilagus transitionalis (Bangs) with a review of social behavior in new world rabbits (Mammalia: Leporidae). Rev. Écol. 1987, 42, 235–276. [Google Scholar] [CrossRef]
- Cooke, B.D.; Robinson, A.J.; Merchant, J.C.; Nardin, A.; Capucci, L. Use of ELISAs in field studies of rabbit haemorrhagic disease (RHD) in Australia. Epid. Infect. 2000, 124, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Cooke, B. Managing the European rabbit: Converging interests between Australian research for rabbit control and European research for their conservation. In Lagomorph Biology: Evolution, Ecology, and Conservation; Springer: Berlin/Heidelberg, Germany, 2008; pp. 317–326. [Google Scholar]
- Calvete, C. Modeling the effect of population dynamics on the impact of Rabbit Hemorrhagic Disease. Conserv. Biol. 2006, 20, 1232–1241. [Google Scholar] [CrossRef]
- Mutze, G.; Cooke, B.; Alexander, P. The initial impact of rabbit hemorrhagic disease on rabbit populations in South Australia. J. Wildl. Dis. 1998, 34, 221–227. [Google Scholar] [CrossRef]
- Mutze, G.; Bird, P.; Cooke, B.; Henzell, R. Geographic and seasonal variation in the impact of rabbit haemorrhagic disease on European rabbits, Oryctolagus cuniculus, and rabbit damage in Australia. In Lagomorph Biology; Alves, P.C., Ferrand, N., Hackländer, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 279–293. [Google Scholar]
- Mutze, G.J.; Sinclair, R.G.; Peacock, D.E.; Capucci, L.; Kovaliski, J. Is increased juvenile infection the key to recovery of wild rabbit populations from the impact of rabbit haemorrhagic disease? Europ. J. Wildl. Res. 2014, 60, 489–499. [Google Scholar] [CrossRef]
- Mutze, G.; Bird, P.; Jennings, S.; Peacock, D.; de Preu, N.; Kovaliski, J.; Cooke, B.; Capucci, L. Recovery of South Australian rabbit populations from the impact of rabbit haemorrhagic disease. Wildl. Res. 2015, 41, 552–559. [Google Scholar] [CrossRef]
- Cooke, B.D.; Taggart, P.; Patel, K. Quantifying resistance to myxomatosis in wild rabbits produces novel evolutionary insights. Epid. Infect. 2023, 151, e182. [Google Scholar] [CrossRef]
