The Anti-SARS-CoV-2 S-Protein IgG, Which Is Detected Using the Chemiluminescence Microparticle Immunoassay (CMIA) in Individuals Having Either a History of COVID-19 Vaccination and/or SARS-CoV-2 Infection, Showed a High-Titer Neutralizing Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
- Over 18 years old and under 65 years old;
- Vaccinated with at least two doses of Sinovac or Biontech vaccines;
- No more than six months have passed since vaccination or infection;
- No chronic or metabolic disease;
- No COVID-19-like symptoms.
- Under 18 years old and over 65 years old;
- Have not been vaccinated with at least two doses of Sinovac or Biontech vaccines;
- More than six months have passed since vaccination or infection;
- Have a chronic or metabolic disease;
- Have COVID-19-like symptoms;
- A PCR test result that has not yet become negative.
2.2. Collection and Storage of Samples
2.3. Chemiluminescence Microparticle Immune Assay (CMIA)
2.4. Surrogate Virus Neutralization Test (sVNT)
2.5. Statistical Method
3. Results
3.1. Demographic Properties of the Study Groups
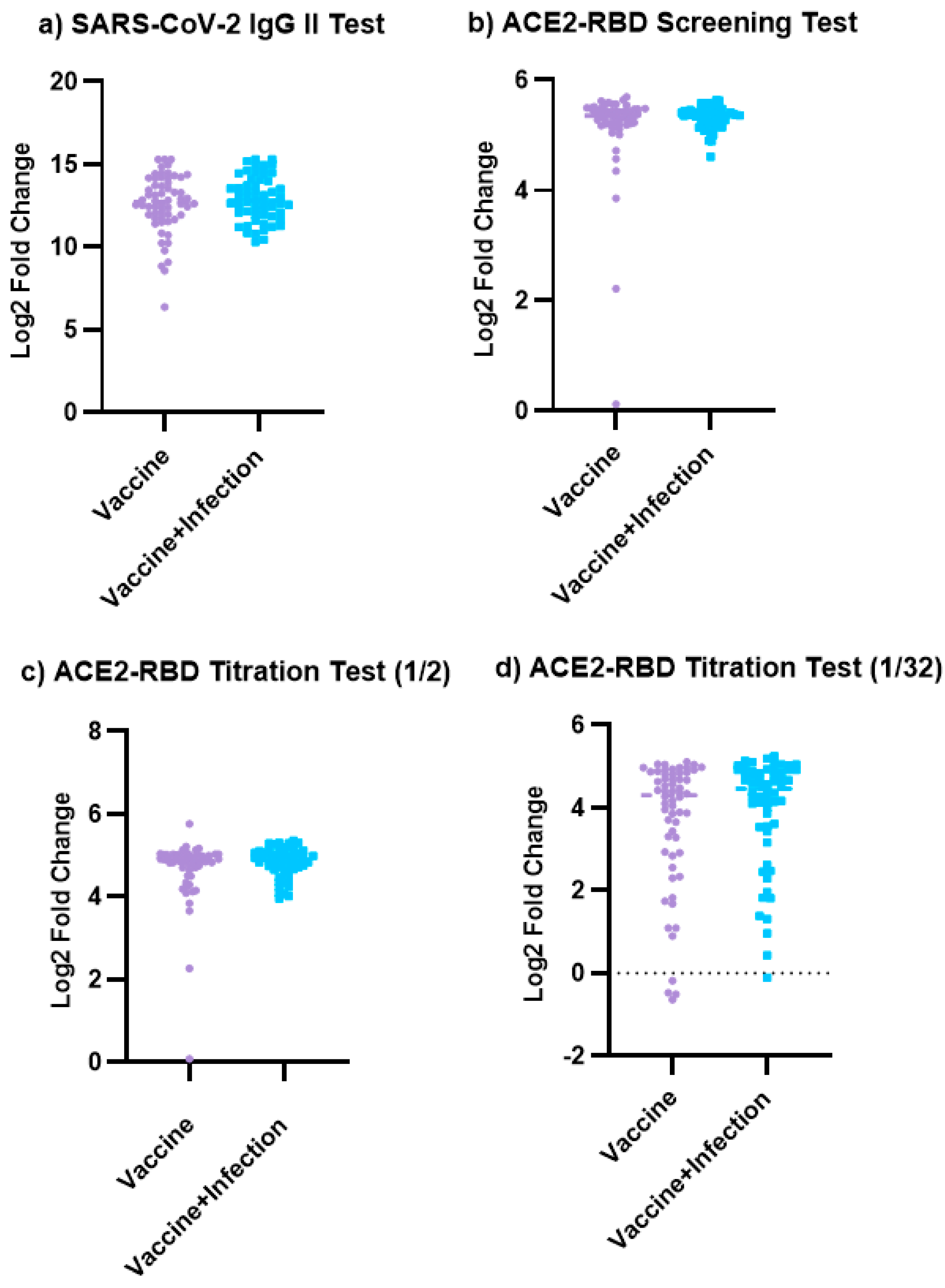
3.2. The Results of the SARS-CoV-2 IgG II Quant Test and ACE2-RBD Neutralization Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Post, N.; Eddy, D.; Huntley, C.; van Schalkwyk, M.C.I.; Shrotri, M.; Leeman, D.; Rigby, S.; Williams, S.V.; Bermingham, W.H.; Kellam, P.; et al. Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS ONE 2020, 15, e0244126. [Google Scholar] [CrossRef] [PubMed]
- Winichakoon, P.; Wipasa, J.; Chawansuntati, K.; Salee, P.; Sudjaritruk, T.; Yasri, S.; Khamwan, C.; Peerakam, R.; Dankai, D.; Chaiwarith, R. Diagnostic performance between in-house and commercial SARS-CoV-2 serological immunoassays including binding-specific antibody and surrogate virus neutralization test (sVNT). Sci. Rep. 2023, 13, 34. [Google Scholar] [CrossRef]
- Burton, D.R.; Williamson, R.A.; Parren, P.W. Antibody and Virus: Binding and Neutralization. Virology 2000, 270, 1–3. [Google Scholar] [CrossRef]
- Cavlek, T.V.; Stevanovic, V.; Kovac, S.; Borko, E.; Bogdanic, M.; Miletic, G.; Hruskar, Z.; Ferenc, T.; Coric, I.; Ferenc, M.V.; et al. Neutralizing Activity of SARS-CoV-2 Antibodies in Patients with COVID-19 and Vaccinated Individuals. Antibodies 2023, 12, 61. [Google Scholar] [CrossRef]
