Abstract
Shrews (Soricidae) are common small wild mammals. Some species of shrews, such as Asian house shrews (Suncus murinus), have a significant overlap in their habitats with humans and domestic animals. Currently, over 190 species of viruses in 32 families, including Adenoviridae, Arenaviridae, Arteriviridae, Astroviridae, Anelloviridae, Bornaviridae, Caliciviridae, Chuviridae, Coronaviridae, Filoviridae, Flaviviridae, Hantaviridae, Hepadnaviridae, Hepeviridae, Nairoviridae, Nodaviridae, Orthoherpesviridae, Orthomyxoviridae, Paramyxoviridae, Parvoviridae, Phenuiviridae, Picobirnaviridae, Picornaviridae, Polyomaviridae, Poxviridae, Rhabdoviridae, Sedoreoviridae, Spinareoviridae, and three unclassified families, have been identified in shrews. Diverse shrew viruses, such as Borna disease virus 1, Langya virus, and severe fever with thrombocytopenia syndrome virus, cause diseases in humans and/or domestic animals, posing significant threats to public health and animal health. This review compiled fundamental information about shrews and provided a comprehensive summary of the viruses that have been detected in shrews, with the aim of facilitating a deep understanding of shrews and the diversity, epidemiology, and risks of their viruses.
1. Introduction of Shrews
Shrews, which are usually termed long-nosed mice, can be found in a variety of habitats, including forests, grasslands, deserts, and wetlands. Some species, such as American water shrews (Sorex palustris), are aquatic and can be found in or near water.
Asian house shrews (Suncus murinus) are the largest shrews, attaining a length of approximately 15 cm and a weight of around 100 g, while Etruscan shrews (Suncus etruscus) could represent the smallest extant terrestrial mammals, measuring a mere 3.5 cm in length and weighing approximately 1.8 g [1].
Shrews, hedgehogs, and moles share the same order, Eulipotyphla. They belong to the families Soricidae, Erinaceidae, and Talpidae, respectively. Eulipotyphla encompasses 593 species, second only to Rodentia (2398 species) and Chiroptera (1449 species) in mammals [2]. These three orders comprise about 9.5%, 38.5%, and 23.2% of all mammalian species, respectively.
Of the known 163 mammalian families, Soricidae encompasses 487 species, only less than Muridae (775 species), Cricetidae (705 species), and Vespertilionidae (520). The 488 species of Soricidae belong to three subfamilies and 28 genera [2]. The three subfamilies are Crocidurinae (white-toothed shrews), Soricinae (red-toothed shrews), and Myosoricinae (African shrews). These three subfamilies comprise 10 genera with 267 species, 15 genera with 195 species, and three genera with 25 species, respectively. The genus Crocidura is the largest genus in Crocidurinae, covering 222 species. The genus Sorex is the largest genus in Soricinae, covering 86 species [2].
Notably, tree shrews in the order Scandentia, otter shrews in the order Afrosoricida, elephant shrews in the order Macroscelidea, and marsupial shrews in the order Dasyuromorphia are not shrews, because they do not fall within the order Eulipotyphla [3]. West Indies shrews were in the order Eulipotyphla, but they were in the family Nesophontidae and have been extinct.
Shrews are distributed across major tropical and temperate landmasses on all continents except Australia and Antarctica (Table 1). Oceania is devoid of shrews, except on two islands of the Mariana Archipelago (Guam and Tinian), where Suncus murinus was introduced following World War II [4]. In South America, shrews are confined to the northern Andes region and were introduced during the Great American Interchange.

Table 1.
Geographic distribution and species number of 26 shrew genera.
Shrews have a lifespan of 12 to 30 months, and they exhibit a relatively high metabolic rate, higher than some mammals of similar body sizes. Consequently, shrews have a relatively large food intake. Shrews do not hibernate but can enter a torpid state. During the winter, many shrews undergo significant changes in morphology, with body weight decreasing by 30% to 50% and both skeletal and organ sizes noticeably shrinking [5]. Shrews are mostly found in cool and humid environments, with many being terrestrial, while some are semi-aquatic or burrowing. They may be active during both day and night or primarily nocturnal. Shrews are typically solitary creatures, coming together only for mating purposes.
Female shrews can give birth to as many as 10 litters each year. In tropical regions, they can reproduce year-round, while in temperate regions, they cease reproduction during the winter. The gestation period for shrews ranges from 17 to 32 days. Female shrews can become pregnant again within one to two days after giving birth and can lactate and nurse their offspring during pregnancy [6].
Certain shrew species, such as American or southern short-tailed shrews (Blarina carolinensis), northern short-tailed shrews (Blarina brevicauda), and long-tailed shrews or rock shrews (Sorex dispar) in North America, and Asiatic short-tailed shrews (Blarinella quadraticauda) in China, secrete venom [7], which contains various compounds. The venom of American short-tailed shrews can kill 200 mice when administered intravenously [8]. In addition, similar to bats and toothed whales, two genera, namely Sorex (long-tailed shrews) and Blarina (short-tailed shrews), possess echolocation abilities [9]. These two genera include Eurasian water shrews (Neomys fodiens), northern short-tailed shrews (Blarina brevicauda), American water shrews, and some others [10].
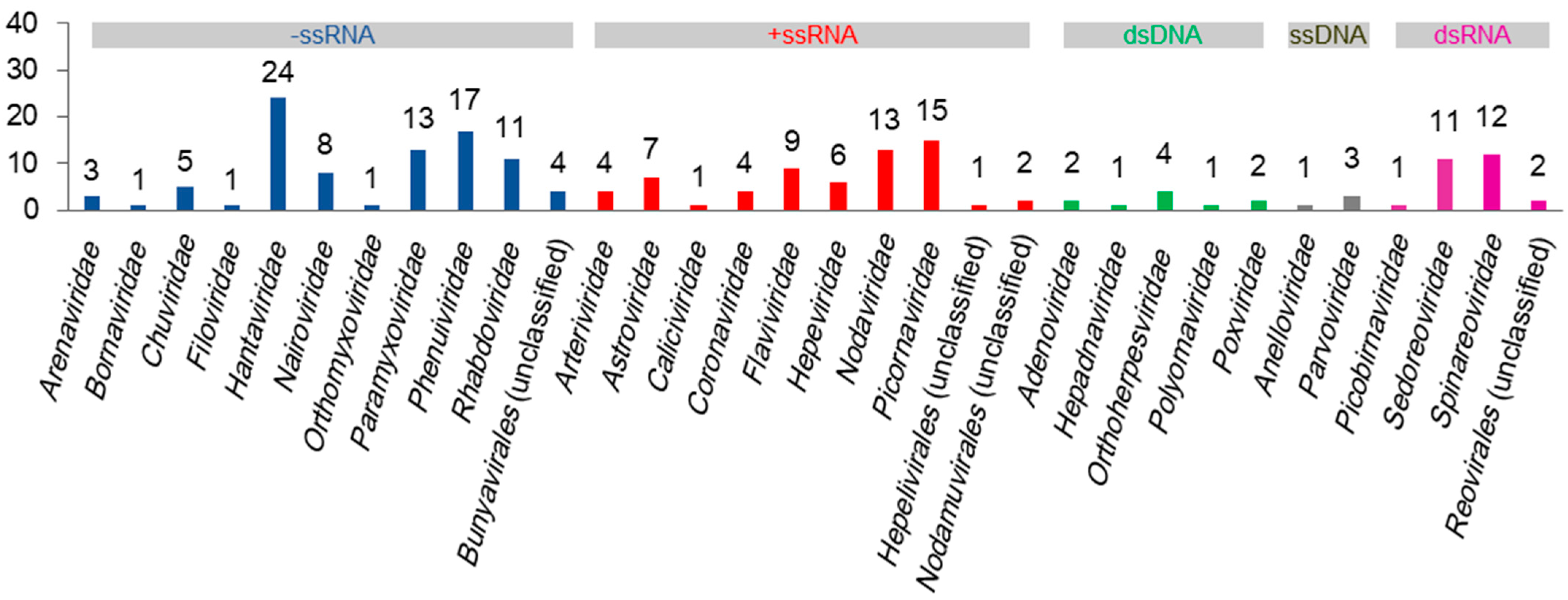
Diverse viruses in shrews have been identified, and many viruses in shrews have yet to be identified [11]. Currently, as detailed below and summarized in Figure 1, at least 190 species of viruses in 32 families have been identified in shrews, excluding plant viruses or invertebrate viruses that might be detected in shrews due to their existence in the food or surroundings of shrews. These viruses can be classified into negative-sense single-stranded RNA (−ssRNA), positive-sense single-stranded RNA (+ssRNA), double-stranded DNA (dsDNA), single-stranded DNA (ssDNA), and double-stranded RNA (dsRNA) viruses (Figure 1). The relevant sequences of these viruses are provided in the supplementary file (Table S1).
