Abstract
The potato leafhopper (Empoasca fabae, PLH) is a serious pest that feeds on a wide range of agricultural crops and is found throughout the United States but is not known to be a vector for plant-infecting viruses. We probed the diversity of virus sequences in field populations of PLH collected from four Midwestern states: Illinois, Indiana, Iowa, and Minnesota. High-throughput sequencing data from total RNAs extracted from PLH were used to assemble sequences of fifteen positive-stranded RNA viruses, two negative-stranded RNA viruses, and one DNA virus. These sequences included ten previously described plant viruses and eight putative insect-infecting viruses. All but one of the insect-specific viruses were novel and included three solemoviruses, one iflavirus, one phenuivirus, one lispivirus, and one ambidensovirus. Detailed analyses of the novel genome sequences and their evolutionary relationships with related family members were conducted. Our study revealed a diverse group of plant viruses circulating in the PLH population and discovered novel insect viruses, expanding knowledge on the untapped virus diversity in economically important crop pests. Our findings also highlight the importance of monitoring the emergence and circulation of plant-infecting viruses in agriculturally important arthropod pests.
1. Introduction
Insects represent a highly diverse group of organisms that have symbiotic and parasitic relationships with plants and animals. Being an integral dietary component of many animals, insects could potentially act as vehicles of virus transport within the animal and plant kingdoms, or between these kingdoms. There have been considerable advancements in research on insect viruses, but this has mainly been skewed towards vector-borne diseases of animals and focused on blood-feeding insects [1]. With advances in sequencing technologies, novel viruses have been discovered from various organisms and challenging environments. Recently, several metagenomic and metatranscriptomic studies have discovered viruses from other insects, including plant feeders such as aphids, beetles, fruit flies, leafhoppers, grasshoppers, lepidopterans, planthoppers, psyllids, soldier flies, stink bugs, thrips, and white flies [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. Yet, the viromes of most insect species remain unexplored.
Leafhoppers are one of the most abundant insect groups belonging to the family Cicadellidae under the order Hemiptera. They fall under the suborder Auchenorrhyncha [18] and have broad host ranges. These plant feeders have mouth parts adapted for piercing and sucking sap from a wide range of plants species. Leafhopper feeding damages plants, causing the ‘burning’ of foliage known as ‘hopper burn’, a characteristic symptom of hopper infestation. Potato leafhoppers (PLHs, Empoasca fabae) are native to North America and are a common and serious pest in the United States, affecting over 100 species of host plants [19]. Potato leafhoppers are migratory pests in the Midwest as they cannot survive the cold winter. During the winter, PLHs migrate and reproduce on the Gulf Coast, and in the spring the winged adults are caught in wind currents and transported back. If PLHs arrive early in the growing season, they feed and survive on the alternate weed hosts in the field and subsequently infest crops once they are established. In late summer when the crops senesce, PLHs move to native plants again and feed until they migrate south for the winter [20].
Some species of leafhoppers can transmit pathogens associated with plant sap, such as bacteria, phytoplasma, and viruses [21,22,23]. More than 50 species of leafhoppers under 25 genera are reported to be vectors of plant viruses, including badnaviruses, geminiviruses, phytoreoviruses, picornaviruses, rhabdoviruses, and waikaviruses [22,24,25,26,27,28,29]. The mechanisms of virus transmission by leafhoppers vary depending on the species of leafhopper and the virus involved. Modes of virus transmission by leafhoppers include semipersistent transmission of foregut-borne viruses, persistent transmission of circulative viruses, and persistent transmission of propagative viruses [22].
Although symptoms induced by PLH resemble those induced by plant viruses, studies have failed to demonstrate the ability of PLH to transmit plant-infecting viruses [23,30]. Our communication is the first report on a broad range of viruses detected in PLH. Also, our study expands knowledge on the diverse viruses present in arthropod pests of common agricultural crops.
2. Materials and Methods
2.1. Potato Leafhopper Collection and Sample Preparation for High-Throughput Sequencing
Leafhoppers were collected using the Midwest Suction Trap Network [31] installed in corn and soybean fields in Illinois (Freeport, Morris, Orr, and Urbana-Champaign); Iowa (Ames, Kanawha, Nashua, and Sutherland); Indiana (Wanatah); and Minnesota (Crookston, Lamberton, Morris, and Rosemount). Among the insects collected in the suction traps, hoppers were separated and PLHs were identified based on the morphological characteristics described [32]. Potato leafhoppers were then stored in 95% ethanol at −20 °C until processed. Samples from Illinois were collected in the 2020 and 2021 growing seasons; samples from Iowa and Indiana were collected in 2020; and samples from Minnesota were collected in 2021. A total of 593 individuals were collected from suction traps in Illinois (480), Indiana (13), Iowa (57), and Minnesota (43). For high-throughput sequencing, 13 (Indiana) to 15 (Iowa and Minnesota) randomly selected individuals were pooled into one sample for each state. Due to the large number of PLHs collected from Illinois, a total of 137 individuals (107 for 2020 and 30 for 2021) were used for total RNA extractions. Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valentia, CA, USA), treated with Turbo DNase (ThermoFisher, Waltham, MA, USA), and depleted of ribosomal RNA (rRNA) with the Illumina Ribo-Zero Plus rRNA Depletion Kit (Illumina, San Diego, CA, USA). A total of five sequencing libraries were prepared separately for each state and each year (libraries for IL, IA and IN for 2020 and libraries for IL and MN for 2021) using the Illumina ScriptSeq RNA-Seq Library Preparation Kit and sequenced on an Illumina NovaSeq 6000 at the Roy J. Carver Biotechnology Center at the University of Illinois as 100 nt paired-end reads. The sequence libraries produced from 7.71 × 107 to 1.62 × 108 paired-end reads each (Table 1).
2.2. Assembly of Virus-Like Sequences from the Leafhopper Transcriptome
Sequence reads from each library were trimmed using Trimmomatic [33] and de novo assembled with Trinity [34]. Contigs were compared to a sequence database that included reference invertebrate and virus amino acid (aa) sequences (NCBI GenBank Release 236) using USEARCH [35]. Contigs with significant similarity to virus sequences were compared to the NCBI nonredundant protein database using BLASTX [36]. The number of reads aligning to each virus sequence and depths of coverage were calculated using Bowtie2 [37] and SAMtools [38]. The raw reads were deposited in the SRA database under the Bioproject accession PRJNA802548. The GenBank accession numbers of the virus genomes assembled from the PLH transcriptome data are presented in Table 2. Sequence data are presented only for contigs larger than 500 nucleotides (nt). The open reading frames (ORFs) of virus genomes were identified using ORF finder [39] and the predicted sizes of the encoded proteins were estimated using Protein Molecular Weight [40]. For phylogenetic analyses, RNA-dependent RNA polymerase (RdRp) sequences of novel viruses and taxonomically related members were aligned using the CLUSTALW program in MEGA 11 [41], and maximum likelihood trees were constructed with 500 bootstrap replications.
3. Results
The transcriptome data of PLH collected from the Midwestern states of Illinois, Iowa, Indiana, and Minnesota contained both plant- and insect-specific virus sequences. The details of the sequence reads obtained from the five sequencing libraries are presented in Table 1.

Table 1.
Numbers of sequence reads for viruses detected in potato leafhoppers’ RNA-seq data.
Table 1.