- Delibes-Mateos, M.; Redpath, S.; Angulo, E.; Ferreras, P.; Villafuerte, R. Rabbits as a keystone species in Southern Europe. Biol. Conserv. 2007, 137, 149–156. [Google Scholar] [CrossRef]
- Ruvoën-Clouet, N.; Ganière, J.P.; André-Fontaine, G.; Blanchard, D.; Le Pendu, J. Binding of rabbit hemorrhagic disease virus to antigens of the ABH histo-blood group family. J. Virol. 2000, 74, 11950–11954. [Google Scholar] [CrossRef]
- Marques, R.M.; Teixeira, L.; Aguas, A.P.; Ribeiro, J.C.; Costa-e-Silva, A.; Ferreira, P.G. Immunosuppression abrogates resistance of young rabbits to Rabbit Haemorrhagic Disease (RHD). Vet. Res. 2014, 45, 14. [Google Scholar] [CrossRef]
- Neave, M.J.; Hall, R.N.; Huang, N.; McColl, K.A.; Kerr, P.; Hoehn, M.; Taylor, J.; Strive, T. Robust Innate Immunity of Young Rabbits Mediates Resistance to Rabbit Hemorrhagic Disease Caused by Lagovirus Europaeus GI.1 But Not GI.2. Viruses 2018, 10, 512. [Google Scholar] [CrossRef]
- Robinson, A.J.; So, P.T.M.; Müller, W.J.; Cooke, B.D.; Capucci, L. Statistical models for the effect of age and maternal antibodies on the development of rabbit haemorrhagic disease in Australian wild rabbits. Wildl. Res. 2002, 29, 663–671. [Google Scholar] [CrossRef]
- Fenner, F.; Marshall, I.D. Passive immunity in myxomatosis of the European rabbit (Oryctolagus cuniculus): The protection conferred on kittens born by immune does. J. Hyg. 1954, 52, 321–336. [Google Scholar] [CrossRef]
- Cooke, B.D.; McPhee, S.; Robinson, A.J.; Capucci, L. Rabbit haemorrhagic disease: Does a pre-existing RHDV-like virus reduce the effectiveness of RHD as a biological control in Australia? Wildl. Res. 2002, 29, 673–682. [Google Scholar] [CrossRef]
- Baratelli, M.; Molist-Badiola, J.; Puigredon-Fontanet, A.; Pascual, M.; Boix, O.; Mora-Igual, F.X.; Woodward, M.; Lavazza, A.; Capucci, L. Characterization of the maternally derived antibody immunity against Rhdv-2 after administration in breeding does of an inactivated vaccine. Vaccines 2020, 8, 484. [Google Scholar] [CrossRef]
- Peri, B.A.; Rothberg, R.M. Transmission of maternal antibody prenatally and from milk into serum of neonatal rabbits. Immunology 1986, 57, 49–53. [Google Scholar]
