Abstract
Larval mortality is the primary symptom of diseased Apis cerana colonies, often attributed to sacbrood virus (SBV) and Melissococcus plutonius. However, the impact of other common honeybee viruses is frequently overlooked, and their pathogenicity to A. cerana remains poorly understood. To investigate the causes of the increasing disease incidence in A. cerana brood, we conducted an epidemiological survey, collecting 70 samples from 19 sites across nine provinces in China. Furthermore, we examined the pathogenicity of Israeli acute paralysis virus (IAPV) in A. cerana brood through artificial inoculation experiments. Our results demonstrate that, besides SBV and M. plutonius, the infection rate and viral load of IAPV in diseased brood are significantly high. Brood artificially inoculated with high concentrations of IAPV exhibited a significant increase in mortality and displayed clinical symptoms similar to those observed in naturally infected colonies. Moreover, a limited resistance to IAPV was observed in A. cerana brood, with some individuals able to restrict viral proliferation. Our study highlights the previously unrecognized pathogenicity of IAPV to A. cerana brood, demonstrating that IAPV poses a significant threat similar to SBV and M. plutonius. We emphasize that IAPV should be recognized as an emerging pathogen causing brood disease in A. cerana and managed accordingly in beekeeping practices.
1. Introduction
Honeybees are important pollinators of plants in natural and agricultural ecosystems. Multiple factors contribute to the poor health of honeybees, including pathogens, exposure to agrochemicals, and inadequate habitat and nutritional resources [1,2]. Among these, viruses are one of the major threats and cause serious concern for researchers and beekeepers. Some of the most common and harmful viruses in honeybee colonies include acute bee paralysis virus (ABPV), black queen cell virus (BQCV), chronic bee paralysis virus (CBPV), deformed wing virus (DWV), Kashmir bee virus (KBV), Israeli acute paralysis virus (IAPV), sacbrood virus (SBV), and Varroa destructor virus-1 (VDV-1) [2]. Over the last decade, our understanding of honeybee viruses has grown dramatically thanks to rapid improvements in and increased accessibility to molecular approaches. However, these advances have mostly been limited to viruses that infect the Western honeybee, Apis mellifera.
The Eastern honeybee, Apis cerana, is one of the two species of honeybee that have been truly domesticated and used in apiculture. It has adapted to diverse environments and has a broad natural distribution. A. cerana is widely found in complex topographic regions with varied habitats, diverse flora, and divergent climates across Asia [3]. Like its western counterpart, A. mellifera, A. cerana also plays a vital role in agricultural production and biodiversity conservation. However, compared to A. mellifera, there is limited research on the health status of A. cerana. A. cerana was considered to harbor far fewer parasites and pathogens, due to its more strongly expressed collective defense mechanisms [4]. The health of A. cerana brood (larvae and pupae) is typically threatened by sacbrood disease caused by SBV, European foulbrood caused by a bacterial pathogen Melissococcus plutonius, and the enemy wax moth (Galleria mellonella). Although SBV infection does not usually result in colony losses in A. mellifera, it poses the greatest threat facing A. cerana [5]. SBV can infect both the brood and adult stages of honeybees’ life cycles; however, larvae around two days old are the most sensitive [5].
Besides SBV, many other viruses infect both A. mellifera and A. cerana, including IAPV. IAPV is a positive-sense, single-stranded RNA virus that can cause systemic infection in honeybees and has been linked with colony losses of A. mellifera [2]. In addition to the typical symptom of paralysis and an inability to fly [6], honeybees infected with IAPV exhibit behavioral and cognitive impairments, such as reduced learning and memory abilities [7], reduced lifespan [8], and an exacerbation of the harmful effects of antibiotics [9].
Except for A. mellifera, IAPV can also infect other pollinating insects such as A. cerana [10,11], bumblebees [12], and wasps [13], as well as various species inside beehives [11,14,15]. IAPV was first detected in Japanese honeybees A. cerana japonica in 2011 [10] and in Chinese honeybees A. cerana cerana in 2012 [11]. Data from Japan suggest the possibility of inter-specific transmission from A. mellifera to A. cerana for IAPV infection [10]. A previous study showed that IAPV was the second most common virus found in V. destructor in A. cerana colonies [16]. Since its first detection, it has been one of the most common viruses detected in A. cerana. In a study conducted in 2021 [17], the overall infection rate of IAPV in A. cerana reached 18.57%, which was higher than earlier reports [18,19,20], suggesting that A. cerana in China may potentially be facing an increasing threat from this virus.
We collected diseased brood of A. cerana from various geographical sources and analyzed the infection levels of M. plutonius and seven common viruses. Considering the high prevalence of IAPV in A. cerana, we hypothesized that IAPV is another pathogen contributing to larval disease in A. cerana. We also inoculated A. cerana larvae with IAPV to primarily study the pathogenicity of IAPV on A. cerana.
2. Materials and Methods
2.1. Sample Collection
Seventy samples of diseased A. cerana brood were collected between May 2017 and May 2020 from 19 regions in 9 provinces in China (Table 1). Samples were collected only from colonies with dead brood in cells. When sampling, the developmental stages of the majority of brood were identified as larvae. However, in a few cases, the developmental stages of dead brood could not be identified because they had rotted. All the samples were collected in the spring, when brood disease is the most prevalent in A. cerana [5]. Each sample consisted of five dead brood, which were placed in a 1.5 mL centrifuge tube and stored in a −80 °C freezer.

Table 1.
Sampling information of the diseased A. cerana brood.
2.2. DNA Extraction, RNA Extraction and cDNA Synthesis of Diseased Brood
The total genomic DNA of brood samples was extracted using the DNA extraction kit (DP304, Tiangen, Beijing, China), following the procedure suggested by the manufacturer, and resuspended in 50 μL buffer TE. The total RNA of the brood samples was extracted with the RNApure Total RNA Kit (RN-03, Aidlab, Beijing, China), following the manufacturer’s instructions. The quality of each RNA sample was checked using a Nanodrop-2000 spectrophotometer (Thermo Fisher, Waltham, MA, USA). From each RNA sample, 800 ng was taken for cDNA synthesis with the ReverTra Ace qPCR RT Master Mix (FSQ-201, Toyobo, Osaka, Japan), according to the manufacturer’s instructions. The cDNAs and genomic DNA of all samples were stored at −20 °C until use. Genomic DNA was utilized for the qualitative detection of M. plutonius, while cDNA was employed for detecting common bee viruses.