Numbers of sequence reads for viruses detected in potato leafhoppers’ RNA-seq data.
| Numbers of Aligned Sequence Reads | |||||
|---|---|---|---|---|---|
| Plant Viruses | IL 2020 1 (n = 107) 2 | IL 2021 (n = 30) | IA 2020 (n = 15) | IN 2020 (n = 13) | MN 2021 (n = 15) |
| Barley yellow dwarf virus-PAV NC_002160.2 | 0 | 0 | 0 | 19 | 0 |
| Clover yellow mosaic virus NC_001753.1 | 13 | 0 | 4 | 0 | 0 |
| Lucerne transient streak virus NC_001696.2 | 522 | 0 | 4 | 0 | 0 |
| Peanut stunt virus RNA2 NC_002039.1 | 18 | 0 | 0 | 0 | 0 |
| Peanut stunt virus RNA1 NC_002038.1 | 19 | 0 | 0 | 0 | 0 |
| Peanut stunt virus RNA3 NC_002040.1 | 17 | 0 | 0 | 0 | 0 |
| Red clover vein mosaic virus NC_012210.1 | 138 | 0 | 8 | 0 | 0 |
| Red clover necrotic mosaic virus RNA1 NC_003756.1 | 0 | 0 | 220 | 0 | 0 |
| Red clover necrotic mosaic virus RNA2 NC_003775.1 | 0 | 0 | 34 | 0 | 0 |
| Soybean carlavirus 1 | 5262 | 0 | 0 | 0 | 0 |
| Turnip vein-clearing virus NC_001873.1 | 130 | 8 | 0 | 25 | 0 |
| White clover mosaic virus NC_003820.1 | 86 | 0 | 3 | 1 | 0 |
| White clover mottle virus NC_031747.1 | 852 | 0 | 0 | 37 | 0 |
| Insect Viruses | |||||
| Empoasca fabae solemovirus 1 | 3498 [3000 nt] 3 | 9149 [3022 nt] | 1811 [2999 nt] | 0 NC 4 | 0 NC |
| Empoasca fabae solemovirus 2 | 24,633 [2935 nt] | 151,541 [2941 nt] | 7 NC | 44 [1734 nt] | 132,567 [2957 nt] |
| Empoasca fabae solemovirus 3 | 116,809 [2820 nt] | 35,666 [2815 nt] | 330,243 [3025 nt] | 132,419 [2818 nt] | 53,827 [2818 nt] |
| Marma virus RNA1 OM817544.1 | 0 NC | 80 [858 nt] | 9 NC | 0 NC | 7 NC |
| Marma virus RNA2 OM817545.1 | 0 NC | 46 [1650 nt] | 2 NC | 0 NC | 0 NC |
| Empoasca fabae iflavirus 1 | 44,850 [10,785 nt] | 192,880 [10,857 nt] | 111,614 [10,877 nt] | 208,341 [10,854 nt] | 40,699 NC |
| Empoasca fabae lispivirus 1 | 49,575 [14,523 nt] | 0 NC | 82 NC | 0 NC | 298 [1380 nt] |
| Empoasca fabae phenuivirus | 165,436 [9330 nt] | 125,122 [9325 nt] | 423,949 [9389 nt] | 212,198 [9350 nt] | 0 NC |
| Empoasca fabae densovirus 1 | 586 [3837 nt] | 303 [1689 nt] | 661 [3676 nt] | 480 [2242 nt] | 598 NC |
| Total sequence reads in library | 7.71 × 107 | 1.29 × 108 | 1.62 × 108 | 1.31 × 108 | 1.31 × 108 |
1 Location and year of collection of leafhopper samples for RNA-seq library preparation. 2 Numbers of potato leafhopper individuals used in the preparation of RNA-seq libraries. 3 Length of longest contig assembled for each virus from each library. 4 No contig (NC) of greater than 500 nt was assembled for the designated virus for the location and year.
3.1. Plant Viruses Discovered from PLH
Sequences from 10 previously identified positive-strand RNA plant-infecting viruses were identified from PLH RNA-seq data (Table 2). These viruses were barley yellow dwarf virus (BYDV)-PAV, clover yellow mosaic virus (ClYMV), lucerne transient streak virus (LTSV), peanut stunt virus (PSV), red clover vein mosaic virus (RCVMV), red clover necrotic mosaic virus (RCNMV), soybean carlavirus 1 (SCV-1), turnip vein-clearing virus (TVCV), white clover mosaic virus (WClMV), and white clover mottle virus (WCMoV). These viruses are either seed-borne, transmitted mechanically, or transmitted by aphids or beetles, but none have been reported to be transmitted by leafhoppers [42,43,44,45,46,47].
Among the plant viruses, nearly complete genomes were assembled for RCNMV and SCV-1 (Table 2). The PLH RCNMV sequence (accession number, PP946270) contained all coding regions of RNA1 of its bipartite genome, which encoded a 27 kDa replication-associated protein, 86 kDa viral polymerase, and a 36 kDa coat protein (CP). The predicted aa sequences of these proteins were 97.6%, 89.4%, and 79.6% identical, respectively, to the corresponding proteins encoded by the reference RCNMV RNA1 sequence (NC_003756.1).
A nearly complete genome (8223 nt) was assembled from PLH for SCV-1 (accession number, PP946266) (Table 2). Soybean carlavirus 1 was originally identified from RNA-seq data from soybean plants [48] and from soybean thrips [15]. Sequence comparison of the insect-derived (PLH and soybean thrips) SCV-1 isolates revealed very high levels of aa sequence identity: 95.7% in replicase, 98.7% in triple gene block (TGB) 1 protein, 100% in TGB2 and TGB3 proteins, and 99% in CP. Contigs representing more than half the genomes of LTSV (2953 nt, accession number, PP946263) and WCMoV (3366 nt, accession number, PP946269) were assembled from the Illinois 2020 PLH RNA-seq data.