Figure 1.
The numbers of virus species in 32 virus families identified in shrews.
2. −ssRNA Viruses Detected in Shrews
Table 2 shows that 88 species of −ssRNA viruses in shrews have been detected.

Table 2.
Detection of −ssRNA viruses in shrews using high-throughput sequencing (HTS), PCR, RT-PCR, serological methods, and/or virus isolation.
2.1. Arenavirid Viruses Detected in Shrews
The family Arenaviridae currently comprises five genera and 69 species, which infect mammals, reptiles, and fish [51,52]. Their genomes consist of two or three single-stranded RNA molecules, some of which encode two proteins in non-overlapping ORFs of opposite polarities. Some viruses in this family, such as lymphocytic choriomeningitis virus and Lassa virus, can cause diseases in humans.
Three species in the genus Mammarenavirus (M. wenzhouense, M. choriomeningitidis, and an unclassified species) of this family were identified in shrews. M. wenzhouense and an unclassified species of Suncus murinus in China were reported in recent years [12,13,15], and M. choriomeningitidis in goliath shrews (Crocidura goliath) in Gabon was reported in 2021 [14]. Of them, M. wenzhouense (Wēnzhōu virus) can infect humans, causing influenza-like symptoms [12,13]. M. choriomeningitidis (lymphocytic choriomeningitis virus) can infect humans and primates, causing infection in the central nervous system. This virus can also cause other illnesses, such as conjunctivitis, hepatitis, pneumonia, meningitis, and sepsis [51,52]. Shrews could be accidental hosts or a minor reservoir of these two virus species, which mainly circulate in rodents [51,52].
2.2. Bornavirid Viruses Detected in Shrews
The family Bornaviridae currently comprises the genera Carbovirus, Cultervirus, and Orthobornavirus, which infect mammals, birds, reptiles, and fish [53]. Several viruses in this family, such as Orthobornavirus bornaense (Borna disease virus 1, BDV-1) and variegated squirrel bornavirus 1, can cause diseases in humans or domestic animals.
BDV-1 in the genus Orthobornavirus of this family causes severe T-cell-mediated meningoencephalitis in horses, sheep, and other animals, especially in central Europe. Recent studies have identified white-toothed shrews (Crocidura leucodon) as the natural host and reservoir of BDV-1 [54,55]. This virus was detected in white-toothed shrews (Crocidura russula) in Germany in recent years (Table 2). BDV-1 can persist in the shrews for long periods without noticeable clinical signs of illness and can be shed through shrew saliva, urine, skin swabs, tears, and feces.
2.3. Chuvirid Viruses Detected in Shrews
The family Chuviridae currently comprises 16 genera and 43 species, which infect arachnids, barnacles, crustaceans, insects, fish, and reptiles in Africa, Asia, Australia, Europe, North America, and South America [56].
Five unclassified species of Chuviridae with unknown pathogenicity were identified in Asian grey shrews (Crocidura attenuata), Smith’s shrews (Chodsigoa smithii), and Ussuri white-toothed shrews (Crocidura lasiura) in China in recent years [11,17].
2.4. Filovirid Viruses Detected in Shrews
The family Filoviridae currently comprises nine genera and 16 species, which infect mammals, reptiles, and fish [57]. Several viruses in this family, such as Ebola virus and Marburg virus, can cause severe diseases in humans and animals.
Ebola virus in the genus Orthoebolavirus of this family is highly pathogenic to humans and has been detected in greater forest shrews (Sylvisorex ollula) in the Central African Republic, but shrews could be intermediate or incidental hosts of this virus [58].
2.5. Hantavirid Viruses Detected in Shrews
The family Hantaviridae currently comprises four subfamilies, eight genera, and 53 species, which infect mammals, birds, reptiles, and fish [59]. Multiple viruses in this family, such as Hantaan virus and Seoul virus, can cause diseases in humans.
One species in the genus Mobatvirus of this family, M. lenaense (Lena virus), was discovered in Laxmann’s shrews (Sorex caecutiens) and flat-skulled shrews (Sorex roboratus) captured between 2018 and 2019 in Russia [19].
Diverse species in the genus Orthohantavirus of this family, such as O. artybashense, O. asikkalaense, O. boweense, O. caobangense, O. jejuense, O. kenkemeense, O. seewisense, O. seoulense, and several unclassified species, were detected in multiple countries (Table 2). Of them, O. seoulense (Seoul virus) is highly pathogenic to humans with a reservoir of rodents. This virus was discovered in Crocidura lasiura captured in 2014 in China [26]. It remains unknown whether shrews are the natural hosts or a reservoir of the virus.
In the genus Orthohantavirus, O. seewisense (Seewis virus) was first detected in Eurasian common shrews (Sorex araneus) captured in 2006 in Switzerland [20]. This virus is widely distributed throughout the geographic ranges of its soricid hosts, including Sorex araneus, tundra shrews (Sorex tundrensis), Siberian large-toothed shrews (Sorex daphaenodon), and Mediterranean water shrews (Neomys anomalus). In addition, some genetic variants of this virus, termed Artybash virus and Amga virus, were detected in Sorex caecutiens [27]. O. caobangense (Cao Bằng virus) was first detected in the lung tissue of Chinese mole shrews (Anourosorex squamipes) captured in 2006 in Vietnam [60]. O. asikkalaense (Asikkala virus) was first discovered in Eurasian pygmy shrews (Sorex minutus) captured in Finland [21]. Subsequently, this virus was identified in shrews in the Czech Republic and Germany. O. boweense (Bowé virus) was discovered in the muscle tissue of Doucet’s musk shrews (Crocidura douceti) captured in 2012 in southwestern Guinea [22]. O. jejuense (Jeju virus) was first discovered in Asian lesser white-toothed shrews (Crocidura shantungensis) captured between 2007 and 2010 in South Korea [24]. O. kenkemeense (Kenkeme virus) was first discovered in Sorex roboratus captured in 2006 in Russia [25].