2.3. Qualitative Detection of Common Bee Viruses and M. plutonius
Normal polymerase chain reaction (PCR) was performed using a high-fidelity enzyme kit (KFX-101, TOYOBO, Osaka, Japan). The total reaction volume of 25 μL consisted of cDNA or genomic DNA (1.0 μL), 2× PCR buffer for KOD FX (12.5 μL), KOD FX (0.5 μL), dNTPs (2 μM, 5 μL), ddH2O (4.5 μL), and forward and reverse primers (10 μM, 0.75 μL each) (primer sets listed in Table 2). The cycling profile was 94 °C for 2 min; followed by 40 cycles of 98 °C for 10 s, 56 °C for 30 s, and 68 °C for 1 min; and 72 °C for 5 min. The PCR products were electrophoresed on a 1.5% agarose gel.

Table 2.
Primer sets of the common pathogens used in this study.
2.4. Quantitative Real-Time PCR (qRT-PCR) Assays for Viral Load Quantification of Five Bee Viruses
qRT-PCR was performed to investigate the difference in viral loads of five common RNA viruses (BQCV, CBPV, DWV, IAPV, and SBV), given their high prevalence in the diseased A. cerana brood. Positive samples of each virus were applied based on the normal PCR detection. The primers for the qRT-PCR are listed in Table 3. The assays were performed in 10 μL volumes containing 1 μL cDNA, 5 μL TB Green Premix Ex Taq II (Takara, Osaka, Japan), 0.5 μL each of the forward and reverse primer, and 3 μL RNase-free water. Amplifications were performed in triplicate with the following PCR conditions: a single cycle at 95 °C for 1 min; 40 cycles at 95 °C for 15 s, 60 °C for 1 min, and 95 °C for 15 s.

Table 3.
Primer sets of the quantitative detection of five viruses.
Viral loads were quantified using absolute quantification methods according to our former study [17]. Briefly, the linear standard curve equation for each virus was established based on standard curves obtained through six ten-fold dilutions of known amounts of plasmids (pMD®18-T Vector, TaKaRa, Osaka, Japan) containing cloned viral target sequences. A linear standard curve was used for each qRT-PCR run. The copy numbers of the viruses were determined by relating the Ct value of each sample to an established standard curve.
2.5. Phylogenetic Analysis of IAPV Sequences
Part of the products of IAPV amplification were sequenced (Sangon Biotech, Shanghai, China) for phylogenetic analysis, and the sequences were deposited in GenBank (https://www.ncbi.nlm.nih.gov/genbank/) under the accession numbers MZ494141–MZ494155, accessed on 1 July 2021. These sequences were individually aligned using the ClustalW program in MEGA 6.0 software [30], with other representative homologous sequences retrieved from GenBank. The phylogenetic tree was constructed in MEGA 6.0 software using the neighbor-joining method, based on the Tamura 3-parameter model [31] and a bootstrap value of 1000 replicates.
2.6. Preparation and Quantification of IAPV Solution
Since phylogenetic analysis did not show a species barrier for IAPV infection [17], A. mellifera workers were used for the preparation of the IAPV solution. After qRT-PCR detection of the five common viruses as described in Section 2.4, 50 adult A. mellifera bees from a colony heavily infected with IAPV were homogenized in 10 mL 1× phosphate-buffered saline (1× PBS buffer). Debris was eliminated using centrifugation (12,000× g, 4 °C for 40 min) and virus preparations were prepared using a 0.2-micron filter. Following the method of Remnant et al. [32], 2 μL of the inoculant was injected into the white-eyed pupae via a self-made injector at a slow rate. All the pupae were maintained in an incubator (STIK, Shanghai, China) at 34 ± 0.5 °C, 70 ± 5% relative humidity for 4–5 days. Then, the pupae were used to prepare the viral inoculant using the approach outlined above. qRT-PCR was conducted on the viral inoculant to detect BQCV, CBPV, DWV, SBV, and IAPV. If any viruses other than IAPV were detected, the viral inoculant was injected into white-eyed pupae for further purification. The purification process was repeated several times until only pure IAPV virions were obtained.
2.7. Virus Inoculation of A. cerana Larvae
Larvae younger than 24 h (day 1) from healthy A. cerana colonies were carefully transferred to 48-well culture plates and fed a total of 150 µL of IAPV-containing food (approximately 1 × 106, 1 × 105, and 1 × 104 genome copy numbers/µL) twice at the following two days, while the control groups were fed the same volume of food with the IAPV solution replaced by PBS buffer. Afterward, the larvae were fed a regular artificial diet (50% royal jelly, 6% glucose, 6% fructose, 1% liquid yeast, and 37% H2O) until their pupation (day 6).
2.8. IAPV Detection of Inoculated A. cerana Brood
At day 5, 7, 9, 12, 15, and 19, six individuals from both the control group and the experimental group (exposed to a concentration of 1 × 106 copies/μL) were collected and stored at −80 °C for further experiments. Additionally, six dead brood from the 1 × 106 copies/μL experimental group were collected on day 9 and 12. The same inoculation experiment was repeated three times. RNA extraction, reverse transcription, and quantitative detection of IAPV from these samples were performed using the methods described above.
2.9. Statistical Analysis
The viral loads were analyzed using one-way ANOVA analysis or/with Tukey’s post hoc test for multiple comparisons. The correlations between the occurrence of viruses were analyzed by Spearman’s rho. The survival curve was analyzed using the Kaplan–Meier test. A p-value of <0.05 was considered statistically significant. All statistical analyses were conducted in SPSS 17.0 software.