3.2. Insect-Specific Virus-Like Sequences Discovered from PLH
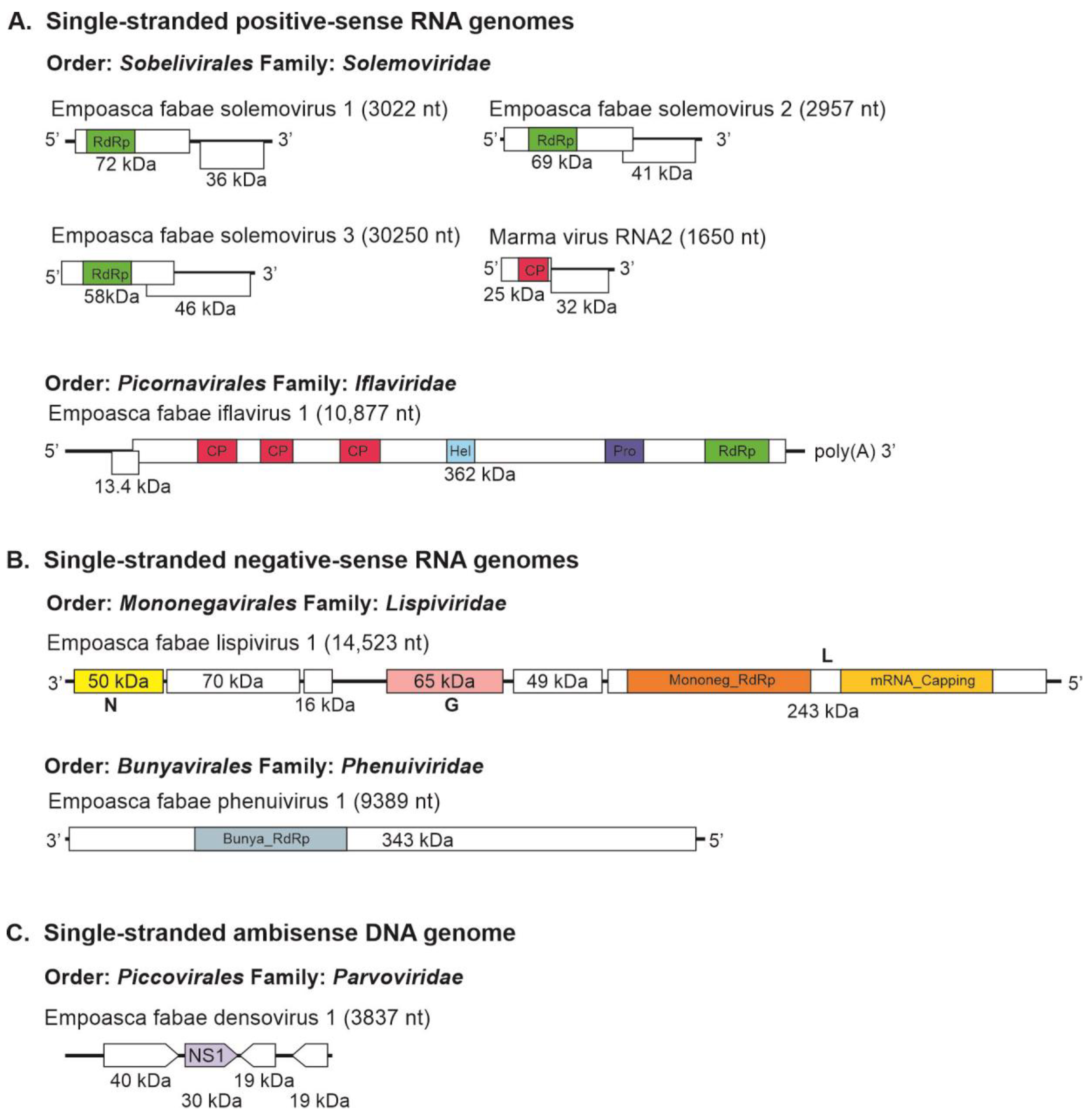
Eight insect-specific virus sequences, representing the genomes of seven novel and one previously reported virus, were assembled from the PLH transcriptome data (Table 2). These included sequences of five positive-strand RNA viruses, two negative-strand RNA viruses, and one DNA virus. Among the positive-strand RNA viruses, four solemovirus genomes were assembled, including one previously described Marma virus. The three novel solemoviruses were tentatively named Empoasca fabae solemovirus (EFSV)1 (independently assembled from three of the five libraries); EFSV-2 (independently assembled from four of the five libraries); and EFSV-3 (independently assembled from all five libraries) (Table 1). The nt sequences of the three isolates of EFSV-1 discovered were 91.2% to 92.6% identical; the four isolates of EFSV-2 were 93.6% to 96.8% identical; and the five isolates of EFSV-3 were 95.1% to 97.1% identical. The genomes of these viruses contained two ORFs, which overlapped in EFSV-2 (accession number, PP946275) and EFSV-3 (accession number, PP946280) but not in EFSV-1 (accession number, PP946277) (Figure 1). The first ORFs of EFSV-1, EFSV-2, and EFSV-3 encoded hypothetical proteins of 72 kDa, 69 kDa, and 58 kDa, respectively. The second ORFs encoded putative replicases of 36 kDa, 41 kDa, and 46 kDa, respectively.

Figure 1.
Genome organization of novel insect-specific viruses identified from potato leafhopper (Empoasca fabae) transcriptome data with single-stranded positive-sense RNA (A), single-stranded negative-sense RNA (B), and single-stranded ambisense DNA (C) genomes. Boxes represent open reading frames. Colored boxes indicate conserved domains: RdRp = RNA-dependent RNA polymerase; Pro = protease; Hel = helicase; CP = capsid protein; Mononeg_RdRp = Mononegavirales RdRp; mRNA_capping = Mononegavirales mRNA-capping region; N = bunyavirus nucleocapsid protein; G = bunyavirus glycoprotein; L = bunyavirus replicase; Bunya_RdRp = bunyavirus RNA-dependent RNA polymerase; NS1 = parvovirus nonstructural protein 1 (NS1) domain.

Table 2.
Viruses discovered from the transcriptome sequencing of potato leafhoppers collected from four Midwestern states in the United States.
Table 2.
Viruses discovered from the transcriptome sequencing of potato leafhoppers collected from four Midwestern states in the United States.
| Viruses | Accession Number | Family | Longest Contig | Best Match (Accession Number) | % aa Identity | % Coverage |
|---|---|---|---|---|---|---|
| Plant viruses | ||||||
| Barley yellow dwarf virus-PAV | PP946271 | Solemoviridae | 467 | Barley yellow dwarf virus-PAV (NC_004750.1) | 97.9 | 8.2 |
| Clover yellow mosaic virus | PP946262 | Alphaflexiviridae | 276 | Clover yellow mosaic virus (NC_001753.1) | 73.2 | 3.9 |
| Lucerne transient streak virus | PP946263 | Solemoviridae | 2953 | Lucerne transient streak virus (NC_001696.2) | 97.5 | 69.0 |
| Peanut stunt virus RNA1 | PP946264 | Bromoviridae | 447 | Peanut stunt virus RNA1 (NC_002038.1) | 96.6 | 13.3 |
| Red clover vein mosaic virus | PP946265 | Betaflexiviridae | 1010 | Red clover vein mosaic virus (NC_012210.1) | 96.8 | 11.7 |
| Red clover necrotic mosaic virus RNA1 | PP946270 | Tombusviridae | 3851 | Red clover necrotic mosaic virus RNA1 (NC_003756.1) | 92.9 | 99.0 |
| Soybean carlavirus 1 | PP946266 | Betaflexiviridae | 8223 | Soybean carlavirus 1 (MW349427.1) | 96.0 | 95.1 |
| Turnip vein-clearing virus | PP946267 | Virgaviridae | 1961 | Turnip vein-clearing virus (NC_001873.1) | 99.4 | 31.1 |
| White clover mosaic virus | PP946268 | Alphaflexiviridae | 1048 | White clover mosaic virus (NC_003820.1) | 98.8 | 17.9 |
| White clover mottle virus | PP946269 | Solemoviridae | 3366 | White clover mottle virus (NC_031747.1) | 98.9 | 54.2 |