In the genus Orthohantavirus, the unclassified Altai virus was first detected in the tissue of Sorex araneus in the Altai Republic of Russia in 2007 [29]. The unclassified Ash River virus was discovered in masked shrews (Sorex cinereus) captured in 1983 and 1994 in Minnesota. The unclassified Jemez Springs virus was discovered in Sorex monticolus captured in 1994 in Colorado and between 1996 and 2000 in New Mexico [31]. The unclassified Artybash virus was first detected in the lung of Sorex caecutiens captured between 2006 and 2014 in eastern Siberia, Russia, and Hokkaido, Japan [20]. The unclassified Azagny virus was first discovered in West African pygmy shrews (Crocidura obscurior) captured in 2009 in Côte d’Ivoire [34]. The unclassified Boginia virus was first reported in 2013. It was discovered in Neomys fodiens captured between 2010 and 2012 in central Poland [33]. The unclassified Camp Ripley virus is the first hantavirus discovered in Blarina brevicauda captured in Minnesota in 1998 [32]. The unclassified Qiān Hú Shān virus was discovered in the lung tissue of stripe-backed shrews (Sorex cylindricauda) captured in 2005 in China [35]. The unclassified Xinyi virus was first discovered in Taiwanese mole shrews (Anourosorex yamashinai) captured in 1985 in Taiwan, China [30]. Phylogenetic analysis suggested that the Xinyi virus shares a common ancestor with the Cao Bằng virus. The unclassified Yákèshí virus was discovered in long-clawed shrews (Sorex unguiculatus) captured in 2006 in Yákèshí, Inner Mongolia. The unclassified Liánghé virus was found in Anourosorex squamipes captured between 2010 and 2011 in China [23].
Four species in the genus Thottimvirus of this family, such as T. imjinense, T. thottapalayamense, and two unclassified species, were detected in shrews (Table 2). Of them, T. thottapalayamense (Thottapalayam virus) was first isolated in 1964 in Suncus murinus in India. This virus represents the earliest isolation of a hantavirus in shrews [37]. T. imjinense (Imjin virus) was first discovered in Crocidura lasiura captured between 2004 and 2005 near the DMZ along the Imjin River in South Korea [36]. The unclassified Kilimanjaro virus and Uluguru virus in Thottimvirus were first discovered in Geata mouse shrews (Myosorex geata) and Kilimanjaro mouse shrews (Myosorex zinki) captured between 1995 and 2002 in Tanzania [39].
Except for Seoul virus, all the above hantanviruses were only found in shrews with unknown pathogenicity.
2.6. Nairovirid Viruses Detected in Shrews
The family Nairoviridae currently comprises seven genera and 51 species, which infect arthropods, birds, and mammals [61]. Several viruses in this family, such as the Crimean-Congo hemorrhagic fever virus and Nairobi sheep disease virus, can cause diseases in humans and/or domestic animals [61].
Six species in the genus Orthonairovirus of this family, namely O. erveense, O. lambarenense, O. thiaforaense, and three unclassified species, were detected in Crocidura spp. in multiple countries (Table 2). Of them, O. erveense (Erve virus) was first detected in 1982 from multiple organs of Crocidura russula trapped in Western France. Serological surveys indicated that Erve virus had a large apparent geographical distribution in France and infects rodents, insectivores, wild boars, red deer, sheep, herring gulls, and humans, leading to neurological symptoms in humans [40]. O. lambarenense (Lamusara virus) and the unclassified Lamgora virus in Orthonairovirus were discovered in Crocidura spp. captured between 2019 and 2020 in Gabon [41]. O. thiaforaense (Thiafora virus) was first discovered in Crocidura sp. captured in 1971 in Senegal [42].
Additionally, the unclassified Cencurut virus in Orthonairovirus was first discovered in Suncus murinus captured between 2012 and 2014 in Singapore [43]. An unclassified species of Orthonairovirus and an unclassified species of the genus Xinspiovirus of this family were detected in the lungs of Taiwanese gray shrews (Crocidura tanakae) [17]. These orthonairoviruses have been found only in shrews with unknown pathogenicity.
2.7. Othomyxovid Viruses Detected in Shrews
The family Orthomyxoviridae currently comprises nine genera and 21 species, which infect mammals and birds [62]. Several viruses in this family, such as influenza A virus and influenza B virus, can cause diseases in humans and/or domestic animals.
H5N6 subtype highly pathogenic avian influenza in the genus Alphainfluenzavirus of this family was detected in the lungs of Anourosorex squamipes [17]. This virus is highly pathogenic to chickens, mainly circulating in wild and domestic birds. It has sporadically infected some humans [63]. The shrew was likely accidentally infected with the avian virus.
2.8. Paramyxovid Viruses Detected in Shrews
The family Paramyxoviridae currently comprises four subfamilies, 17 genera, and 78 species [64]. Numerous viruses in this family, such as measles virus and canine distemper virus, cause diseases in humans or domestic animals.
Gamak virus and Daeryong virus in the genus Henipavirus were first reported in South Korea in recent years. They were both discovered in Crocidura lasiura and Crocidura shantungensis. These two viruses were exclusively detected in shrews. Gamak virus can infect and replicate in human hypotriploid alveolar basal epithelial cell lines and elicit the production of type I/III interferon, interferon-stimulated genes, and proinflammatory cytokines [44].
Twelve unclassified species in the genus Henipavirus of this family have been identified. Among them, Langya virus was first reported in China in 2022 with the reservoir hosts of shrews. The virus was detected in Crocidura lasiura and Crocidura shantungensis. The virus is pathogenic to humans, and 35 acute Langya virus cases manifested influenza-like symptoms. Previous research encompassed 25 wild animal species and indicated a high virus infection rate among the shrews (71/262, 27%) [45].
In Henipavirus, Melian virus was first detected in large-headed shrews (Crocidura grandiceps) captured in 2018 in Guinea [46]. Denwin virus was first detected in Crocidura russula captured in 2019 in Belgium [46]. Ninorex virus was first detected in Sorex minutus captured in 2020 in Belgium [47]. Phylogenetic analysis of these viruses and some other relevant paramyxoviruses suggested that both genera could be divided into two clades, one covering bat-borne viruses and the other covering rodent- and shrew-borne viruses [47].
Beilong virus in the genus Jeilongvirus of this family has been detected in rodents and shrews. A study in China showed a positivity rate of Beilong virus 28.57% (2/7) in the Asian lesser white-toothed shrews (Crocidura shantungensis) and 17.57% (13/74) in the Suncus murinus [48]. Currently, there is no evidence of human infection with Beilong virus, but its potential risk to humans and livestock should not be underestimated [65].