3. Results
3.1. Qualitative Detection of Pathogens in A. cerana Diseased Brood
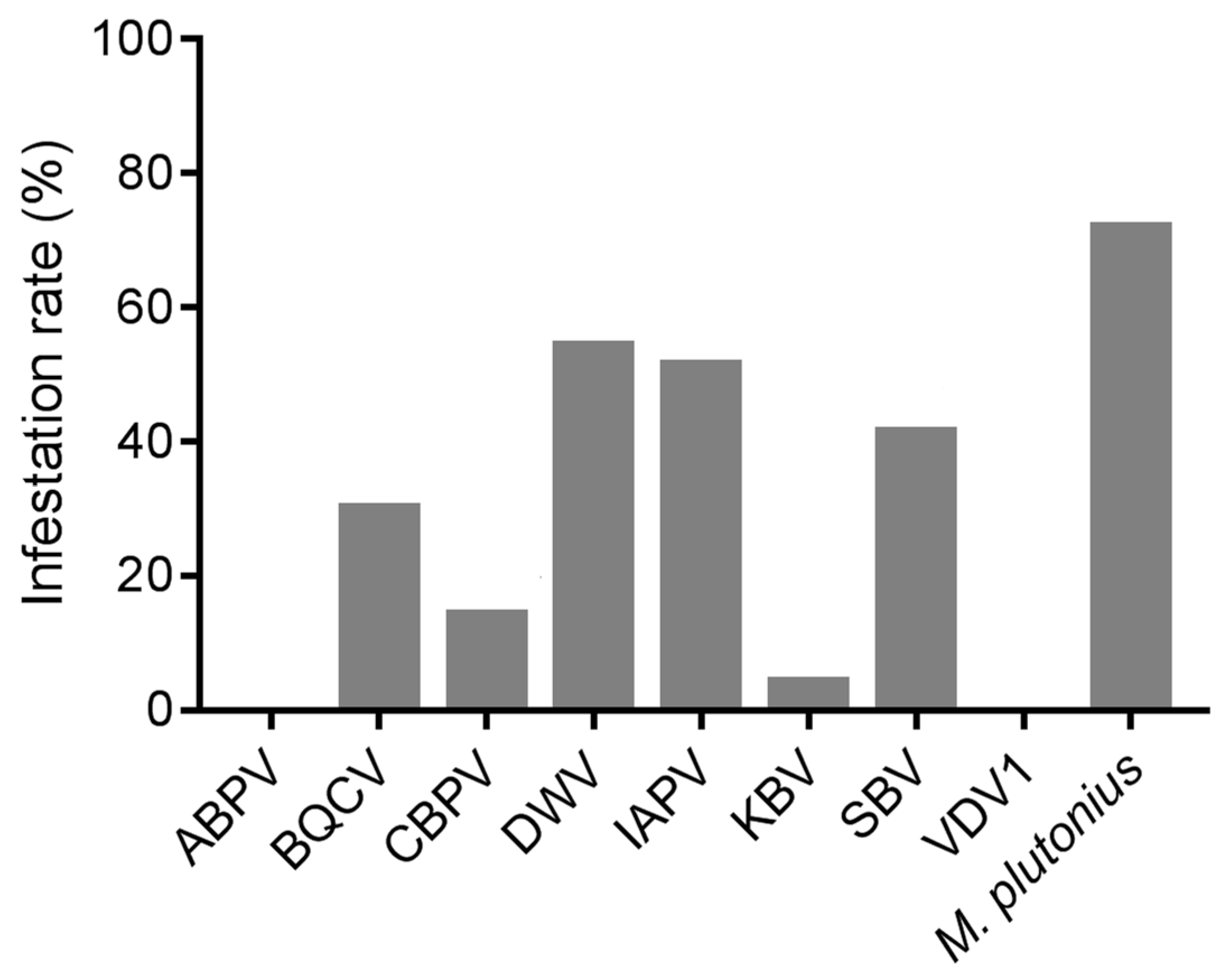
As one of the most prevalent pathogens of A. cerana brood, the infection rate of M. plutonius was 71.93% among 70 samples of diseased brood (Figure 1). Apart from ABPV and VDV1, neither of which was detected, DWV (54.29%) was the most prevalent virus, followed by IAPV (51.43%), SBV (41.43%), BQCV (30.00%), CBPV (14.29%), and KBV (4.29%).
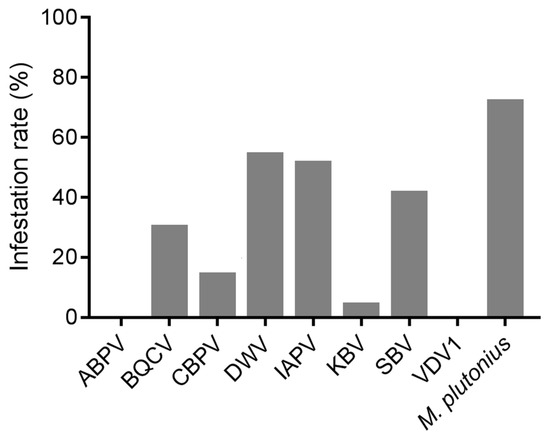
Figure 1.
The infection rates of the detected pathogens in the diseased brood of A. cerana.
Among all the samples, 21.43% of the samples were not infected with any bee virus, while almost two-thirds (62.86%; Table 4) were infected with at least two viruses, with a maximum of five viruses simultaneously infecting a sample. Among these viruses, co-infection of BQCV, DWV, and IAPV (8.57%) was the most common, followed by the co-infections of BQCV and DWV; IAPV and SBV; and BQCV, DWV, IAPV, and SBV (each 7.14%). The correlation analysis on their occurrence indicated that IAPV was the virus most strongly correlated with other viruses, showing significant positive correlations with CBPV, DWV, and SBV (Spearman’s rho, p < 0.05) (Table S1).

Table 4.
The multiple infections of viruses in the diseased brood of A. cerana.