| Insect viruses | ||||||
| Empoasca fabae solemovirus 1 | PP946277 | Solemoviridae | 3022 | Amygdalus persica sobemo-like virus (QKI29237.1) | 45.1 | 92.8 |
| Empoasca fabae solemovirus 2 | PP946275 | Solemoviridae | 2957 | Amygdalus persica sobemo-like virus (QKI29238.1) | 47.8 | 90.8 |
| Empoasca fabae solemovirus 3 | PP946280 | Solemoviridae | 3025 | Solemoviridae sp. (QXV86398.1) | 50.5 | 100.2 |
| Marma virus RNA 1 | PP946293 | Solemoviridae | 858 | Marma virus RNA1 (OM817544.1) | 99.8 | 27.3 |
| Marma virus RNA 2 | PP946286 | Solemoviridae | 1650 | Marma virus RNA2 (OM817545.1) | 99.6 | 101.5 |
| Empoasca fabae iflavirus 1 | PP946291 | Iflaviridae | 10,877 | Scaphoideus titanus iflavirus 1 (QIJ56901.1) | 46.6 | 101.4 |
| Empoasca fabae lispivirus 1 | PP946292 | Lispiviridae | 14,523 | Hemipteran arli-related virus OKIAV95 (QPL15300.1) | 40.1 | 192.8 |
| Empoasca fabae phenuivirus 1 | PP946285 | Phenuiviridae | 9330 | Blattodean phenui-related virus OKIAV266 (QMP82340.1) | 30.5 | 130.7 |
| Empoasca fabae densovirus 1 | PP946287 | Parvoviridae | 3837 | Motacilla cinerea parvoviridae sp. (QTE03821.1) | 40.0 | 71.1 |
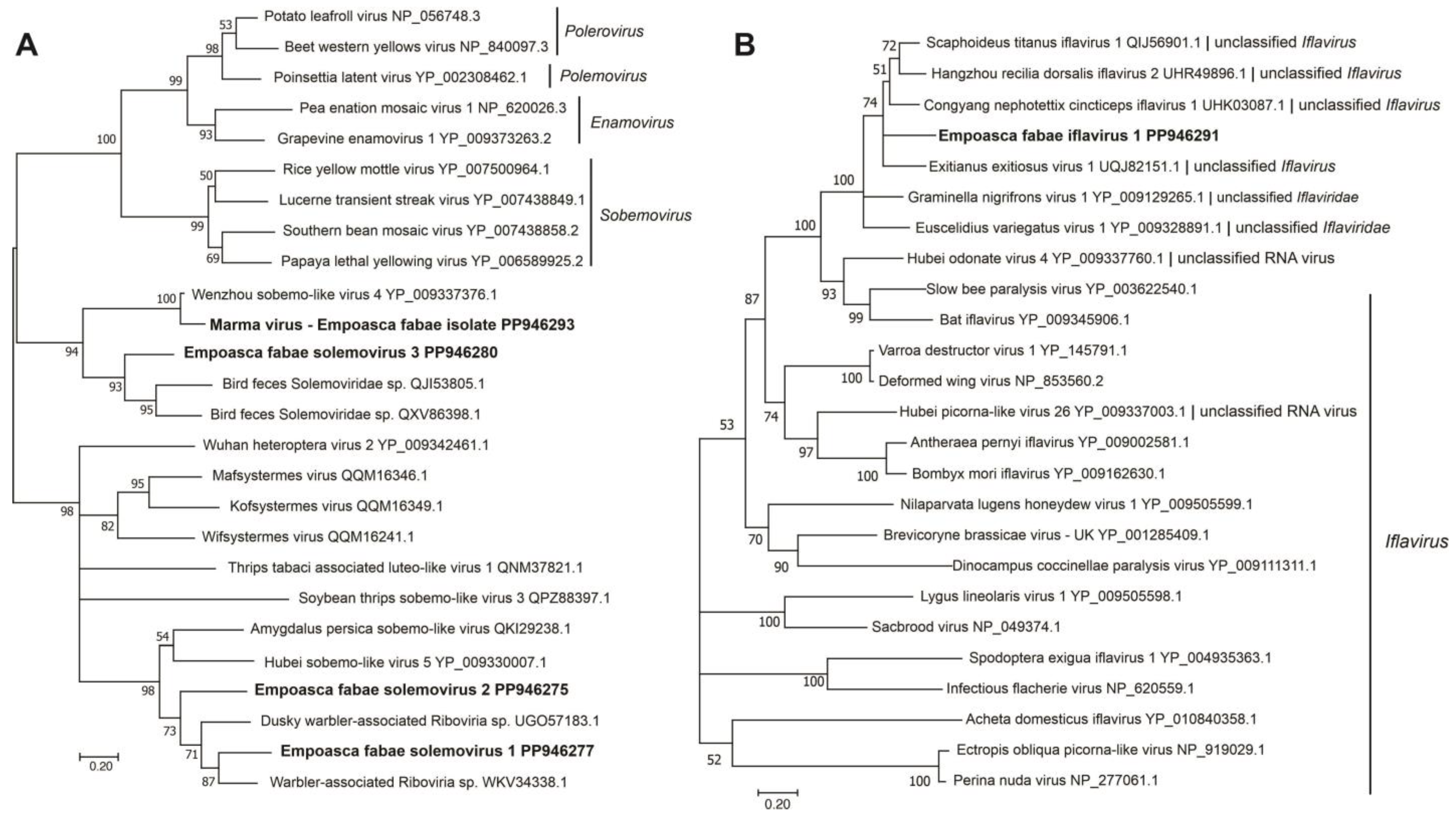
A contig representing a near full-length sequence for RNA2 of Marma virus (accession number, PP946286) was also obtained from the Illinois 2021 PLH samples. Marma virus is an unclassified member of Solemoviridae associated with mosquitoes [49]. This sequence contained coding regions of its 25 kDa CP and 32 kDa hypothetical proteins. Five contigs (191 nt, 394 nt, 618 nt, 733, nt, and 858 nt) representing 86% coverage of Marma virus RNA1 were also assembled from the Illinois 2021 RNA-seq data. In phylogenetic analysis, the previously described plant-infecting members of Solemoviridae and the unclassified solemo-like viruses reported from arthropods formed two main clades in which the PLH solemovirus sequences branched with arthropod-associated viruses (Figure 2A).

Figure 2.
Phylogenetic analysis of predicted RNA-dependent RNA polymerase amino acid sequences encoded by contigs assembled from potato leafhopper (Empoasca fabae) transcriptome data and related viruses in the families Solemoviridae (A) and Iflaviridae (B). Predicted amino acid sequences containing RNA-dependent RNA polymerase domains were aligned using MUSCLE. Phylogenetic analyses were performed using the maximum likelihood method in MEGA11. Numbers at nodes indicate percent bootstrap support (500 replicates). Nodes with less than 50% bootstrap support were collapsed to the next higher level. GenBank accession numbers and taxonomic designation (where available) are indicated after each sequence name. Sequences identified from potato leafhopper transcriptome data are indicated in bold.
Genome sequences of greater than 10 kb were assembled for a novel iflavirus from four of the five RNA-seq libraries (Table 1 and Table 2) that was tentatively named as Empoasca fabae iflavirus 1 (EFIV-1). The EFIV-1 genome (10,877 nt, accession number, PP946291) contained a small ORF, uORF, upstream of the main ORF capable of encoding a 13.4 kDa protein (Figure 1). The uORF was present in all four EFIV-1 isolate sequences discovered from PLH data. Some other members of the Picornavirales have small ORFs upstream of the initiation codon for their primary ORFs [50,51]. In enteroviruses, uORFs have been shown to modulate virus infection in gut epithelial cells [52]. The EFIV-1 primary ORF was predicted to encode a 362 kDa polyprotein with structural protein domains proximal to the amino terminus and nonstructural protein domains proximal to the carboxyl terminus. In phylogenetic analysis, EFIV-1 grouped with bat iflavirus, slow bee paralysis virus, and several iflavirus sequences from arthropods (Figure 2B).