2.9. Phenuivirid Viruses Detected in Shrews
The family Phenuiviridae currently comprises 23 genera and 159 species, which infect vertebrates, including mammals and birds, invertebrates, plants, and fungi [66]. Some viruses in this family, such as sand-fly fever Naples virus and Chikungunya virus, can cause diseases in humans and/or domestic animals. Phenuivirid genomes consist of two or three single-stranded RNA molecules, some of which encode two proteins in non-overlapping ORFs of opposite polarities.
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging hemorrhagic fever caused by SFTS virus (SFTSV), a species (Dabie Bandavirus) in the genus Bandavirus in Phenuiviridae [49]. SFTS has been reported in humans in China, South Korea, and Japan since 2010 [67]. The main clinical manifestations of SFTS include acute fever, thrombocytopenia, leukopenia, and gastrointestinal and neurological symptoms [6,7,8]. Moreover, multiple organ failure may occur in severe cases, with a maximum mortality rate of 30%. A survey suggested that shrews are more likely than other wild animals to be the reservoir of SFTSV because the prevalence of SFTSV in Suncus murinus is significantly higher than in other animals [49].
Diverse unclassified species in Phenuiviridae have been identified in Anourosorex squamipes, Crocidura attenuata, and Chodsigoa smithii in China in recent years [11]. It remains unknown whether these viruses were shrew viruses or the viruses of plants or fungi in shrew food.
2.10. Rhabdovirid Viruses Detected in Shrews
The family Rhabdoviridae currently comprises 56 genera that are assigned to four subfamilies and 434 species, which infect vertebrates, invertebrates, and plants [68]. A few rhabdoviruses, such as rabies virus and vesicular stomatitis virus, can cause diseases in humans and domestic animals.
Members of the genus Lyssavirus of this family infect a variety of mammals causing rabies-like diseases [69]. The species L. mokola was detected in Nigeria in 1968 from pooled lung, liver, spleen, kidney, and heart of shrews (Crocidura sp.) [69]. The species L. rabies (rabies virus) was identified in the lungs of Suncus murinus in China using HTS [17]. This finding is of public health significance, as it suggests that shrews could be involved in the ecology and epidemiology of rabies virus.
An unclassified species in the genus Tupavirus and eight unclassified species in unclassified genera of Rhabdoviridae were identified in shrews, such as Chodsigoa smithii, Anourosorex squamipes, Crocidura attenuata, Blarinella griselda (Indochinese short-tailed shrews), and Anourosorex squamipes, in China [11,17]. The pathogenicity of these shrew viruses remains unknown.
3. +ssRNA Viruses Detected in Shrews
Table 3 shows that 62 species of +ssRNA viruses in shrews have been detected.

Table 3.
Detection of +ssRNA viruses in shrews using high-throughput sequencing (HTS), PCR, RT-PCR, serological methods, and/or virus isolation.
3.1. Arterivirid Viruses Detected in Shrews
The family Arteriviridae currently comprises six subfamilies, 13 genera, and 23 species, which infect vertebrates, such as pigs, horses, and non-human primates [84]. Several significant veterinary pathogens, such as equine arteritis virus and porcine respiratory and reproduction syndrome virus 2, can cause severe diseases in domestic animals and/or non-human primates.
The genus Muarterivirus of Arteriviridae contains a shrew arterivirus, Olivier’s shrew virus 1, which was first detected in Olivier’s shrews (Crocidura olivieri guineensis) in 2016 in Guinea [70]. Additionally, six unclassified species of shrew arteriviruses were detected in Crocidura shantungensis and Anourosorex squamipes in China in recent years [11,17].
3.2. Astrovirid Viruses Detected in Shrews
The family Astroviridae currently comprises two genera and 22 species, which infect mammals (19 species) and birds (3 species). Some astroviruses, such as human astrovirus and duck astrovirus, can cause diseases in humans or domestic animals [85]. Infection with astroviruses often involves damage to livers, kidneys, or immune system. Duck astrovirus causes highly fatal hepatitis in ducklings [86].
Three unclassified species in the genus Bastrovius, three unclassified species in the genus Mamastrovirus, and one species in an unclassified genus of Astroviridae were detected in shrews, such as Anourosorex squamipes, Crociduraattenuata, Chodsigoa smithii, Episoriculus leucops (long-tailed brown-toothed shrews), and Suncus murinus in China in recent years.
3.3. Calicivirid Viruses Detected in Shrews
The family Caliciviridae currently comprises 11 genera, each including one or two species, which infect mammals, birds, or fish [87]. Some viruses in this family, such as norovirus and feline calicivirus, can cause diseases in humans or domestic animals.
Rabbit hemorrhagic disease virus in the genus Lagovirus of this family is highly pathogenic to rabbits. A survey identified this virus in a dead Mediterranean pine vole and two white-toothed shrews [72], which suggested that shrews could be victims of this virus.
3.4. Coronavirid Viruses Detected in Shrews
The family Coronaviridae currently comprises four subfamilies, six genera, and 54 species, which infect vertebrates (mammals, birds, amphibians, and fish) [88]. Some coronaviruses, such as severe acute respiratory syndrome-related coronavirus, infectious bronchitis virus, and porcine epidemic diarrhea virus, can cause diseases in humans or domestic animals.
Three unclassified shrew coronaviruses in the genus Alphacoronavirus of this family were detected in Sorex araneus and Suncus murinus captured in recent years in China [11,75]. Another unclassified shrew coronavirus in the genus Alphacoronavirus was detected in Sorex araneus captured in the United Kingdom between 2008 and 2015 [73]. These shrew viruses have been found only in shrews with unknown pathogenicity.
3.5. Flavivirid Viruses Detected in Shrews
The family Flaviviridae currently comprises four genera and 97 species, which infect various mammals [89,90]. Most members of the genus Orthoflavivirus are arthropod-borne. Some flaviviruses, such as yellow fever virus, Dengue virus, Zika virus, human hepatitis C virus, Japanese encephalitis virus, West Nile virus, tick-borne encephalitis virus, and Tambusu virus, can cause diseases in humans or domestic animals.
Tick-borne encephalitis virus (TBEV) in the genus Orthoflavivirus of this family can infect humans and cause meningitis, encephalitis, or meningoencephalitis. It circulates between ticks and small mammals. Hedgehogs, shrews, moles, and certain rodents are hosts of ticks and reservoirs of this virus. This virus was detected in 1967 in Sorex araneus in Slovakia [76].
Usutu virus in Orthoflavivirus is a Culex-associated mosquito-borne flavivirus that was also found in Crocidura sp. captured between 2012 and 2013 in Senegal. This virus was initially discovered in 1959 and is associated with mosquitoes as its vectors. Currently, the virus has been isolated from birds, arthropods, and humans in Europe and Africa, and subsequently detected in bats and horses [79].
Powassan virus 2 in Orthoflavivirus can lead to encephalitis in humans and is primarily transmitted by arthropod vectors. This virus has been reported not only in humans and other animals but also detected in Blarina brevicauda captured between 2018 and 2020 in Massachusetts and Rhode Island in the United States. Early studies suggested that groundhog ticks and squirrel ticks naturally maintain this virus with woodchucks and other medium-sized mammals, such as skunks and raccoons, or squirrels. These animals likely serve as the reservoir of the virus [78].
Shrews could be minor reservoirs or accidental hosts of the aforementioned TBEV, Usutu virus, and Powassan virus.