3.2. Quantification of Viral Loads in Diseased Brood
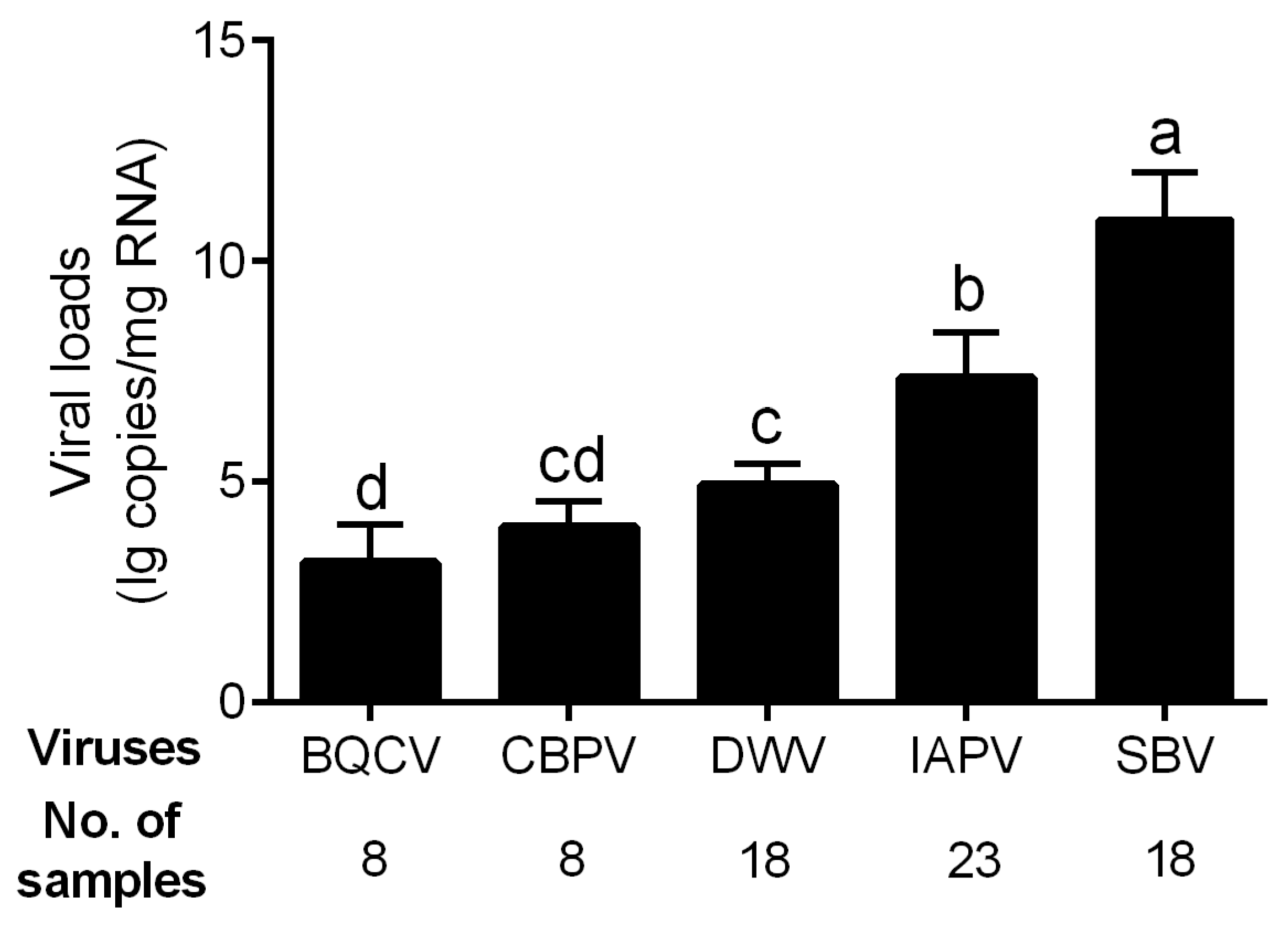
The viral loads of the five bee viruses with high infection rates, i.e., BQCV, CBPV, DWV, IAPV, and SBV, were determined. The genome copy number of SBV was high in all infected samples, reaching 10.94 ± 1.06 lg copies/mg RNA in average (Figure 2), which was significantly higher than those of other viruses (p < 0.01 for all comparisons). The viral load of IAPV in the diseased brood was 7.36 ± 1.02 lg copies/mg RNA on average and significantly higher than those of the other three viruses (p < 0.01 for all comparisons). The infection levels of BQCV (3.18 ± 0.86), CBPV (3.98 ± 0.59), and DWV (4.93 ± 0.48) were relatively low.
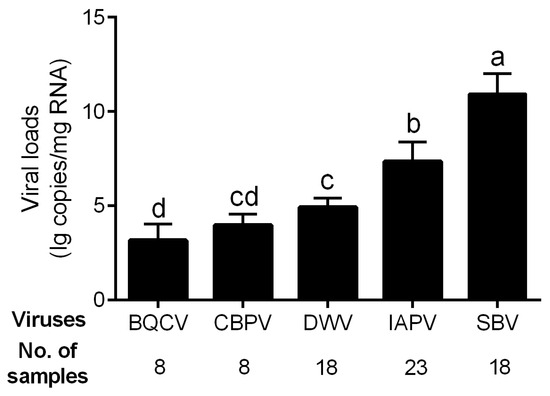
Figure 2.
Viral loads of five common viruses in the positive samples of A. cerana. Different letters mean significantly different statistics levels (p < 0.05).
3.3. Phylogenetic Tree of IAPV Isolates
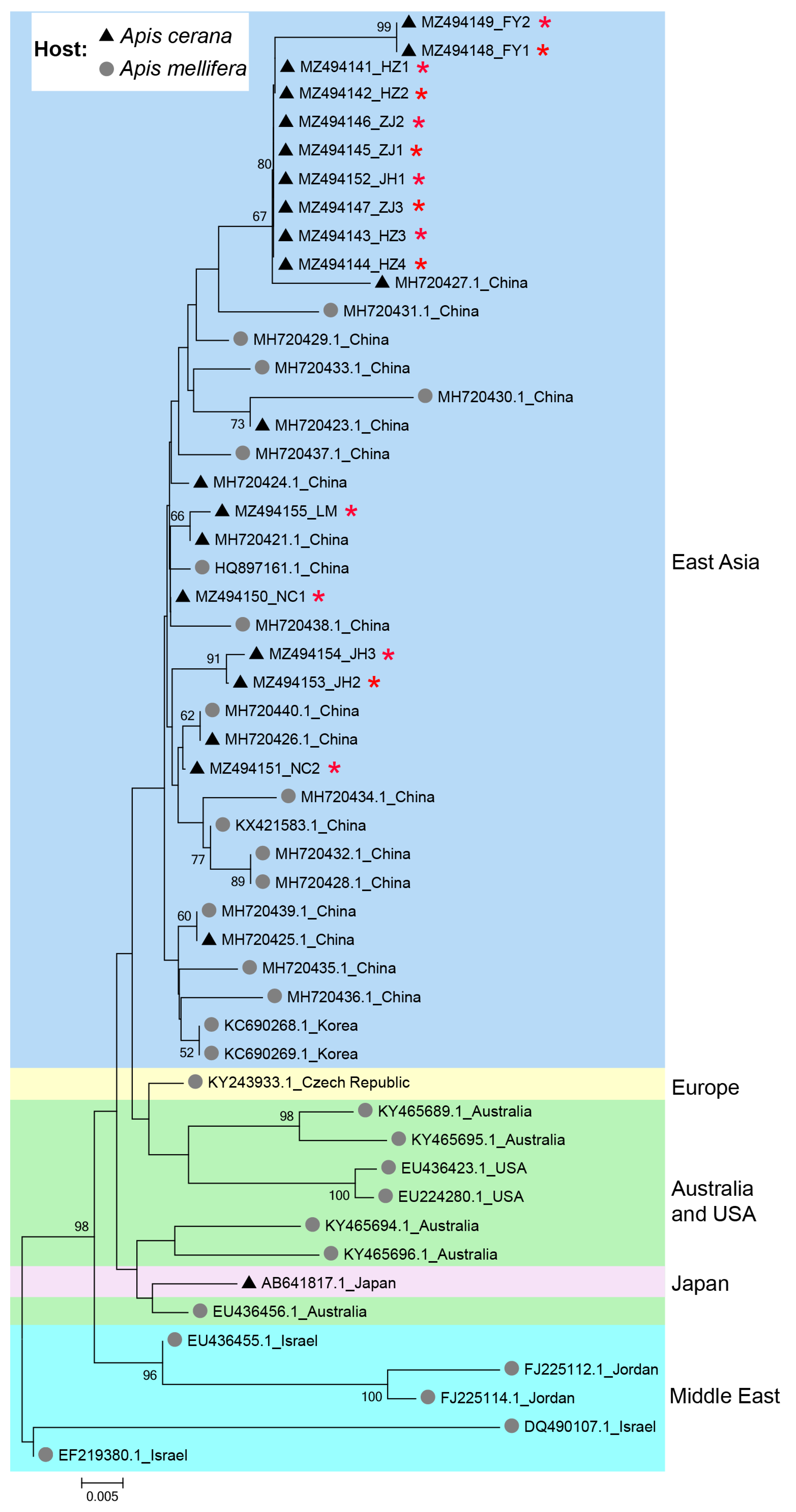
The phylogeny of IAPV clearly showed a geographical pattern, with isolates from nearby countries tending to cluster together. Isolates from A. cerana were phylogenetically clustered with those from A. mellifera, without forming distinct clades based on host species in China (Figure 3).