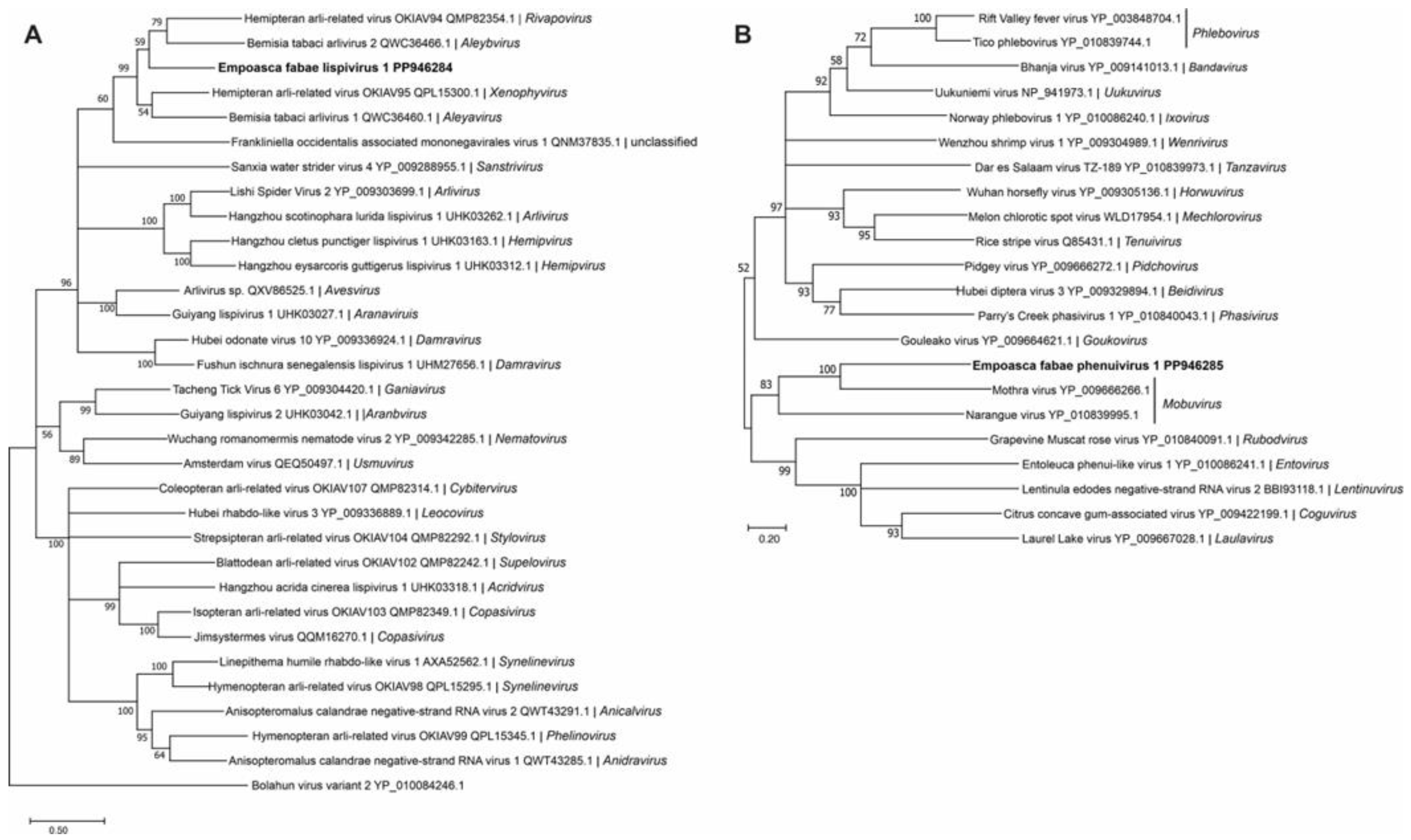
One of the two novel negative-stranded RNA virus genomes discovered from PLH was a lispivirus (accession number, PP946292) tentatively named as Empoasca fabae lispivirus 1 (EFLV-1). A genome of 14.5 kb was assembled from the 49,575 reads obtained from the Illinois 2020 RNA-seq library. Low numbers of sequence reads for the virus were also detected in the Iowa data (82 reads) and the Minnesota (298 reads) data (Table 1). The genome contained six ORFs (Figure 1), which was predicted to encode proteins of 50 kDa, 70 kDa, 16 kDa, 65 kDa, 49 kDa, and 243 kDa. The predicted aa sequences of ORFs 4 and 6 showed similarity to the glycoprotein and RdRp, respectively, of the viruses in Lispiviridae family in order Mononegavirales.
The predicted amino acid sequence of the EFLV-1 RdRp grouped most closely with members of the Aleyavirus, Aleybvirus, Rivapovirus, and Xenophyvirus genera in the family Lispiviridae (Figure 3A). An L- segment of greater than 9.3 kb (accession number, PP946285) of a new putative member of the family Phenuiviridae, Empoasca fabae phenuivirus 1 (EFPV-1), was assembled from all five libraries (Table 1). The RNA was predicted to encode a 343 kDa RdRp (Figure 1). Phenuiviridae members have tripartite negative-stranded RNA genomes with L, M, and S segments. Most are transmitted by arthropod vectors. In phylogenetic analyses, EFPV-1 grouped with Mothra virus and Narangue virus of the genus Mobuvirus in the Phenuiviridae family (Figure 3B).

Figure 3.
Phylogenetic analysis of predicted RNA-dependent RNA polymerase amino acid sequences encoded by contigs assembled from potato leafhopper (Empoasca fabae) transcriptome data and related negative-sense RNA viruses in the families Lispiviridae (A) and Phenuiviridae (B). See Figure 2 legend for details.