Rat pegivirus in Pegivirus was initially identified in the sera of desert woodrats in 2013, which was also discovered in Suncus murinus captured between 2013 and 2015 in China. This virus primarily targets lymphocytes and causes asymptomatic infections in humans and other animals [80].
An unclassified shrew flavivirus in the genus Pegivirus, three unclassified shrew flaviviruses in the genus Hepacivirus, and an unclassified shrew flavivirus in the genus Pestivirus were detected in Suncus murinus, Chodsigoa smithii, or Crocidura attenuata in China in recent years through HTS [11,80,91].
3.6. Hepevirid Viruses Detected in Shrews
The family Hepeviridae currently comprises six genera and 39 species, five genera and ten species, which infect mammals, birds, and fish [92]. Some viruses in this family, such as human hepatitis E virus and rabbit hepatitis E virus, can cause diseases in humans and/or domestic animals.
Three unclassified shrew hepevirus species were detected in Crocidura attenuata, Chodsigoa smithii, and Crocidura attenuata in China in recent years through HTS [11,17]. An unclassified shrew hepevirus species was detected in Crocidura russula in Germany in recent years through HTS as suggested by a sequence reported to GenBank.
3.7. Nodavirid Viruses Detected in Shrews
The family Nodaviridae currently comprises two genera, each including four or more species, which infect insects (Alphanodavirus) or fish (Betanodavirus) [93]. Some viruses in this family, such as the striped jack nervous necrosis virus, can cause diseases in animals.
Multiple unclassified species in this family with unknown pathogenicity were detected in Anourosorex squamipes, Chodsigoa smithii, Crocidura attenuata, and Crocidura shantungensis in China in recent years through HTS [11].
3.8. Picornavirid Viruses Detected in Shrews
The family Picornaviridae currently comprises 63 genera and 147 species which infect vertebrates (at least six of the seven classes) [94]. Numerous viruses in this family, such as poliovirus and foot-and-mouth disease virus, can cause diseases in humans or domestic animals.
An unclassified species in the genus of Dicipivirus of this family was detected in the lungs of Crocidura lasiura in China using HTS [17].
Hepatitis A virus (HAV) in the genus Hepatovirus of Picornaviridae is an ancient and ubiquitous human pathogen recovered previously only in primates. Human HAV could originate from the virus in rodents [82]. Although HAV-like viruses have been identified in Sorex araneus in Germany and in Suncus murinus in China, shrew HAVs are distinct from human HAV, and its pathogenicity to humans remains unknown [11,82]. Patterns of shrew HAV infection in shrews mimicked those of human HAV in hepatotropism, fecal shedding, acute nature, and extinction of the virus in a closed host population [74].
One unclassified species in the genus Parechovirus of Picornaviridae was detected in Crocidura leucodon and Sorex antinorii (Valais shrews) in Italy using RT-PCR [83]. Two unclassified species in the genus Mischivirus, one unclassified species in the genus Parobovirus, and eight species of unclassified genera in Picornaviridae from Anourosorex squamipes, Blarinella griselda, Chodsigoa smithii, Crocidura attenuata, Crocidura lasiura, Sorex caecutiens, and Suncus murinus in China were detected using the HTS [11,17]. The pathogenicity of these shrew viruses remains unknown.
4. dsDNA Viruses Detected in Shrews
Table 4 shows that seven species of dsDNA viruses in shrews have been detected.

Table 4.
Detection of dsDNA viruses in shrews using high-throughput sequencing (HTS), PCR, RT-PCR, serological methods, and/or virus isolation.
4.1. Adenovirid Viruses Detected in Shrews
The family Adenoviridae currently comprises six genera and 109 species, which infect mammals, birds, reptiles, amphibians, and fish [100]. Some adenoviruses, such as human adenovirus 1 and canine adenovirus 1, can cause diseases in humans or domestic animals.
Two unclassified species with unknown pathogenicity in the genus Mastadenovirus of this family were detected in Suncus murinus in China and Sylvisorex sp. in Cameron, respectively, using PCR [95,96].
4.2. Hepadnavirid Viruses Detected in Shrews
The family Hepadnaviridae currently comprises five genera and 18 species, which infect mammals, birds, and fish [87]. Some viruses in this family, such as human hepatitis B virus and duck hepatitis B virus, can cause diseases in humans or domestic animals.
Shrew hepatitis B virus with unknown pathogenicity in the genus Orthoepadnavirus of this family has been detected in Anourosorex squamipes, Crocidura attenuata, and Crocidura lasiura, but not in Suncus murinus, captured in China in recent years [97]. Phylogenetic analysis revealed that shrew HBVs were closely related to a bat hepatitis B virus with a similar genome structure.
4.3. Orthoherpesvirid Viruses Detected in Shrews
The family Orthoherpesviridae currently comprises 17 genera and 118 species, which infect mammals, birds, and reptiles [101]. Some viruses in this family, such as Kaposi’s sarcoma-associated herpesvirus and pseudorabies virus, can cause diseases in humans or domestic animals.
Four shrew species with unknown pathogenicity in an unclassified genus of this subfamily were detected in Crocidura spp. in Cameroon and Congo [74].
4.4. Polyomavirid Viruses Detected in Shrews
The family Polyomaviridae currently comprises six genera and 112 species, which infect mammals, birds, and fish [102]. Some viruses in this family, such as JC virus and SV40, can cause diseases in humans or domestic animals.
An unclassified polyomavirus with unknown pathogenicity in Sorex araneus and Sorex coronatus (Millet’s shrews) was detected in Germany and Norway through PCR [98].
4.5. Poxvirid Viruses Detected in Shrews
The family Poxviridae currently comprises 22 genera and 83 species, which infect vertebrates and arthropods [103]. Some viruses in this family, such as variola virus and chicken poxvirus, can cause diseases in humans and/or domestic animals.
Monkeypox virus in the genus Orthopoxvirus of this family is zoonotic and has caused human infections in numerous countries in recent years. A survey in Zambia suggested that 14 of 42 shrews had antibodies against this virus [99]. However, the role of shrews in the epidemiology of this virus remains enigmatic. An unclassified species in this genus was detected in lung samples of Sorex araneus in Norway using PCR [54].
5. ssDNA Viruses Detected in Shrews
Table 5 shows that four species of ssDNA viruses in shrews have been detected.

Table 5.
Detection of ssDNA viruses in shrews using high-throughput sequencing (HTS), PCR, RT-PCR, serological methods, and/or virus isolation.
5.1. Anellovirid Viruses Detected in Shrews
The family Anelloviridae currently comprises 34 genera and 173 species, which infect mammals, birds, and reptiles [108]. Some viruses in this family, such as Torque teno virus and Torque teno mini virus, are prevalent in humans and/or domestic animals. They could be associated with liver or respiratory diseases, hematological disorders, cancer, and immune suppression.
The species Rhotorquevirus murid 1 (Torque teno rodent virus) was detected in Suncus murinus captured in China in recent years through PCR [104].
5.2. Parvovirid Viruses Detected in Shrews
The family Parvoviridae currently comprises two subfamilies (Parvovirinae and Densovirinae), 13 genera, and > 75 species. Viruses in Parvovirinae infect vertebrates, including mammals, birds, and reptiles. Viruses in Densovirinae infect insects, crustacea, and echinoderms [109]. Some parvoviruses, such as parvovirus B19 and canine parvovirus, can cause diseases in humans or domestic animals.