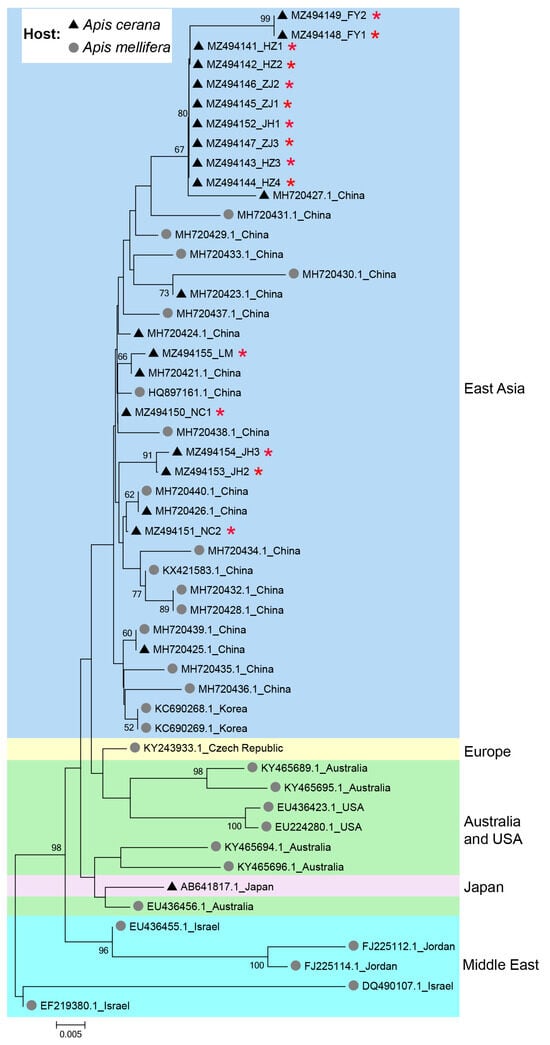
Figure 3.
Phylogenetic tree illustrating the genetic relationship of IAPV isolates from different countries and different hosts. The sequences obtained in this study were indicated by red asterisks (*). Numbers at each node represent bootstrap values as percentages of 100; only bootstrap values greater than 50% are shown.
3.4. Mortality and Viral Load of A. cerana Brood Inoculated with IAPV
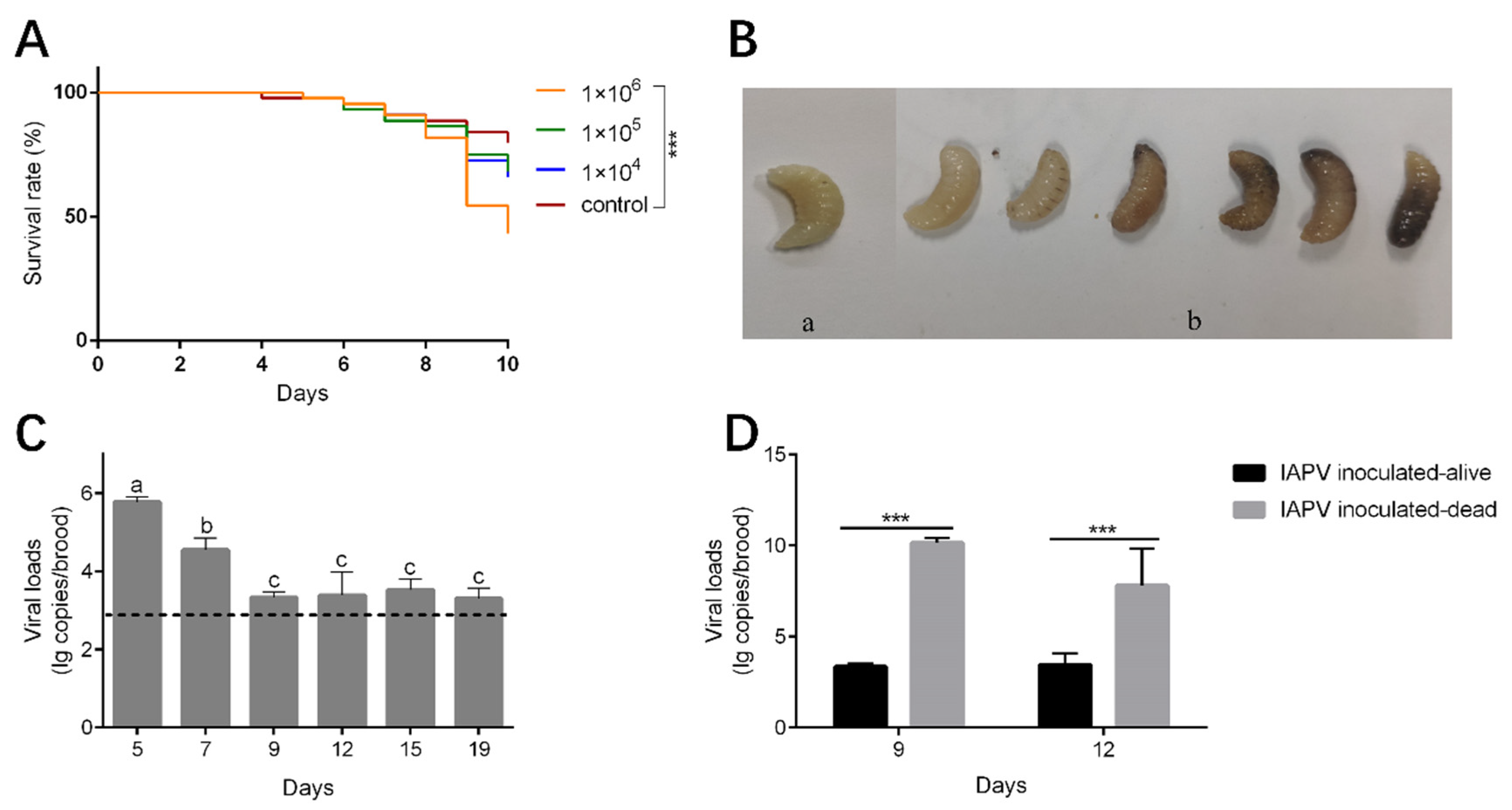
The mortality rate of the brood inoculated with IAPV at titers of 1 × 104 and 1 × 105 genome copies/uL was relatively higher, but not significantly different from those of the control group (Kaplan–Meier test, p = 0.184 and p = 0.244, respectively). However, when inoculated with high titers of IAPV (1 × 106 genome copies/uL), high mortality occurred at day 9 at the prepupae stage (Kaplan–Meier test, p < 0.001) (Figure 4A). The dead brood appeared dark brown or milky white, some showing fluid accumulation under the skin or developmental malformations (Figure 4B). These symptoms are similar to those observed in the diseased larvae we collected. The viral load of the survival brood decreased significantly from day 5 to day 9 (Tukey’s post hoc test, p < 0.05) (Figure 4C). Meanwhile, the viral loads of dead brood collected on day 9 and day 12 were significantly higher than those of survived brood at the corresponding day ages (one-way ANOVA analysis, p < 0.001) (Figure 4D).
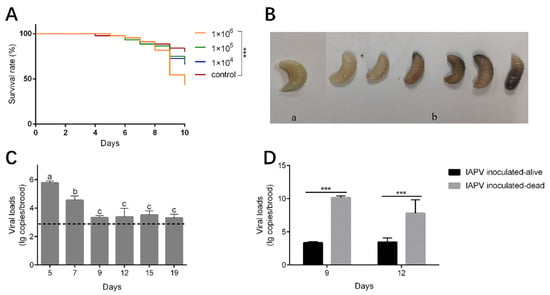
Figure 4.
Impact of IAPV inoculation on survival and viral load of A. cerana larvae. (A) The survival curve of A. cerana brood inoculated with IAPV. ***, p < 0.001. (B) The brood after IAPV inoculation. (a) Alive A. cerana brood. (b) Dead A. cerana brood. (C) The viral loads of alive A. cerana brood after inoculation. The dashed line indicates the mean viral loads of the control groups at the six time points. Low titer of IAPV can be detected in control groups due to the ubiquitous virus in honeybee colonies. Different letters mean significantly different statistical levels (p < 0.01). (D) The viral loads of alive and dead A. cerana brood after IAPV inoculation at day 9 and 12. ***, p < 0.001.
4. Discussion
The primary symptom of diseased A. cerana colonies is larval mortality, commonly attributed to SBV and M. plutonius. This attribution often overlooks other viruses present in A. cerana. Certainly, the mere presence of pathogens does not necessarily indicate they are the causative agents of the disease, but the prevalence and loads of these pathogens may provide significant clues. Moreover, studies on honeybee viruses have predominantly focused on A. mellifera, with limited research addressing the effects of viruses on A. cerana. Therefore, it is essential to conduct epidemiological investigations and studies on viral pathogenicity to address the rising incidence of brood diseases in A. cerana.