- Chvatal-Medina, M.; Mendez-Cortina, Y.; Patino, P.J.; Velilla, P.A.; Rugeles, M.T. Antibody Responses in COVID-19: A Review. Front. Immunol. 2021, 12, 633184. [Google Scholar] [CrossRef]
- Ozcurumez, M.K.; Ambrosch, A.; Frey, O.; Haselmann, V.; Holdenrieder, S.; Kiehntopf, M.; Neumaier, M.; Walter, M.; Wenzel, F.; Wolfel, R.; et al. SARS-CoV-2 Antibody Testing—Questions to be Asked. Allergy Clin. Immunol. 2020, 146, 35–43. [Google Scholar] [CrossRef]
- Ru, Z.; Zhang, Y.; Wu, J.; Huang, H.; Liang, Y.; Yang, X.; Wu, J.; Lou, J. Comparison of the SARS-CoV-2 Surrogate Virus Neutralization Test (sVNT) Assay and Direct Binding ELISA (S-IgG) with the Cytopathic Effect Assay (CPE) in Analyzing the Neutralization Antibody of Vaccination People. J. Clin. Immunol. Immunother. 2021, 7, 063. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Ruben, O.D.; Koup, R.A.; Fong, Y.; Plotkin, S.A.; Folmen, D. A COVID-19 Milestone Attained-A Correlate of Protection for Vaccines. N. Engl. J. Med. 2022, 387, 2203–2206. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, Y.; Greig, M.; Liu, G.; Driedger, M.; Langlois, M.A. Humoral Responses and Serological Assays in SARS-CoV-2 Infections. Front. Immunol. 2020, 11, 610688. [Google Scholar] [CrossRef]
- Riepler, L.; Rössler, A.; Falch, A.; Volland, A.; Borena, W.; von Laer, D.; Kimpel, J. Comparison of Four SARS-CoV-2 Neutralization Assays. Vaccines 2020, 9, 13. [Google Scholar] [CrossRef]
- Souiri, A.; Lemriss, S.; El Maliki, B.; Falahi, H.; El-Fahime, E.; El-Kabbaj, S. SARS-CoV-2-Neutralizing Antibody Response and Correlation of Two Serological Assays with Microneutralization. Vaccines 2023, 11, 590. [Google Scholar] [CrossRef] [PubMed]
- Kohmer, N.; Westhaus, S.; Rühl, C.; Ciesek, S.; Rabenau, H.F. Clinical performance of different SARS-CoV-2 IgG antibody tests. J. Med. Virol. 2020, 92, 2243–2247. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Mao, Q.; Tan, D.; Liu, J.; Zhang, X.; Li, L.; Liu, M.; Wang, Z.; Cheng, F.; Cui, B.; et al. Establishment of national standard for anti-SARS-CoV-2 neutralizing antibody in China: The first National Standard calibration traceability to the WHO International Standard. Front. Immunol. 2023, 14, 1107639. [Google Scholar] [CrossRef]