The species Rodent protoparvovirus 1 (Mpulungu bufavirus) in the genus Protoparvovirus of this family was detected in lesser red musk shrews (Crocidura hirta) captured between 2011 and 2013 in Zambia using HTS and PCR [105].
A new genotype of porcine bocavirus in the genus Bocaparvovirus of Parvoviridae can cause respiratory and gastrointestinal diseases in pigs. This virus was detected in Suncus murinus captured between 2015 and 2017 in China, but shrews are unlikely its reservoir [106].
Adeno-associated virus in the genus Dependoparvovirus of Parvoviridae can infect humans and multiple domestic and wild animals, including pigs, cows, goats, chickens, snakes, bats, and rodents. It was detected in Suncus murinus captured between 2015 and 2017 in China [107].
6. dsRNA Viruses Detected in Shrews
Table 6 shows that 26 species of dsRNA viruses in shrews have been detected.

Table 6.
Detection of dsRNA viruses in shrews using high-throughput sequencing (HTS), PCR, RT-PCR, serological methods, and/or virus isolation.
6.1. Picobirnavirid Viruses Detected in Shrews
The family Picobirnaviridae currently comprises one genus and three species, which infect vertebrates and invertebrates [112]. Some picobirnaviruses are widely distributed geographically among humans and other mammals. They have been mainly identified in fecal specimens and in raw sewage samples. The pathogenicity of picobirnaviruses has not been established. An unclassified picobirnavirus was identified in lungs of Crocidura lasiura, and Crocidura shantungensis in China using HTS [17].
6.2. Sedoreovirid Viruses Detected in Shrews
The family Sedoreoviridae currently comprises six genera and 39 species, which infect mammals, birds, crustaceans, arthropods, algae, and plants [113]. Some viruses in this family, such as rotavirus A, bluetongue virus, and African horse sickness virus, can cause diseases in humans or domestic animals.
Bluetongue virus in the genus Orbivirus of this family primarily infects sheep and various ruminant animals, with a high fatality rate in sheep. This virus can also infect rodents and carnivores and can be transmitted by certain mosquitoes. This virus was found in shrews in Africa in the 1990s [110]. The role of shrews in the epizootiology of bluetongue needs elucidation.
Rotavirus A in the genus Rotavirus of this family can infect humans and diverse domestic or wild animals. This virus and five unclassified species in this genus in Anourosorex squamipes, Blarinella griselda, Chodsigoa smithii, Crocidura shantungensis, Crocidura attenuata, and Suncus murinus captured in China in recent years were detected through HTS [11,111]. Additionally, three unclassified species of shrew sedoreoviruses in Chodsigoa smithii and Crocidura attenuate captured in China, which were distinct from known genera of this family, were detected in recent years through HTS [11,111].
6.3. Spinareovirid Viruses Detected in Shrews
The family Spinareoviridae currently comprises nine genera and 58 species, which infect mammals, aquatic animals (fish, mammals, crustaceans, and molluscs), birds, reptiles, arthropods, fungi, and plants [114]. Some spinareoviruses, such as human orthoreovirus and turkey orthoreovirus, can cause diseases in humans and domestic animals.
Two unclassified species in the genus Cypovirus, two unclassified species in the genus Orthoreovirus, and eight unclassified species distinct from known genera of this family were detected in Chodsigoa smithii, Crocidura attenuata, and Suncus murinus captured in China in recent years through HTS [11].
7. Discussion and Outlook
This review shows that shrew populations host at least 32 families and 190 species of viruses. Two recent publications regarding the large-scale detection of shrew viruses in China greatly expanded the knowledge about shrew virome or shrew viral diversity [11,17].
Dozens of other species of viruses have also been identified in shrews, but they were plant viruses or invertebrate viruses, and hence have not been described in this review [11,17]. They were detected in shrew samples, possibly due to their existence in shrew food or shrew surroundings.
Shrews have been identified with compelling evidence as the reservoirs of the zoonotic Langya virus, TBEV, SFTSV, and BDV-1. Shrews could be intermediate or incidental hosts or victims of Ebola virus, bluetongue virus, bufavirus, Usutu virus, tick-borne encephalitis virus, monkeypox virus, avian influenza virus, and RHDV, which are pathogenic to humans or domestic animals. Nevertheless, these infections have demonstrated that shrews are significant in the epidemiology and control of zoonosis and pose considerable threats to public health and animal health because shrews share overlapping habitats with humans and livestock. This highlights the surveys of shrew viruses for public health and animal welfare.
Dozens of virus species, such as Thottapalayam virus, Seewis virus, Thiafora virus, Wencheng shrew virus, Suncus murinus hepacivirus, and Olivier’s shrew virus 1, have been identified only in shrews. This suggests that shrews are likely the reservoirs of these species of viruses.
At least 24 species of hantaviruses, 13 species of paramyxoviruses, 17 species of phenuiviruses, 11 species of phabdoviruses, 9 species of flaviviruses, 13 species of nodaviruses, 13 species of nairoviruses, 11 species of sedoreoviruses, and 12 species of spinareoviruses in shrews have been identified (Figure 1). This suggests that shrews are likely the reservoirs of some species of these virus families, as it is unlikely that multiple species of the same virus family all occasionally infect shrews.