Our results indicated that, among all the detected pathogens, M. plutonius was the most prevalent pathogen (Figure 1), while SBV exhibited the highest viral load in diseased A. cerana brood (Figure 2). This aligns with previous reports that M. plutonius and SBV are the major pathogenic microorganisms threatening A. cerana [33,34]. Notably, IAPV warrants attention considering its high prevalence both in A. mellifera and A. cerana [17]. Among all the tested common honeybee viruses, it showed the second highest prevalence (Figure 1) and viral load (Figure 2) in diseased A. cerana brood. When multiple viruses concurrently infect honeybees, IAPV may preferentially proliferate under certain conditions [8,35]. Additionally, IAPV can affect all developmental stages of honeybees, with brood being more susceptible than adult bees [36]. Despite the health status of A. cerana being generally better than A. mellifera, thanks to its high resistance to the ectoparasite V. destructor at colony level, the brood of A. cerana is more susceptible to both biological and mechanical stimuli [37,38]. Our survey indicated that IAPV is likely a previously unrecognized pathogen capable of causing disease in A. cerana brood.
Phylogenetic analysis indicated that the clustering of IAPV is not influenced by host species but by geographical locations, suggesting that cross-species transmission of IAPV occurs between A. mellifera and A. cerana, which aligns with previous studies [11,17,19]. This further raises the risk of IAPV to A. cerana, since both A. mellifera and A. cerana are widely kept in Asia; thus, cross-species transmission of the virus may occur frequently [27].
Artificial inoculation of the virus was conducted to further assess the pathogenicity of IAPV to A. cerana larvae. A dosage effect of IAPV inoculation by oral administration was observed, with a concentration of 1 × 106 copies/μL of IAPV leading to significant mortality in A. cerana brood. A previous study has demonstrated that as few as 104 copies/μL of IAPV can induce mortality in A. mellifera pupae within 96 h by injection [8]. This inconsistency can be explained by the different methodologies, the different pathogenicity of IAPV isolates, or the different resistance to IAPV of the two honeybee species. The virus loads of IAPV in dead inoculated brood were 106 times higher than those in alive inoculated brood, suggesting the virus proliferated fast before or after the death of some larvae, while, in others, virus proliferation was inhibited by the hosts. The decrease in viral load from day 5 to day 9 of survived larvae suggest that the host’s innate immune system is triggered and activated to restrict virus replication and clear pathogens [39]. A high mortality rate was observed in the groups of honeybees inoculated with a high titer of IAPV at day 9 before pupation, suggesting that A. cerana larvae are more susceptible to IAPV infection at this stage.