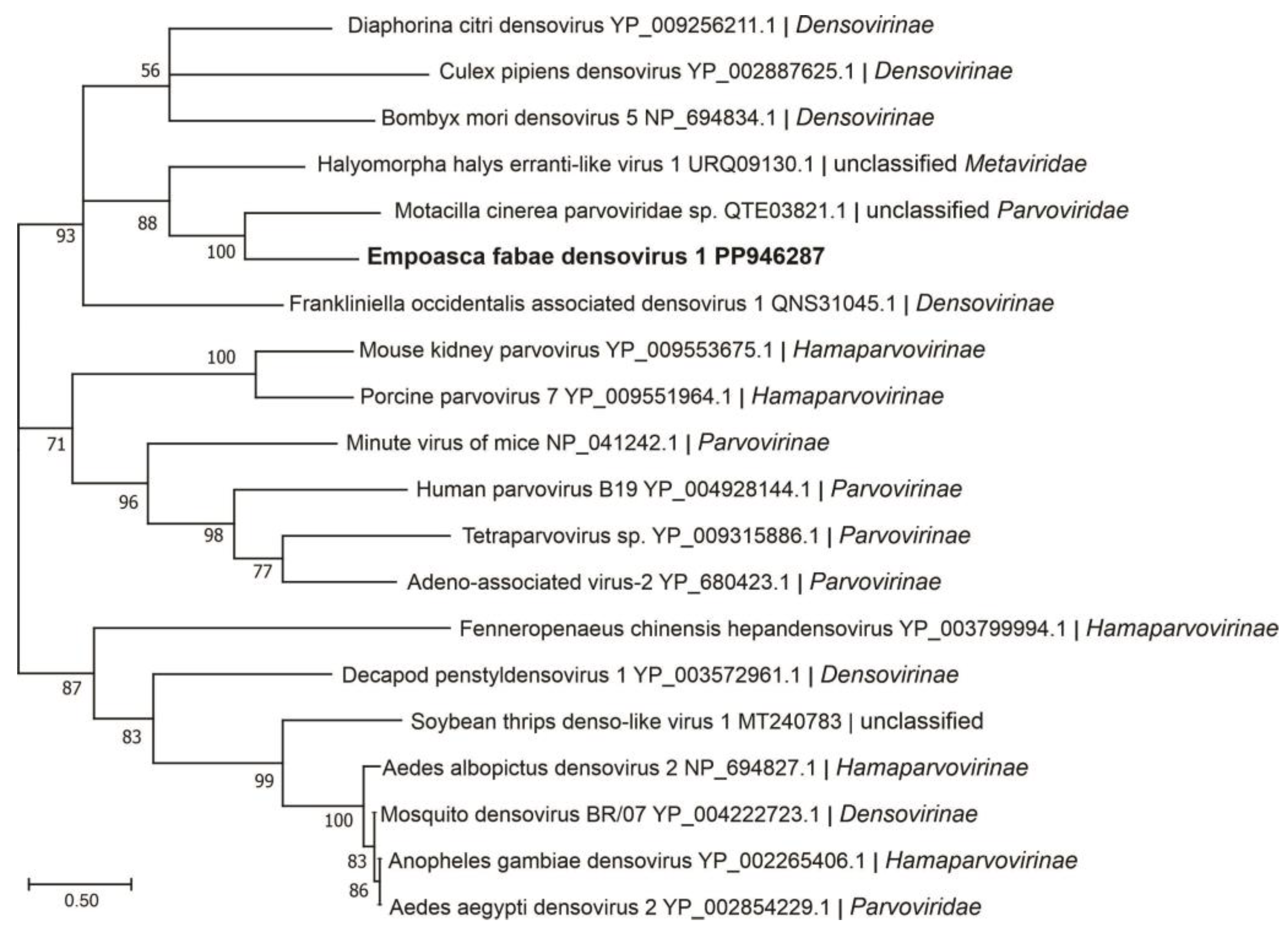
Four assembled contigs of a DNA virus resembling the genomes of ambidensoviruses were discovered from the samples of PLH and the virus was provisionally named as Empoasca fabae densovirus 1 (EFDV-1). Densoviruses are small DNA viruses with single-stranded linear genomes of 4–6 kb and are grouped in the Densovirinae subfamily of the Parvoviridae family. The Densovirinae subfamily contains five genera, among which members of the Ambidensovirus genus have protein-coding regions on both DNA strands. The nonstructural proteins are encoded in the 5′-proximal of the positive strand, while the structural proteins are encoded in the 5′-proximal of the negative strand [53]. EFDV-1 sequence (3837 nt, accession number, PP946287), contained four ORFs (Figure 1), ORFs 1 and 2 are on the positive strand while ORFs 3 and 4 are on the negative strand, and encoded proteins of 40 kDa, 30 kDa, 19 kDa, and 19 kDa, respectively. Among these proteins, only the ORF2-encoded protein (non-structural protein 1) showed a relationship with members of Parvoviridae (Table 2). In phylogenetic analysis, EFDV-1 grouped with members of the Parvoviridae family in the subfamily Densovirinae (Figure 4).

Figure 4.
Phylogenetic analysis of predicted NS1 amino acid sequences encoded by contigs assembled from potato leafhopper (Empoasca fabae) transcriptome data and related viruses in the family Parvoviridae. See Figure 2 legend for details.
3.3. Virus Distribution in PLH Collected from Midwestern States
Among the plant viruses detected in PLH data, all except two, BYDV-PAV and RCNMV, were present in PLH collected from Illinois (Table 1). No plant viruses were detected in PLH from Minnesota. Four of the viruses were detected only in a single state: BYDV-PAV only in Indiana; PSV and SCV-1 only in Illinois; and RCNMV only in Iowa.
Compared to plant viruses, insect-specific virus sequences had much higher read counts and most were detected in all five RNA-seq libraries with a few exceptions. EFSV-1 was not present in PLH collected from Indiana and Minnesota; Marma virus and EFLV-1 were not detected in Indiana; and EFPV-1 was not detected in Minnesota.
4. Discussion
Because of their importance for human health, studies of insect viral diversity have been biased towards blood-feeding arthropods. Few studies have reported on viral diversity in agriculturally relevant arthropods that include vectors and non-vectors of plant viruses [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. Potato leafhoppers are widespread, economically important pests affecting a wide range of crops in the US. Leafhoppers feed on the phloem, xylem, and mesophyll cells of plants [54], and in the process PLH could potentially acquire, harbor, and/or transmit pathogens including viruses. Studies have shown that some species in the genus Empoasca are vectors of plant viruses [55]. An early study described PLH as a non-vector of plant viruses [56], and there have been no subsequent reports of PLH vectoring plant viruses.
We discovered ten previously described plant viruses from PLH transcriptome data (Table 2). Six of these (BYDV-PAV, LTSV, PDSV, RCVMV, SCV-1, and WClMoV) belong to families of viruses transmitted by aphids or beetles and four (ClYMV, RCNMV, TVCV, and WClMV) to families whose members do not have biological vectors.
In the United States, LTSV was first discovered in soybean thrips [15] and reported naturally infecting alfalfa [57]. White clover mottle virus, an unclassified member of Solemoviridae, has not been previously detected in North America. There are no published reports of the occurrence of WCMoV. However, detection of WCMoV in white clover samples from South Korea has been documented in GenBank (accession number, NC031747). Its presence in PLH indicates the existence of WCMoV in either crop or weed species in North America. Because of their migratory nature and extensive host range, plant viruses discovered from PLH could possibly include those that were acquired from crop and weed plants, locally as well as from their overwintering regions. Potato leafhoppers have not been reported to transmit the plant viruses detected in our study or any other plant viruses. Also, it is interesting to note that no known economically important viruses of the major host plants of PLH in the US were detected in the RNA-seq data. These observations could indicate that, while PLHs can acquire plant viruses, they are unable to transmit viruses to host plants. As illustrated by the detection of plant-infecting viruses not previously reported in North America from leafhoppers, monitoring the viromes of polyphagous insect pests like PLH may provide insights into the populations of plant-infecting viruses circulating in a geographic region that would otherwise be difficult to achieve without sampling a large number of plant species.
Two promising uses of insect viruses are their use as gene delivery and expression vectors in insects and their use as biological control agents. Further studies are needed to determine the distribution of the novel viruses in the field populations of PLH, the impact of these viruses on their physiology and survival, and their potential uses in biological studies and as control agents for PLH management.
Our study discovered eight insect-specific viruses, seven novel viruses, and a mosquito-associated Marma virus [49] from PLHs. Potato leafhoppers could have acquired Marma virus directly from plants on which Marma virus-infected mosquitoes had fed or, like some other invertebrate viruses, Marma virus may have a broad host range that includes both PLH and mosquitoes. The absence of certain viruses in certain states under study could be due to the small sizes of PLH populations analyzed in those states or differences in circulating viruses in the PLH population in those areas. Additional experiments will be required to determine whether these viruses can infect/replicate in PLH or if they were merely just acquired during PLH feeding.
5. Conclusions
Viruses are abundant microorganisms that have the potential to parasitize any living organism. Diversity in many agricultural virus pests is unexplored. Through metatranscriptome analysis, we discovered a broad range of viruses, including novel arthropod viruses and existing plant viruses in PLH for the first time (Supplementary Materials).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v16081305/s1. Virus sequences generated and analyzed in this study: Supplemantary_data_1.xlsx.