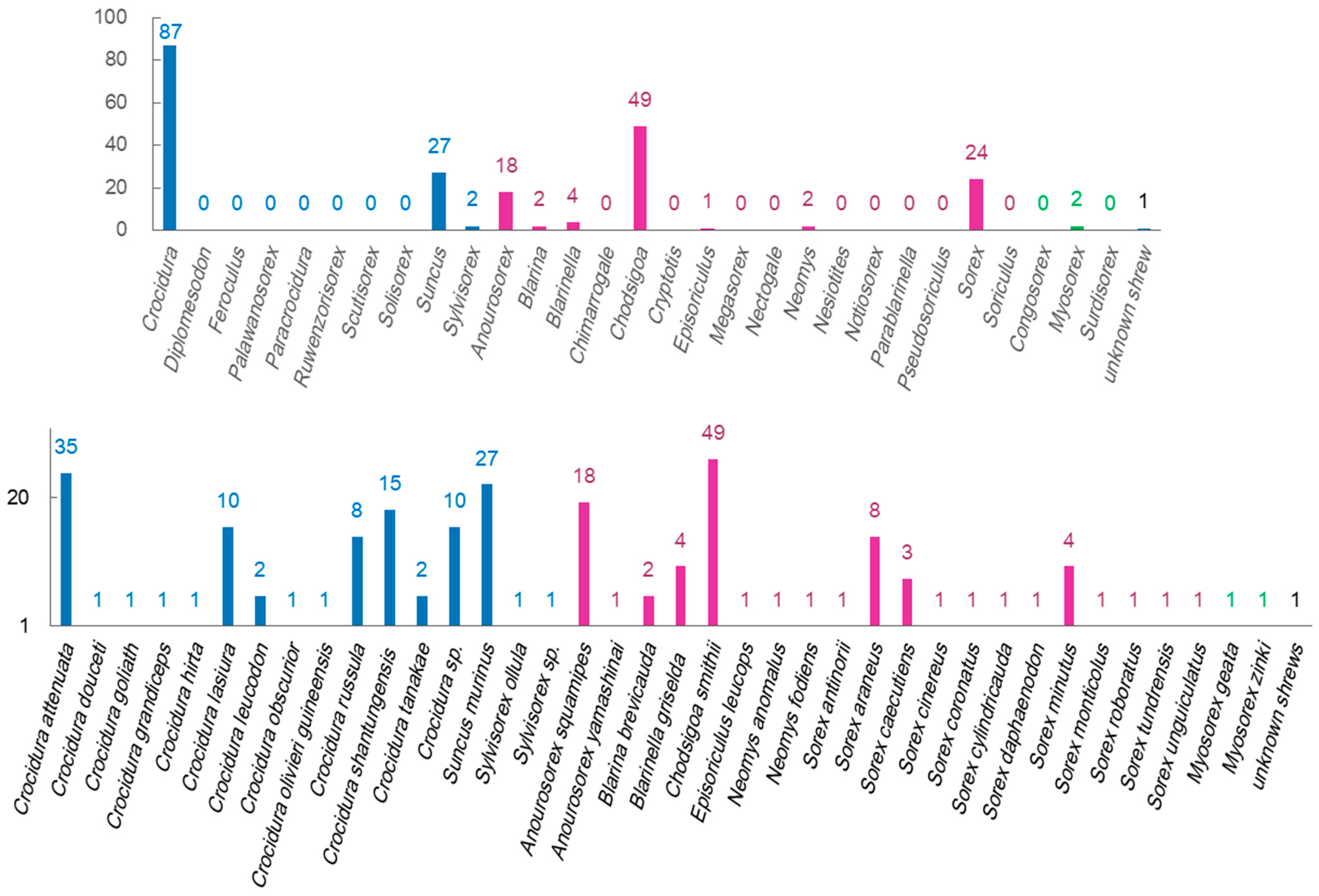
The counts of identified virus species were significantly different among shrew genera and shrew species (Figure 2). The shrew genus Crocidura hosts more shrew species than other shrew genera, and the identified virus species were more from this shrew genus than from other shrew genera. Chodsigoa smithii, Crocidura attenuate, Suncus murinus, Anourosorex squamipes, Crocidura shantungensis, Sorex araneus, and Crocidura lasiura have been identified as being infected with more species of viruses than other shrew species. The factors, such as the abundance and distribution of these shrew genera or species, as well as the difficulty of capturing shrews of these genera or species, can affect the counts of identified virus species.
Figure 2.
Numbers of virus species that have been identified in certain shrew genera and shrew species. The blue, purple, and green colors represent genera or species in the shrew subfamilies of Crocidurinae, Soricinae, and Myosoricinae, respectively.
A database showed that 237, 201, and 204 species of viruses have been detected in Mus musculus, Sus scrofa, and Bos taurus, respectively [115]. With the consideration of the genetic diversity of shrews, most shrew viruses have yet to be identified so far. Meanwhile, much information regarding shrew viruses is based on viral sequences rather than viral isolates. The paucity of knowledge regarding virus diversity and viral isolates from shrews has hampered progress in demonstrating their pathogenic potential.
By virtue of their small size, voracious appetite, high metabolic rate, and aggressive behavior, most shrew species are difficult to handle and breed. However, it is desirable to develop efficient methods to breed them in laboratories because shrews are needed to investigate the transmission, pathogenicity, and potential control measures of shrew viruses, particularly those with biomedical significance [116].
Future research should assess the transmission risks of shrew viruses from shrews to humans and domestic animals and conduct broader surveys to delve into the diversity, evolution, and potential pathogenicity of these viruses. This will enhance our capacity to respond swiftly to the potential outbreaks of shrew viruses in humans or domestic animals with effective measures.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v16091441/s1, Table S1. Detailed information regarding the viruses having been identified in shrews.
Author Contributions
H.-Y.G., R.-X.C., S.-M.T. and X.W. wrote the first version of this manuscript under the direction of J.-M.C., Y.-L.Z., M.L. and H.-Y.G. drew the tables and figures. J.-M.C., Y.-L.Z. and M.L. conceived, designed, and financially supported this study and revised this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the High-Level Talent Fund of Foshan University [No. 20210036] and the Open Competition Program of Top Ten Critical Priorities of Agricultural Science and Technology Innovation for the 14th Five-Year Plan of Guangdong Province [2023SDZG02]. The APC was funded by the second funder.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author Ji-Ming Chen upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jürgens, K.D. Etruscan shrew muscle: The consequences of being small. J. Exp. Biol. 2002, 205, 2161–2166. [Google Scholar] [CrossRef] [PubMed]
- Integrated Taxonomic Information System. Available online: https://www.itis.gov (accessed on 20 July 2024).
- Zhang, Z. Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness (Addenda 2013). Zootaxa 2013, 3703, 1–82. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.D. Suncus murinus, a Recent Introduction to Guam. J. Mammal. 1956, 37, 278–279. [Google Scholar] [CrossRef]
- Chung, D.J.; Madison, G.P.; Aponte, A.M.; Singh, K.; Li, Y.; Pirooznia, M.; Bleck, C.K.E.; Darmani, N.A.; Balaban, R.S. Metabolic design in a mammalian model of extreme metabolism, the North American least shrew (Cryptotis parva). J. Physiol. 2022, 600, 547–567. [Google Scholar] [CrossRef] [PubMed]
- Bedford, J.M.; Mock, O.B.; Goodman, S.M. Novelties of conception in insectivorous mammals (Lipotyphla), particularly shrews. Biol. Rev. Camb. Philos. Soc. 2004, 79, 891–909. [Google Scholar] [CrossRef]
- Kowalski, K.; Rychlik, L. Venom use in Eulipotyphlans: An evolutionary and ecological approach. Toxins 2021, 13, 231. [Google Scholar] [CrossRef]
- Liao, Z.; Tang, X.; Chen, W.; Jiang, X.; Chen, Z.; He, K.; Li, Q.; Duan, Z.; He, X.; Kamau, P.M.; et al. Shrew’s venom quickly causes circulation disorder, analgesia and hypokinesia. Cell Mol. Life Sci. 2022, 79, 35. [Google Scholar] [CrossRef]
- Chai, S.; Tian, R.; Rong, X.; Li, G.; Chen, B.; Ren, W.; Xu, S.; Yang, G. Evidence of echolocation in the common shrew from molecular convergence with other echolocating mammals. Zool. Stud. 2020, 59, e4. [Google Scholar]
- Siemers, B.M.; Schauermann, G.; Turni, H.; von Merten, S. Why do shrews twitter? Communication or simple echo-based orientation. Biol. Lett. 2009, 5, 593–596. [Google Scholar] [CrossRef]
- Chen, Y.M.; Hu, S.J.; Lin, X.D.; Tian, J.H.; Lv, J.X.; Wang, M.R.; Luo, X.Q.; Pei, Y.Y.; Hu, R.X.; Song, Z.G.; et al. Host traits shape virome composition and virus transmission in wild small mammals. Cell 2023, 186, 4662–4675.e4612. [Google Scholar] [CrossRef]