To the best of our knowledge, this is the first preliminary study revealing the pathogenesis of IAPV to honeybee species other than A. mellifera. This work emphasizes that IAPV should be recognized as an emerging pathogen causing brood disease in A. cerana. However, our current knowledge is scarce and further studies are needed to fully elucidate the pathogenesis of IAPV to A. cerana and the host defense of A. cerana against the virus.
5. Conclusions
Our study reveals the previously unrecognized pathogenicity of IAPV to A. cerana brood, which partially explains the increasing incidence of brood diseases in A. cerana. IAPV has posed a significant threat to A. cerana, with high infection rates and viral loads similar to those of SBV and M. plutonius in diseased brood. We emphasize that, in beekeeping practice, IAPV should be considered to be an emerging pathogen responsible for brood disease in A. cerana. Further studies are necessary to gain a full understanding of the interactions between IAPV and A. cerana to finally develop technologies to mitigate the impact of IAPV to A. cerana.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v16091395/s1, Table S1: Spearman correlation coefficients among viral detections.
Author Contributions
Conceptualization, Y.X., S.W. and H.Z.; methodology, Y.X., S.W. and J.D.; software, S.W.; validation, Y.X.; formal analysis, Y.X., S.W. and J.D.; investigation, Y.X., X.S. and J.D.; data curation, Y.X., Z.H. and J.D.; writing—original draft preparation, Y.X. and S.W.; writing—review and editing, Y.X., S.W., Y.L. and H.Z.; visualization, Y.X., S.W. and J.D.; supervision, Y.X. and H.Z.; funding acquisition, H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by China Agriculture Research System of MOF and MARA (grant number CARS-44) and the Zhejiang Collaborative Extension Plan of Major Agricultural Technologies (grant number 2023ZDXT17-01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and analyzed during the current study are available upon reasonable request.
Acknowledgments
We are very grateful to the colleagues in the China Bee Industry Research System and beekeepers for generously providing samples. We are also very grateful to the Experimental Teaching Centre, College of Animal Sciences, Zhejiang University, for providing access to their instruments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Evans, J.; Schwarz, R. Bees brought to their knees: Microbes affecting honey bee health. Trends Microbiol. 2011, 19, 614–620. [Google Scholar] [CrossRef]
- McMenamin, A.; Genersch, E. Honey bee colony losses and associated viruses. Curr. Opin. Insect Sci. 2015, 8, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, H.; Radloff, S. (Eds.) Honeybees of Asia; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Lin, Z.; Page, P.; Li, L.; Qin, Y.; Zhang, Y.; Hu, F.; Neumann, P.; Zheng, H.; Dietemann, V. Go east for better honey bee health: Apis cerana is faster at hygienic behavior than A. mellifera. PLoS ONE 2016, 11, e0162647. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Cao, L.; Feng, Y.; Chen, Y.; Chen, G.; Zheng, H. Sacbrood virus: A growing threat to honeybees and wild pollinators. Viruses 2022, 14, 1871. [Google Scholar] [CrossRef] [PubMed]
- Maori, E.; Lavi, S.; Mozes-Koch, R.; Gantman, Y.; Peretz, Y.; Edelbaum, O.; Tanne, E.; Sela, I. Isolation and characterization of Israeli acute paralysis virus, a dicistrovirus affecting honeybees in Israel: Evidence for diversity due to intra- and inter-species recombination. J. Gen. Virol. 2007, 12, 3428–3438. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, Y.; Zhang, S.; Chen, S.; Li, W.; Yan, L.; Shi, L.; Wu, L.; Sohr, A.; Su, S. Viral infection affects sucrose responsiveness and homing ability of forager honey bees, Apis mellifera L. PLoS ONE 2013, 8, e77354. [Google Scholar] [CrossRef]
- Boncristiani, H.; Evans, J.; Chen, Y.; Pettis, J.; Murphy, C.; Lopez, D.; Simone-Finstrom, M.; Strand, M.; Tarpy, D.; Rueppell, O. In vitro infection of pupae with Israeli acute paralysis virus suggests disturbance of transcriptional homeostasis in honey bees (Apis mellifera). PLoS ONE 2013, 8, e73429. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, S.; Wu, Y.; Zhou, N.; Xie, Y.; Wei, R.; Huang, Z.; Chen, Y.; Hu, F.; Zheng, H. Tetracycline-induced gut community dysbiosis and Israeli Acute Paralysis Virus infection synergistically negatively affect honeybees. Ecotoxicol. Environ. Saf. 2024, 282, 116706. [Google Scholar] [CrossRef]
- Kojima, Y.; Toki, T.; Morimoto, T.; Yoshiyama, M.; Kimura, K.; Kadowaki, T. Infestation of Japanese native honey bees by tracheal mite and virus from non-native European honey bees in Japan. Microb. Ecol. 2011, 62, 895–906. [Google Scholar] [CrossRef]
- Yañez, O.; Zheng, H.; Hu, F.; Neumann, P.; Dietemann, V. A scientific note on Israeli acute paralysis virus infection of Eastern honeybee Apis cerana and vespine predator Vespa velutina. Apidologie 2012, 43, 587–589. [Google Scholar] [CrossRef]
- Meeus, I.; de Miranda, J.; de Graaf, D.; Wäckers, F.; Smagghe, G. Effect of oral infection with Kashmir bee virus and Israeli acute paralysis virus on bumblebee (Bombus terrestris) reproductive success. J. Invertebr. Pathol. 2014, 121, 64–69. [Google Scholar] [CrossRef]
- Yang, S.; Gayral, P.; Zhao, H.; Wu, Y.; Jiang, X.; Wu, Y.; Bigot, D.; Wang, X.; Yang, D.; Herniou, E.; et al. Occurrence and molecular phylogeny of honey bee viruses in Vespids. Viruses 2019, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Levitt, A.; Singh, R.; Cox-Foster, D.; Rajotte, E.; Hoover, K.; Ostiguy, N.; Holmes, E. Cross-species transmission of honey bee viruses in associated arthropods. Virus Res. 2013, 176, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Traiyasut, P.; Mookhploy, W.; Kimura, K.; Yoshiyama, M.; Khongphinitbunjong, K.; Chantawannakul, P.; Buawangpong, N.; Saraithong, P.; Burgett, M.; Chukeatirote, E. First detection of honey bee viruses in wax moth. Chiang Mai J. Sci. 2016, 43, 695–698. [Google Scholar]