- Asgari, S.; Hardy, J.R.; Sinclair, R.G.; Cooke, B.D. Field evidence for mechanical transmission of rabbit haemorrhagic disease virus (RHDV) by flies (Diptera: Calliphoridae) among wild rabbits in Australia. Virus Res. 1998, 54, 123–132. [Google Scholar] [CrossRef]
- Hall, R.N.; King, T.; O’Connor, T.W.; Read, A.J.; Vrankovic, S.; Piper, M.; Strive, T. Passive Immunisation against RHDV2 Induces Protection against Disease but Not Infection. Vaccines 2021, 9, 1197. [Google Scholar] [CrossRef]
- Massad, E.; Raimundo, S.M.; Silveira, A.S.B. A continuous function model for the age-related force of infection. Math. Comput. Model. 1990, 13, 101–112. [Google Scholar] [CrossRef]
- Wells, K.; Brook, B.W.; Lacy, R.C.; Mutze, G.J.; Peacock, D.E.; Sinclair, R.G.; Schwensow, N.; Cassey, P.; O’Hara, R.B.; Fordham, D.A. Timing and severity of immunizing diseases in rabbits is controlled by seasonal matching of host and pathogen dynamics. J. Roy. Soc. Interface 2015, 12, 20141184. [Google Scholar] [CrossRef]
- Cooke, B.D.; Duncan, R.P.; McDonald, I.; Liu, J.; Capucci, L.; Mutze, G.J.; Strive, T. Prior exposure to non-pathogenic calicivirus RCV-A1 reduces both infection rate and mortality from rabbit haemorrhagic disease in a population of wild rabbits in Australia. Transbound. Emerg. Dis. 2018, 65, e470–e477. [Google Scholar] [CrossRef]
- Myers, K. Influence of density on fecundity, growth rates, and mortality in the wild rabbit. CSIRO Wildl. Res. 1964, 9, 134–137. [Google Scholar] [CrossRef]
- De Jong, M.; Diekmann, O.; Heesterbeek, J.A.P. How does transmission of infection depend on population size? In Epidemic Models: Their Structure and Relation to Data; Cambridge University Press: Cambridge, UK, 1995; pp. 84–94. [Google Scholar]
- Kolokolnikov, T.; Iron, D. Law of mass action and saturation in SIR model with application to Coronavirus modelling. Infect. Dis. Model. 2021, 6, 91–97. [Google Scholar] [CrossRef]
- Kovaliski, J. Monitoring the spread of rabbit hemorrhagic disease virus as a new biological agent for control of wild European rabbits in Australia. J. Wildl. Dis. 1998, 34, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Elsworth, P.G.; Kovaliski, J.; Cooke, B.D. Rabbit haemorrhagic disease: Are Australian rabbits (Oryctolagus cuniculus) evolving resistance to infection with Czech CAPM 351 RHDV? Epidemiol. Infect. 2012, 140, 1972–1981. [Google Scholar] [CrossRef] [PubMed]