Author Contributions
Conceptualization, D.L.-K., G.L.H. and L.L.D.; methodology, T.T.-V., D.L.-K. and N.K.M.; formal analysis, T.T.-V., D.L.-K. and L.L.D.; investigation, T.T.-V., D.L.-K. and N.K.M.; data curation, L.L.D.; writing—original draft preparation, T.T.-V. and L.L.D.; writing—review and editing, T.T.-V., L.L.D., D.L.-K., S.J.C., N.K.M. and G.L.H.; supervision, G.L.H., S.J.C. and L.L.D.; project administration, G.L.H., S.J.C. and L.L.D.; funding acquisition, G.L.H. and L.L.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the United States Department of Agriculture—Agriculture and Food Research Initiative [grant number 09290] and the United States Department of Agriculture—Agricultural Research Service [project number 5012-22000-022-000-D].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw sequence reads used in this study were deposited in the SRA database under the Bioproject accession PRJNA802548.
Acknowledgments
Mention of a trademark, proprietary product, or vendor does not constitute a guarantee or warranty of the product by the United States Department of Agriculture and does not imply its approval to the exclusion of other products or vendors that may also be suitable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brinkmann, A.; Nitsche, A.; Kohl, C. Viral Metagenomics on Blood-Feeding Arthropods as a Tool for Human Disease Surveillance. Int. J. Mol. Sci. 2016, 17, 1743. [Google Scholar] [CrossRef] [PubMed]
- Asselin, A.K.; Etebari, K.; Furlong, M.J.; Johnson, K.N. A new dicistro-like virus from soldier fly, Inopus flavus (Diptera: Stratiomyidae), a pest of sugarcane. Arch. Virol. 2021, 166, 2841–2846. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ji, N.; Bai, L.; Ma, J.; Li, Z. Aphid viruses: A brief view of a long history. Front. Insect Sci. 2022, 2, 846716. [Google Scholar] [CrossRef] [PubMed]
- Guy, P.L.; Toriyama, S.; Fuji, S. Occurrence of a picorna-like virus in planthopper species and its transmission in Laodelphax striatellus. J. Invertebr. Pathol. 1992, 59, 161–164. [Google Scholar] [CrossRef]
- Huang, H.-J.; Ye, Z.-X.; Wang, X.; Yan, X.-T.; Zhang, Y.; He, Y.-J.; Qi, Y.-H.; Zhang, X.-D.; Zhuo, J.-C.; Lu, G.; et al. Diversity and infectivity of the RNA virome among different cryptic species of an agriculturally important insect vector: Whitefly Bemisia tabaci. NPJ Biofilms Microbiomes 2021, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Käfer, S.; Paraskevopoulou, S.; Zirkel, F.; Wieseke, N.; Donath, A.; Petersen, M.; Jones, T.C.; Liu, S.; Zhou, X.; Middendorf, M.; et al. Re-assessing the diversity of negative strand RNA viruses in insects. PLoS Pathog. 2019, 15, e1008224. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sappington, T.W.; Coates, B.S.; Bonning, B.C. Nudivirus sequences identified from the southern and western corn rootworms (Coleoptera: Chrysomelidae). Viruses 2021, 13, 269. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, C.M.; Busch, A.K.; Cole, E.A.; Trebicki, P.; Bernardo, P.; Brown, A.K.; Landis, D.A.; Werling, B.P. Emerging wild virus of native grass bioenergy feedstock is well-established in the Midwestern USA and associated with premature stand senescence. GCB Bioenergy 2022, 14, 463–480. [Google Scholar] [CrossRef]
- Medd, N.C.; Fellous, S.; Waldron, F.M.; Xuéreb, A.; Nakai, M.; Cross, J.V.; Obbard, D.J. The virome of Drosophila suzukii, an invasive pest of soft fruit. Virus Evol. 2018, 4, vey009. [Google Scholar] [CrossRef]
- Nouri, S.; Salem, N.; Nigg, J.C.; Falk, B.W. Diverse array of new viral sequences identified in worldwide populations of the Asian citrus psyllid (Diaphorina citri) using viral metagenomics. J. Virol. 2016, 90, 2434–2445. [Google Scholar] [CrossRef]
- Qi, Y.-H.; Ye, Z.-X.; Zhang, C.-X.; Chen, J.-P.; Li, J.-M. Diversity of RNA viruses in agricultural insects. Comput. Struct. Biotechnol. J. 2023, 21, 4312–4321. [Google Scholar] [CrossRef]
- Quito-Avila, D.F.; Reyes-Proaño, E.; Armijos-Capa, G.; Alcalá Briseño, R.I.; Alvarez, R.; Flores, F.F. Analysis of a new negevirus-like sequence from Bemisia tabaci unveils a potential new taxon linking nelorpi- and centiviruses. PLoS ONE 2024, 19, e0303838. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.-P.; Ye, Z.-X.; Wang, S.-N.; Li, J.-M.; Chen, J.-P.; Zhang, C.-X.; Lu, J.-B. Complete genome analysis of a novel chuvirus from a southern green stink bug (Nezara viridula). Arch. Virol. 2022, 167, 2423–2427. [Google Scholar] [CrossRef]
- Shu, R.; Meng, Q.; Miao, L.; Liang, H.; Chen, J.; Xu, Y.; Cheng, L.; Jin, W.; Qin, Q.; Zhang, H. Genome analysis of a novel clade b betabaculovirus isolated from the legume pest Matsumuraeses phaseoli (Lepidoptera: Tortricidae). Viruses 2020, 12, 1068. [Google Scholar] [CrossRef]
- Thekke-Veetil, T.; Lagos-Kutz, D.; McCoppin, N.K.; Hartman, G.L.; Ju, H.-K.; Lim, H.-S.; Domier, L.L. Soybean thrips (Thysanoptera: Thripidae) harbor highly diverse populations of arthropod, fungal and plant viruses. Viruses 2020, 12, 1376. [Google Scholar] [CrossRef]
- Wu, H.; Pang, R.; Cheng, T.; Xue, L.; Zeng, H.; Lei, T.; Chen, M.; Wu, S.; Ding, Y.; Zhang, J.; et al. Abundant and diverse RNA viruses in insects revealed by RNA-seq analysis: Ecological and evolutionary implications. mSystems 2020, 5, e00039-20. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, J.; Lin, X.; Shi, W.; Cao, C. Identification of diverse viruses associated with grasshoppers unveils the parallel relationship between host phylogeny and virome composition. Virus Evol. 2022, 8, veac057. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.P.; Dietrich, C.H.; Friedrich, F.; Beutel, R.G.; Wipfler, B.; Peters, R.S.; Allen, J.M.; Petersen, M.; Donath, A.; Walden, K.K.O.; et al. Phylogenomics and the evolution of hemipteroid insects. Proc. Natl. Acad. Sci. USA 2018, 115, 12775–12780. [Google Scholar] [CrossRef] [PubMed]
- Lamp, W.O.; Morris, M.J.; Armbrust, E.J. Suitability of common weed species as host plants for the potato leafhopper, Empoasca fabae. Entomol. Exp. Appl. 1984, 36, 125–131. [Google Scholar] [CrossRef]
- Taylor, R.A.J.; Shields, E.J. Revisiting Potato Leafhopper, Empoasca fabae (Harris), Migration: Implications in a World Where Invasive Insects are All Too Common. Am. Entomol. 2018, 64, 44–51. [Google Scholar] [CrossRef]
- Dakhil, H.A.; Hammad, E.A.-F.; El-Mohtar, C.; Abou-Jawdah, Y. Survey of leafhopper species in almond orchards infected with almond witches’-broom phytoplasma in Lebanon. J. Insect Sci. 2011, 11, 60. [Google Scholar] [CrossRef]