- Liu, K.T.; Han, Y.J.; Wu, G.H.; Huang, K.Y.A.; Huang, P.N. Overview of Neutralization Assays and International Standard for Detecting SARS-CoV-2 Neutralizing Antibody. Viruses 2022, 14, 1560. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.T.; Li, Z.; Samson, R.; Samavarchi-Tehrani, P.; Valcourt, E.J.; Wood, H.; Wood, H.; Budylowskiet, P.; Il, D.A.P.; Girardin, R.C.; et al. A Simple Protein-Based Surrogate Neutralization Assay for SARS-CoV-2. JCI Insight 2020, 5, e142362. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Reimerink, J.; Torriani, G.; Brouwer, F.; Godeke, G.J.; Yerly, S.; Hoogerwerf, M.; Vuilleumier, N.; Kaiser, L.; Eckerle, I.; et al. Validation and Clinical Evaluation of a SARS-CoV-2 Surrogate Virus Neutralisation Test (sVNT). Emerg. Microbes Infect. 2020, 9, 2394–2403. [Google Scholar] [CrossRef]
- Luo, J.; Klett, J.; Gabert, J.; Lipp, T.; Karbach, J.; Jäger, E.; Borte, S.; Hoffmann, R.; Milkovska-Stamenova, S. A Quantitative ELISA to Detect Anti-SARS-CoV-2 Spike IgG Antibodies in Infected Patients and Vaccinated Individuals. Microorganisms 2022, 10, 1812. [Google Scholar] [CrossRef]
- Lo Sasso, B.; Agnello, L.; Giglio, R.V.; Gambino, C.M.; Ciaccio, A.M.; Vidali, M.; Ciaccio, M. Longitudinal analysis of anti-SARS-CoV-2 S-RBD IgG antibodies before and after the third dose of the BNT162b2 vaccine. Sci. Rep. 2022, 12, 8679. [Google Scholar] [CrossRef]
- Santos da Silva, E.; Servais, J.-Y.; Kohnen, M.; Arendt, V.; Staub, T.; the CON-VINCE Consortium; the CoVaLux Consortium; Krüger, R.; Fagherazzi, G.; Wilmes, P.; et al. Validation of a SARS-CoV-2 Surrogate Validation of a SARS-CoV-2 Surrogate Neutralization Test Detecting Neutralizing Antibodies against the Major Variants of Concern. Int. J. Mol. Sci. 2023, 24, 14965. [Google Scholar] [CrossRef]
- Favresse, J.; Gillot, C.; Di Chiaro, L.; Eucher, C.; Elsen, M.; Van Eeckhoudt, S.; David, C.; Morimont, L.; Dogné, J.-M.; Douxfils, J. Neutralizing Antibodies in COVID-19 Patients and Vaccine Recipients after Two Doses of BNT162b2. Viruses 2021, 13, 1364. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.C.; Tiu, C.; Hu, Z.; Chen, V.C.-V.; Young, B.E.; Wan, R.S.; et al. A SARS-CoV-2 Surrogate Virus Neutralization Test Based on Antibody-Mediated Blockage of ACE2–Spike Protein–Protein Interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Dolscheid-Pommerich, R.; Bartok, E.; Renn, M.; Kümmerer, B.M.; Schulte, B.; Schmithausen, R.M.; Stoffel-Wagner, B.; Streeck, H.; Saschenbrecker, S.; Steinhagen, K.; et al. Correlation Between a Quantitative Anti-SARS-CoV-2 IgG ELISA and Neutralization Activity. J. Med. Virol. 2022, 94, 388–392. [Google Scholar] [CrossRef]