- Elsworth, P.; Cooke, B.D.; Kovaliski, J.; Sinclair, R.; Holmes, E.C.; Strive, T. Increased virulence of rabbit haemorrhagic disease virus associated with genetic resistance in wild Australian rabbits (Oryctolagus cuniculus). Virology 2014, 464–465, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Delibes-Mateos, M.; Farfán, M.A.; Rouco, C.; Olivero, J.; Márquez, A.L.; Fa, J.; Vargas, J.M.; Villafuerte, R. A large-scale assessment of European rabbit damage to agriculture in Spain. Pest Manag. Sci. 2017, 74, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Mahar, J.E.; Hall, R.N.; Peacock, D.; Kovaliski, J.; Piper, M.; Mourant, R.; Huang, N.; Campbell, S.; Gu, X.; Read, A.; et al. Rabbit Hemorrhagic Disease Virus 2 (RHDV2; GI.2) is replacing endemic strains of RHDV in the Australian Landscape within 18 months of its arrival. J. Virol. 2018, 92, e01374-17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santoro, S.; Aguayo-Adán, J.A.; Rouco, C. Comparison of the Impact between Classical and Novel Strains of Rabbit Haemorrhagic Disease on Wild Rabbit Populations in Spain. Biology 2023, 12, 728. [Google Scholar] [CrossRef] [PubMed]
- Dalton, K.P.; Nicieza, I.; Balseiro, A.; Muguerza, M.A.; Rosell, J.M.; Casais, R.; Álvarez, Á.L.; Parra, F. Variant rabbit hemorrhagic disease virus in young rabbits, Spain. Emerg. Infect. Dis. 2012, 18, 2009–2012. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.N.; King, T.; O’Connor, T.; Read, A.J.; Arrow, J.; Trought, K.; Duckworth, J.; Piper, M.; Strive, T. Age and infectious dose significantly affect disease progression after RHDV2 infection in naïve domestic rabbits. Viruses 2021, 13, 1184. [Google Scholar] [CrossRef] [PubMed]
- U.S. Fish and Wildlife Service. Rabbit Hemorrhagic Disease Confirmed for First Time in Endangered Riparian Brush Rabbits. 2022. Available online: https://www.fws.gov/press-release/2022-05/rabbit-hemorrhagic-disease-confirmed-first-time-endangered-riparian-brush (accessed on 30 December 2023).