- Nault, L.R.; Ammar, E.D. Leafhopper and planthopper transmission of plant viruses. Annu. Rev. Entomol. 1989, 34, 503–529. [Google Scholar] [CrossRef]
- Nielson, M.W. The Leafhopper Vectors of Phytopathogenic Viruses (Homoptera, Cicadellidae): Taxonomy, Biology, and Virus Transmission; US Agricultural Research Service: Washington, DC, USA, 1968; pp. 1–386. [Google Scholar]
- Ammar, E.-D.; Nault, L.R. Virus transmission by leafhoppers, planthoppers and treehoppers (auchenorrhyncha, homoptera). In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2002; Volume 36, pp. 141–167. [Google Scholar]
- Bhat, A.I.; Rao, G.P. Transmission of viruses by leafhoppers. In Characterization of Plant Viruses: Methods and Protocols; Bhat, A.I., Rao, G.P., Eds.; Springer: New York, NY, USA, 2020; pp. 77–82. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Long, Z.; Yang, H.; Wei, T. Viral release threshold in the salivary gland of leafhopper vector mediates the intermittent transmission of rice dwarf virus. Front. Microbiol. 2021, 12, 639445. [Google Scholar] [CrossRef]
- Klein, M. Transmission of Viruses by Leafhoppers and Thrips. In Virus and Virus-Like Diseases of Potatoes and Production of Seed-Potatoes; Loebenstein, G., Berger, P.H., Brunt, A.A., Lawson, R.H., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 227–236. [Google Scholar]
- Todd, J.C.; Ammar, E.-D.; Redinbaugh, M.G.; Hoy, C.; Hogenhout, S.A. Plant host range and leafhopper transmission of Maize fine streak virus. Phytopathology 2010, 100, 1138–1145. [Google Scholar] [CrossRef]
- Yi, G.; Wu, W.; Wei, T. Delivery of rice gall dwarf virus into plant phloem by its leafhopper vectors activates callose deposition to enhance viral transmission. Front. Microbiol. 2021, 12, 662577. [Google Scholar] [CrossRef] [PubMed]
- Poos, F.W.; Wheeler, N.H. Studies on Host Plants of the Leafhoppers of the Genus Empoasca; United States Department of Agriculture: Washington, DC, USA, 1943; p. 51. [Google Scholar]
- Lagos-Kutz, D.; Voegtlin, D.J.; Onstad, D.; Hogg, D.; Ragsdale, D.; Tilmon, K.; Hodgson, E.; DiFonzo, C.; Groves, R.; Krupke, C.; et al. The soybean aphid suction trap network: Sampling the aerobiological “Soup”. Am. Entomol. 2020, 66, 48–55. [Google Scholar] [CrossRef]
- Potato Leafhopper. 2020. Available online: https://hort.extension.wisc.edu/articles/potato-leafhopper/ (accessed on 23 January 2023).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Trinity: Reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Subgroup, G.P.D.P. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.L.; Church, D.M.; Federhen, S.; Lash, A.E.; Madden, T.L.; Pontius, J.U.; Schuler, G.D.; Schriml, L.M.; Sequeira, E.; Tatusova, T.A.; et al. Database resources of the National Center for Biotechnology. Nucleic Acids Res. 2003, 31, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Stothard, P. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques 2000, 28, 1102–1104. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R.; Plumb, R. The occurrence and effects of red clover necrotic mosaic virus in red clover (Trifolium pratense). Ann. Appl. Biol. 2008, 91, 227–236. [Google Scholar] [CrossRef]
- Brunt, A.A.; Crabtree, K.; Dallwitz, M.; Gibbs, A.; Watson, L. Viruses of Plants; CABI International: Wageningen, The Netherlands, 1996. [Google Scholar]
- Hebert, T.T. Peanut stunt virus (peanut stunt). In CABI Compendium; CABI: Wallingford, UK, 2021; p. 45670. [Google Scholar] [CrossRef]
- Larsen, R.C. Diseases caused by viruses. In Compendium of Alfalfa Diseases and Pests, 3rd ed.; Samac, D.A., Rhodes, L.H., Lamp, W.O., Eds.; The American Phytopathological Societ: St. Paul, MN, USA, 2015; pp. 66–71. [Google Scholar]
- Melcher, U. Turnip vein-clearing virus, from pathogen to host expression profile. Mol. Plant Pathol. 2003, 4, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.A.; Hampton, R.O. Transmission of two purified carlaviruses by the pea aphid. Phytopathology 1980, 70, 631–633. [Google Scholar] [CrossRef]
- Thekke-Veetil, T.; McCoppin, N.K.; Hobbs, H.A.; Hartman, G.L.; Lambert, K.N.; Lim, H.-S.; Domier, L.L. Discovery of a novel member of the Carlavirus genus from soybean (Glycine max L. Merr.). Pathogens 2021, 10, 223. [Google Scholar] [CrossRef]
- Batson, J.; Dudas, G.; Haas-Stapleton, E.; Kistler, A.L.; Li, L.M.; Logan, P.; Ratnasiri, K.; Retallack, H. Single mosquito metatranscriptomics identifies vectors, emerging pathogens and reservoirs in one assay. eLife 2021, 10, e68353. [Google Scholar] [CrossRef]
- Pelletier, J.; Flynn, M.E.; Kaplan, G.; Racaniello, V.; Sonenberg, N. Mutational analysis of upstream AUG codons of poliovirus RNA. J. Virol. 1988, 62, 4486–4492. [Google Scholar] [CrossRef] [PubMed]
- Pestova, T.V.; Hellen, C.U.; Wimmer, E. A conserved AUG triplet in the 5′ nontranslated region of poliovirus can function as an initiation codon in vitro and in vivo. Virology 1994, 204, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Lulla, V.; Dinan, A.M.; Hosmillo, M.; Chaudhry, Y.; Sherry, L.; Irigoyen, N.; Nayak, K.M.; Stonehouse, N.J.; Zilbauer, M.; Goodfellow, I.; et al. An upstream protein-coding region in enteroviruses modulates virus infection in gut epithelial cells. Nat. Microbiol. 2019, 4, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Cotmore, S.F.; Agbandje-McKenna, M.; Chiorini, J.A.; Mukha, D.V.; Pintel, D.J.; Qiu, J.; Soderlund-Venermo, M.; Tattersall, P.; Tijssen, P.; Gatherer, D.; et al. The family Parvoviridae. Arch. Virol. 2014, 159, 1239–1247. [Google Scholar] [CrossRef]
- Stafford, C.A.; Walker, G.P.; Ullman, D.E. Infection with a plant virus modifies vector feeding behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 9350–9355. [Google Scholar] [CrossRef] [PubMed]
- García-Cámara, I.; Tapia-Tussell, R.; Magaña-Álvarez, A.; Cortés Velázquez, A.; Martín-Mex, R.; Moreno-Valenzuela, O.; Pérez-Brito, D. Empoasca papayae (Hemiptera: Cicadellidae)-mediated transmission of papaya meleira virus-Mexican variant in Mexico. Plant Dis. 2019, 103, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Knull, D.J. Studies on host plants of the leafhoppers of the genus Empoasca. Ann. Entomol. Soc. Am. 1943, 36, 655. [Google Scholar] [CrossRef]
- Nemchinov, L.G.; Irish, B.M.; Grinstead, S.; Shao, J.; Vieira, P. Diversity of the virome associated with alfalfa (Medicago sativa L.) in the U.S. Pacific Northwest. Sci. Rep. 2022, 12, 8726. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).