- Malipiero, G.; D’Agaro, P.; Segat, L.; Moratto, A.; Villalta, D. Long-Term Decay of Anti-RBD IgG Titers after BNT162b2 Vaccination is not Mirrored by Loss of Neutralizing Bioactivity Against SARS-CoV-2. Clin. Chim. Acta 2022, 524, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zhao, Q. Perspectives on Therapeutic Neutralizing Antibodies Against the Novel Coronavirus SARS-CoV-2. Int. J. Biol. Sci. 2020, 16, 1718–1723. [Google Scholar] [CrossRef] [PubMed]
- Lokida, D.; Karyana, M.; Kosasih, H.; Mardian, Y.; Sugiyono, R.I.; Arlinda, D.; Lukman, N.; Salim, G.; Butar, D.P.B.; Naysilla, A.M.; et al. Performance and correlation of ten commercial immunoassays for the detection of SARS-CoV-2 antibodies. Heliyon 2022, 8, e12614. [Google Scholar] [CrossRef]
- Bonelli, F.; Sarasini, A.; Zierold, C.; Calleri, M.; Bonetti, A.; Vismara, C.; Blocki, F.A.; Pallavicini, L.; Chinali, A.; Campisi, D.; et al. Clinical and Analytical Performance of An Automated Serological Test That Identifies S1/S2-Neutralizing IgG in COVID-19 Patients Semiquantitatively. J. Clin. Microbiol. 2020, 58, e01224-20. [Google Scholar] [CrossRef]
- Yin, Q.L.; Zhang, Y.C.; Lian, L.J.; Qu, Y.Y.; Wu, W.; Chen, Z.; Pei, R.J.; Chen, T.Y.; Sun, L.N.; Li, C.; et al. Chemiluminescence Immunoassay Based Serological Immunoassays for Detection of SARS-CoV-2 Neutralizing Antibodies in COVID-19 Convalescent Patients and Vaccinated Population. Viruses 2021, 13, 1508. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef]
- Mouna, L.; Razazian, M.; Duquesne, S.; Roque-Afonso, A.M.; Vauloup-Fellous, C. Validation of a SARS-CoV-2 Surrogate Virus Neutralization Test in Recovered and Vaccinated Healthcare Workers. Viruses 2023, 15, 426. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Imai, K.; Matsuoka, M.; Fukada, A.; Kubota, K.; Sato, M.; Takada, T.; Noguchi, S.; Tarumoto, N.; Maesakiet, S.; et al. Evaluation of the Correlation between the Access SARS-CoV-2 IgM and IgG II Antibody Tests with the SARS-CoV-2 Surrogate Virus Neutralization Test. J. Med. Virol. 2022, 94, 335–341. [Google Scholar] [CrossRef]