- Russell, R.E.; Dusek, R.J.; Prevost, S.; Clifford, D.L.; Moriarty, M.E.; Takahashi, F. Modeling the response of an endangered rabbit population to RHDV2 and vaccination. Cons. Sci. Prac. 2024, 6, e13072. [Google Scholar] [CrossRef]
- Kerr, P.J.; Cattadori, I.M.; Liu, J.; Sim, D.G.; Dodds, J.W.; Brooks, J.W.; Kennett, M.J.; Holmes, E.C.; Read, A.F. Next step in the ongoing arms race between myxoma virus and wild rabbits in Australia is a novel disease phenotype. Proc. Natl. Acad. Sci. USA 2017, 114, 9397–9402. [Google Scholar] [CrossRef]
- Alves, J.M.; Carneiro, M.; Cheng, J.Y.; Lemos de Matos, A.; Rahman, M.M.; Loog, L.; Campos, P.F.; Wales, N.; Eriksson, A.; Manica, A.; et al. Parallel adaptation of rabbit populations to myxoma virus. Science 2019, 363, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
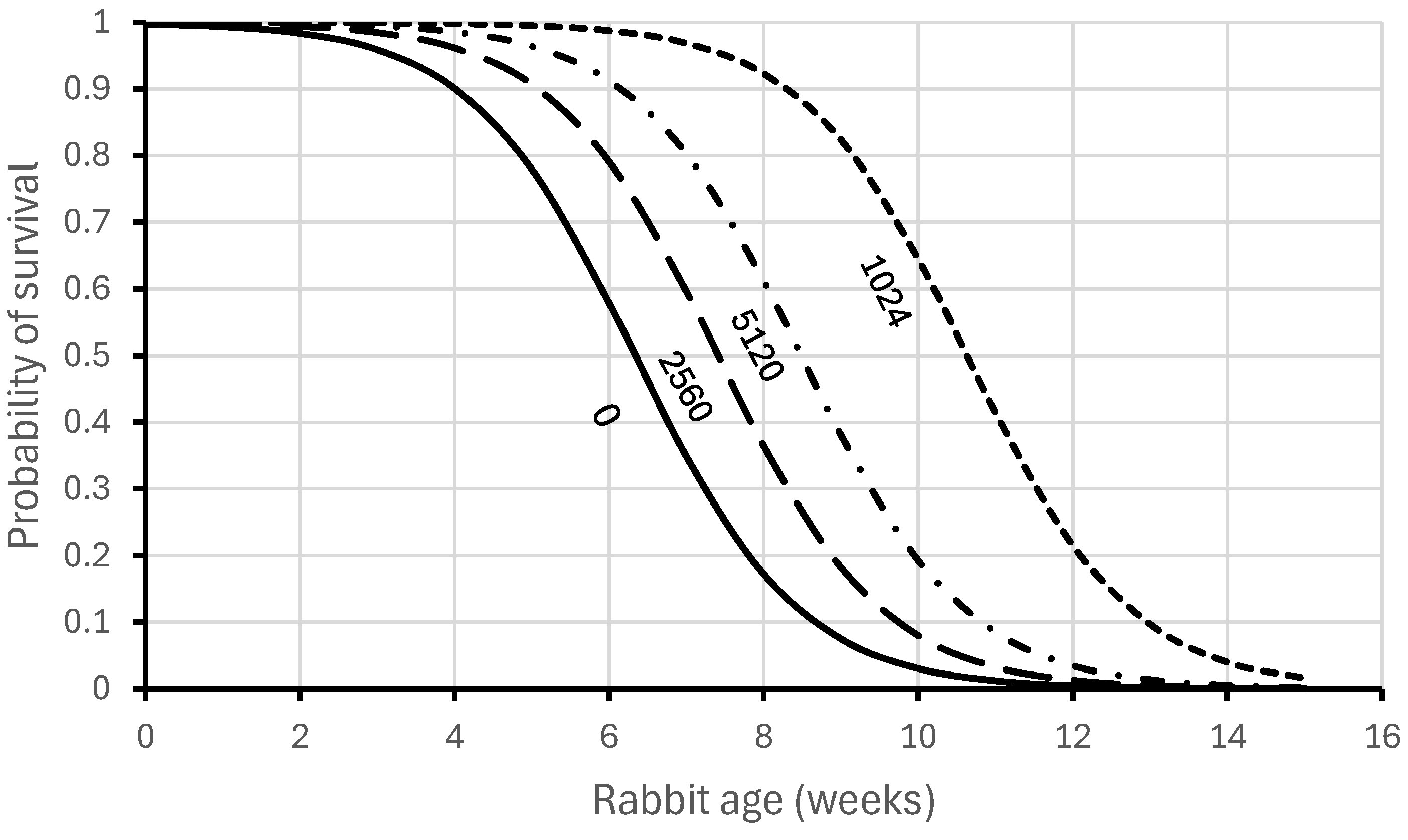
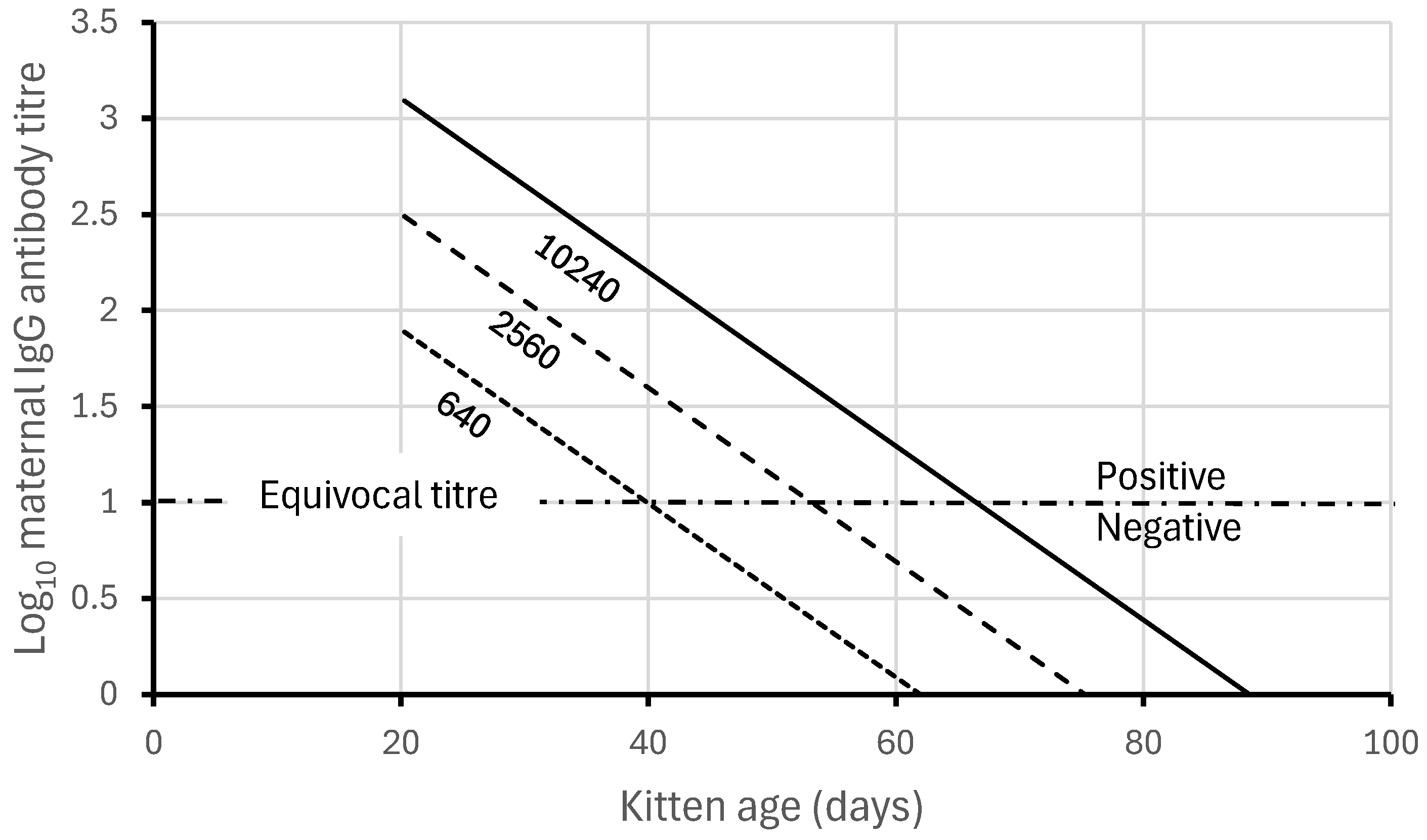
| Antibody Categories in February | |||||
|---|---|---|---|---|---|
| RHDV/MYXV Antibodies | +/+ | +/− | −/+ | −/− | Total |
| Rabbits captured February ‘97 | 4 | 23 | 1 | 20 | 48 |
| Rabbits in each category recaptured June ‘97 | 3 | 8 | 0 | 1 | 12 |
| Four-Month Survival Probability ± s.e. | |||
|---|---|---|---|
| With Antibodies | Without Antibodies | Probability of Survival from Each Disease | |
| RHDV | 0.392 ± 0.060 | 0.054 ± 0.034 | 0.14 |
| MYXV | 0.470 ± 0.119 | 0.220 ± 0.039 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooke, B. Practical Suggestions for Assessing Rabbit Haemorrhagic Disease Virus 2 Risk to Endangered Native Lagomorphs in North America and Southern Africa. Viruses 2024, 16, 1299. https://doi.org/10.3390/v16081299
Cooke B. Practical Suggestions for Assessing Rabbit Haemorrhagic Disease Virus 2 Risk to Endangered Native Lagomorphs in North America and Southern Africa. Viruses. 2024; 16(8):1299. https://doi.org/10.3390/v16081299
Chicago/Turabian StyleCooke, Brian. 2024. "Practical Suggestions for Assessing Rabbit Haemorrhagic Disease Virus 2 Risk to Endangered Native Lagomorphs in North America and Southern Africa" Viruses 16, no. 8: 1299. https://doi.org/10.3390/v16081299