- Wang, S.; Chen, G.; Lin, Z.; Wu, Y.; Hu, F.; Zheng, H. Occurrence of multiple honeybee viruses in the ectoparasitic mites Varroa spp. in Apis cerana colonies. J. Invertebr. Pathol. 2019, 166, 107225. [Google Scholar] [CrossRef]
- Chen, G.; Wu, Y.; Deng, J.; Wen, Z.; Wang, S.; Chen, Y.; Hu, F.; Zheng, H. Seasonal variation of viral infections between the eastern honey bee (Apis cerana) and the western honey bee (Apis mellifera). MicrobiologyOpen 2021, 10, e1162. [Google Scholar] [CrossRef] [PubMed]
- Ai, H.; Yan, X.; Han, R. Occurrence and prevalence of seven bee viruses in Apis mellifera and Apis cerana apiaries in China. J. Invertebr. Pathol. 2012, 109, 160–164. [Google Scholar] [CrossRef]
- Yang, B.; Peng, G.; Li, T.; Kadowaki, T. Molecular and phylogenetic characterization of honey bee viruses, Nosema microsporidia, protozoan parasites, and parasitic mites in China. Ecol. Evol. 2013, 3, 298–311. [Google Scholar] [CrossRef]
- Eva, F.; Shi, W.; Ding, G.; Liu, Z.; Toan, V.; Phuong, T.; Tuan, A.; Tam, Q.; Ingemar, F. Preliminary observations on possible pathogen spill-over from Apis mellifera to Apis cerana. Apidologie 2015, 46, 265–275. [Google Scholar] [CrossRef]
- Benjeddou, M.; Leat, N.; Allsopp, M.; Davison, S. Detection of acute bee paralysis virus and black queen cell virus from honeybees by reverse transcriptase PCR. Appl. Environ. Microbiol. 2001, 67, 2384–2387. [Google Scholar] [CrossRef]
- Ribière, M.; Triboulot, C.; Mathieu, L.; Aurières, C.; Faucon, J.P.; Pépin, M. Molecular diagnosis of chronic bee paralysis virus infection. Apidologie 2002, 33, 339–351. [Google Scholar] [CrossRef]
- Tentcheva, D.; Gauthier, L.; Zappulla, N.; Dainat, B.; Cousserans, F.; Colin, M.E.; Bergoin, M. Prevalence and seasonal variations of six bee viruses in Apis mellifera L. and Varroa destructor mite populations in France. Appl. Environ. Microbiol. 2004, 70, 7185–7191. [Google Scholar] [CrossRef]
- Di Prisco, G.; Pennacchio, F.; Caprio, E.; Boncristiani, H., Jr.; Evans, J.; Chen, Y. Varroa destructor is an effective vector of Israeli acute paralysis virus in the honeybee, Apis mellifera. J. Gen. Virol. 2011, 92, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Stoltz, D.; Shen, X.; Boggis, C.; Sisson, G. Molecular diagnosis of Kashmir bee virus infection. J. Apic. Res. 1995, 34, 153–160. [Google Scholar] [CrossRef]
- Ma, M.; Li, M.; Cheng, J.; Yang, S.; Wang, S.; Li, P. Molecular and biological characterization of Chinese sacbrood virus LN isolate. Int. J. Genomics. 2011, 1, 409386. [Google Scholar] [CrossRef]
- Yanez, O.; Zheng, H.; Su, X.; Hu, F.; Neumann, P.; Dietemann, V. Potential for virus transfer between the honey bees Apis mellifera and A. cerana. J. Apic. Res. 2015, 54, 179–191. [Google Scholar] [CrossRef]
- Govan, V.; Brözel, V.; Allsopp, M.; Davison, S. A PCR detection method for rapid identification of Melissococcus pluton in honeybee larvae. Appl. Environ. Microbiol. 1998, 64, 1983–1985. [Google Scholar] [CrossRef]
- Blanchard, P.; Guillot, S.; Antùnez, K.; Köglberger, H.; Kryger, P.; de Miranda, J.; Franco, S.; Chauzat, M.; Thiéry, R.; Ribière, M. Development and validation of a real-time two-step RT-qPCR TaqMan® assay for quantitation of Sacbrood virus (SBV) and its application to a field survey of symptomatic honey bee colonies. J. Virol. Methods. 2014, 197, 7–13. [Google Scholar] [CrossRef]
- Thompson, J.; Gibson, T.; Plewniak, F.; Jeanmougin, F.; Higgins, D. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Remnant, E.J.; Mather, N.; Gillard, T.L.; Yagound, B.; Beekman, M. Direct transmission by injection affects competition among RNA viruses in honeybees. Proc. R. Soc. B 2019, 286, 20182452. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Guo, J.; Tang, Y.; Chen, Y.; Wu, J.; Li, J. Chinese sacbrood virus infection in Asian honey bees (Apis cerana cerana) and host immune responses to the virus infection. J. Invertebr. Pathol. 2017, 150, 63–69. [Google Scholar] [CrossRef]
- Zhou, T.; Feng, F.; Dong, B. Study on the pathogen of European foulbrood in the Chinese honey bee (Apis cerana cerana F.). Acta Entomol. Sin. 2000, 43, 104–108. [Google Scholar] [CrossRef]
- Carrillo-Tripp, J.; Dolezal, A.; Goblirsch, M.; Miller, W.; Toth, A.; Bonning, B. In vivo and in vitro infection dynamics of honey bee viruses. Sci. Rep. 2016, 6, 22265. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pettis, J.; Corona, M.; Chen, W.; Li, C.; Spivak, M.; Visscher, P.; DeGrandi-Hoffman, G.; Boncristiani, H.; Zhao, Y.; et al. Israeli acute paralysis virus: Epidemiology, pathogenesis and implications for honey bee health. PLoS Pathog. 2014, 10, e1004261. [Google Scholar] [CrossRef] [PubMed]
- Page, P.; Lin, Z.; Buawangpong, N.; Zheng, H.; Hu, F.; Neumann, P.; Chantawannakul, P.; Dietemann, V. Social apoptosis in honey bee superorganisms. Sci. Rep. 2016, 6, 27210. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Qin, Y.; Page, P.; Wang, S.; Li, L.; Wen, Z.; Hu, F.; Neumann, P.; Zheng, H.; Dietemann, V. Reproduction of parasitic mites Varroa destructor in original and new honeybee hosts. Ecol. Evol. 2018, 8, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Brutscher, L.M.; Daughenbaugh, K.F.; Flenniken, M.L. Antiviral defense mechanisms in honey bees. Curr. Opin. Insect Sci. 2015, 10, 71–82. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).