- Infantino, M.; Manfredi, M.; Stacchini, L.; Cosma, C.; Grossi, V.; Lari, B.; Russo, E.; Amedei, A.; Benucci, M.; Veneziani, F.; et al. The Role of Neutralizing Antibodies by sVNT after Two Doses of BNT162b2 mRNA Vaccine in a Cohort of Italian Healthcare Workers. Clin. Chem. Lab. Med. 2022, 60, 934–940. [Google Scholar] [CrossRef] [PubMed]
- González-Stegmaier, R.; Cereceda, K.; Briones, J.; Beltran-Pávez, C.; Oyarzún-Arrau, A.; Riquelme-Barrios, S.; Selman, C.; Yarad, F.; Mahave, M.; Caglevic, C.; et al. Seroconversion and Abundance of IgG Antibodies Against S1-RBD of SARS-CoV-2 and Neutralizing Activity in the Chilean population. J. Immunol. Res. 2021, 19, 6680337. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Du, L. SARS-CoV-2 spike protein: A key target for eliciting persistent neutralizing antibodies. Signal Transduct. Target. Ther. 2021, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.H.; Porrot, F.; Staropoli, I.; Père, H.; et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef]
- Qu, P.; Faraone, J.; Evans, J.P.; Zou, X.; Zheng, Y.M.; Carlin, C.; Bednash, J.S.; Lozanski, G.; Mallampalli, R.K.; Saif, L.J.; et al. Neutralization of the SARS-CoV-2 Omicron BA.4/5 and BA.2.12.1 Subvariants. N. Engl. J. Med. 2022, 386, 2526–2528. [Google Scholar] [CrossRef]
- Carreño, J.M.; Alshammary, H.; Tcheou, J.; Singh, G.; Raskin, A.J.; Kawabata, H.; Sominsky, L.A.; Clark, J.J.; Adelsberg, D.C.; Bielak, D.A.; et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature 2022, 602, 682–688. [Google Scholar] [CrossRef]
- Higdon, M.M.; Baidya, A.; Walter, K.K.; Patel, M.K.; Issa, H.; Espie, E.; Feikin, D.R.; Knoll, M.D. Duration of effectiveness of vaccination against COVID-19 caused by the omicron variant. Lancet Infect. Dis. 2022, 22, 1114–1116. [Google Scholar] [CrossRef]
- Michlmayr, D.; Hansen, C.H.; Gubbels, S.M.; Valentiner-Branth, P.; Bager, P.; Obel, N.; Drewes, B.; Møller, C.H.; Møller, F.T.; Legarth, R.; et al. Observed protection against SARS-CoV-2 reinfection following a primary infection: A Danish cohort study among unvaccinated using two years of nationwide PCR-test data. Lancet Reg. Health Eur. 2022, 20, 100452. [Google Scholar] [CrossRef]
- Global Initiative on Sharing All Influenza Data (GISAID); hCoV-19 Variants Dashboard. Available online: https://gisaid.org/hcov-19-variants-dashboard/ (accessed on 17 July 2024).

| ACE-RBD Titration Test | Vaccinated Group (n = 60) | Vaccinated + Previously Infected Group (n = 60) | ||
|---|---|---|---|---|
| SARS-CoV-2 IgG II Test | SARS-CoV-2 IgG II Test | |||
| 50–5000 AU/mL | >5000 AU/mL | 50–5000 AU/mL | >5000 AU/mL | |
| Low | 1 (1.7%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Medium | 3 (5%) | 0 (0%) | 1 (1.7%) | 0 (0%) |
| High | 0 (0%) | 56 (93.3%) | 0 (0%) | 59 (98.3%) |
| ACE-2 RBD Neutralization Assay | ||||||
|---|---|---|---|---|---|---|
| Screening | 1/2 Titration | 1/4 Titration | 1/32 Titration | |||
| Vaccinated | SARS-CoV-2 IgG II | r | 0.083 | −0.068 | −0.036 | 0.622 |
| p | 0.529 | 0.604 | 0.786 | <0.001 | ||
| Vaccinated + Previously Infected | SARS-CoV-2 IgG II | r | −0.131 | −0.223 | −0.105 | 0.529 |
| p | 0.319 | 0.087 | 0.423 | <0.001 | ||
| Correlation | ||||||
|---|---|---|---|---|---|---|
| ACE2-RBD Screening | 1/2 Titration | 1/4 Titration | 1/32 Titration | |||
| Male | SARS-CoV-2 IgG II | r | −0.092 | −0.101 | −0.028 | 0.529 |
| p | 0.489 | 0.447 | 0.833 | <0.001 | ||
| Female | SARS-CoV-2 IgG II | r | 0.007 | −0.215 | −0.117 | 0.610 |
| p | 0.957 | 0.097 | 0.370 | <0.001 | ||
| Correlation | ||||||
|---|---|---|---|---|---|---|
| ACE2-RBD Screening | 1/2 Titration | 1/4 Titration | 1/32 Titration | |||
| 18–40 Age | SARS-CoV-2 IgG II | r | 0.030 | −0.297 | −0.056 | 0.542 |
| p | 0.819 | 0.021 | 0.669 | <0.001 | ||
| 41–65 Age | SARS-CoV-2 IgG II | r | −0.120 | −0.028 | −0.073 | 0.566 |
| p | 0.363 | 0.835 | 0.578 | <0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cin, D.; Soguksu, P.; Oren, M.M.; Ozgulnar, N.; Agacfidan, A.; Mese, S. The Anti-SARS-CoV-2 S-Protein IgG, Which Is Detected Using the Chemiluminescence Microparticle Immunoassay (CMIA) in Individuals Having Either a History of COVID-19 Vaccination and/or SARS-CoV-2 Infection, Showed a High-Titer Neutralizing Effect. Viruses 2024, 16, 1409. https://doi.org/10.3390/v16091409
Cin D, Soguksu P, Oren MM, Ozgulnar N, Agacfidan A, Mese S. The Anti-SARS-CoV-2 S-Protein IgG, Which Is Detected Using the Chemiluminescence Microparticle Immunoassay (CMIA) in Individuals Having Either a History of COVID-19 Vaccination and/or SARS-CoV-2 Infection, Showed a High-Titer Neutralizing Effect. Viruses. 2024; 16(9):1409. https://doi.org/10.3390/v16091409
Chicago/Turabian StyleCin, Dilan, Pinar Soguksu, Meryem Merve Oren, Nuray Ozgulnar, Ali Agacfidan, and Sevim Mese. 2024. "The Anti-SARS-CoV-2 S-Protein IgG, Which Is Detected Using the Chemiluminescence Microparticle Immunoassay (CMIA) in Individuals Having Either a History of COVID-19 Vaccination and/or SARS-CoV-2 Infection, Showed a High-Titer Neutralizing Effect" Viruses 16, no. 9: 1409. https://doi.org/10.3390/v16091409
APA StyleCin, D., Soguksu, P., Oren, M. M., Ozgulnar, N., Agacfidan, A., & Mese, S. (2024). The Anti-SARS-CoV-2 S-Protein IgG, Which Is Detected Using the Chemiluminescence Microparticle Immunoassay (CMIA) in Individuals Having Either a History of COVID-19 Vaccination and/or SARS-CoV-2 Infection, Showed a High-Titer Neutralizing Effect. Viruses, 16(9), 1409. https://doi.org/10.3390/v16091409