- Guo, L.; Liu, S.; Song, J.; Han, L.; Zhang, H.; Wu, C.; Wang, C.; Zhou, H.; Wang, J. Seroprevalence of Wenzhou virus in China. Biosaf. Health 2020, 2, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lin, X.D.; Wang, W.; Shi, M.; Guo, W.P.; Zhang, X.H.; Xing, J.G.; He, J.R.; Wang, K.; Li, M.H.; et al. Isolation and characterization of a novel arenavirus harbored by rodents and shrews in Zhejiang province, China. Virology 2015, 476, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, Y.; Abe, H.; Ozeki, T.; Ondo, G.N.; Mbadinga, M.; Bikangui, R.; Nze-Nkogue, C.; Akomo-Okoue, E.F.; Ella, G.W.E.; Koumba, L.B.M.; et al. Identification of potential novel hosts and the risk of infection with lymphocytic choriomeningitis virus in humans in Gabon, Central Africa. Int. J. Infect. Dis. 2021, 105, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Cai, C.L.; Li, B.; Zhang, W.; Zhu, Y.; Chen, W.H.; Zhuo, F.; Shi, Z.L.; Yang, X.L. Detection and characterization of three zoonotic viruses in wild rodents and shrews from Shenzhen city, China. Virol. Sin. 2017, 32, 290–297. [Google Scholar] [CrossRef]
- Hilbe, M.; Herrsche, R.; Kolodziejek, J.; Nowotny, N.; Zlinszky, K.; Ehrensperger, F. Shrews as reservoir hosts of borna disease virus. Emerg. Infect. Dis. 2006, 12, 675–677. [Google Scholar] [CrossRef]
- Zhang, J.T.; Hu, Z.Y.; Tang, F.; Liu, Y.T.; Tan, W.L.; Ma, X.F.; Zhang, Y.F.; Si, G.Q.; Zhang, L.; Zhang, M.Q.; et al. Decoding the RNA viromes in shrew lungs along the eastern coast of China. NPJ Biofilms Microbiomes 2024, 10, 68. [Google Scholar] [CrossRef]
- Morvan, J.M.; Deubel, V.; Gounon, P.; Nakouné, E.; Barrière, P.; Murri, S.; Perpète, O.; Selekon, B.; Coudrier, D.; Gautier-Hion, A.; et al. Identification of Ebola virus sequences present as RNA or DNA in organs of terrestrial small mammals of the Central African Republic. Microbes Infect. 1999, 1, 1193–1201. [Google Scholar] [CrossRef]
- Yashina, L.N.; Abramov, S.A.; Zhigalin, A.V.; Smetannikova, N.A.; Dupal, T.A.; Krivopalov, A.V.; Kikuchi, F.; Senoo, K.; Arai, S.; Mizutani, T.; et al. Geographic distribution and phylogeny of soricine shrew-borne Seewis virus and Altai virus in Russia. Viruses 2021, 13, 1286. [Google Scholar] [CrossRef]
- Arai, S.; Kang, H.J.; Gu, S.H.; Ohdachi, S.D.; Cook, J.A.; Yashina, L.N.; Tanaka-Taya, K.; Abramov, S.A.; Morikawa, S.; Okabe, N.; et al. Genetic diversity of Artybash virus in the Laxmann’s shrew (Sorex caecutiens). Vector Borne Zoonotic Dis. 2016, 16, 468–475. [Google Scholar] [CrossRef]
- Radosa, L.; Schlegel, M.; Gebauer, P.; Ansorge, H.; Heroldová, M.; Jánová, E.; Stanko, M.; Mošanský, L.; Fričová, J.; Pejčoch, M.; et al. Detection of shrew-borne hantavirus in Eurasian pygmy shrew (Sorex minutus) in Central Europe. Infect. Genet. Evol. 2013, 19, 403–410. [Google Scholar] [CrossRef]
- Gu, S.H.; Nicolas, V.; Lalis, A.; Sathirapongsasuti, N.; Yanagihara, R. Complete genome sequence and molecular phylogeny of a newfound hantavirus harbored by the Doucet’s musk shrew (Crocidura douceti) in Guinea. Infect. Genet. Evol. 2013, 20, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.P.; Lin, X.D.; Wang, W.; Tian, J.H.; Cong, M.L.; Zhang, H.L.; Wang, M.R.; Zhou, R.H.; Wang, J.B.; Li, M.H.; et al. Phylogeny and origins of hantaviruses harbored by bats, insectivores, and rodents. PLoS Pathog. 2013, 9, e1003159. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Gu, S.H.; Baek, L.J.; Tabara, K.; Bennett, S.N.; Oh, H.S.; Takada, N.; Kang, H.J.; Tanaka-Taya, K.; Morikawa, S.; et al. Divergent ancestral lineages of newfound hantaviruses harbored by phylogenetically related crocidurine shrew species in Korea. Virology 2012, 424, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Arai, S.; Hope, A.G.; Cook, J.A.; Yanagihara, R. Novel hantavirus in the flat-skulled shrew (Sorex roboratus). Vector Borne Zoonotic Dis. 2010, 10, 593–597. [Google Scholar] [CrossRef]
- Sun, X.F.; Zhao, L.; Zhang, Z.T.; Liu, M.M.; Xue, Z.F.; Wen, H.L.; Ma, D.Q.; Huang, Y.T.; Sun, Y.; Zhou, C.M.; et al. Detection of Imjin virus and Seoul virus in crocidurine shrews in Shandong province, China. Vector Borne Zoonotic Dis. 2017, 17, 425–431. [Google Scholar] [CrossRef]
- Song, J.W.; Gu, S.H.; Bennett, S.N.; Arai, S.; Puorger, M.; Hilbe, M.; Yanagihara, R. Seewis virus, a genetically distinct hantavirus in the Eurasian common shrew (Sorex araneus). Virol. J. 2007, 4, 114. [Google Scholar] [CrossRef]
- Schlegel, M.; Radosa, L.; Rosenfeld, U.M.; Schmidt, S.; Triebenbacher, C.; Löhr, P.W.; Fuchs, D.; Heroldová, M.; Jánová, E.; Stanko, M.; et al. Broad geographical distribution and high genetic diversity of shrew-borne Seewis hantavirus in Central Europe. Virus Genes 2012, 45, 48–55. [Google Scholar] [CrossRef]
- Kang, H.J.; Gu, S.H.; Yashina, L.N.; Cook, J.A.; Yanagihara, R. Highly divergent genetic variants of soricid-borne Altai virus (Hantaviridae) in Eurasia suggest ancient host-switching events. Viruses 2019, 11, 857. [Google Scholar] [CrossRef]
- Gu, S.H.; Arai, S.; Yu, H.T.; Lim, B.K.; Kang, H.J.; Yanagihara, R. Genetic variants of Cao Bang hantavirus in the Chinese mole shrew (Anourosorex squamipes) and Taiwanese mole shrew (Anourosorex yamashinai). Infect. Genet. Evol. 2016, 40, 113–118. [Google Scholar] [CrossRef]
- Arai, S.; Bennett, S.N.; Sumibcay, L.; Cook, J.A.; Song, J.W.; Hope, A.; Parmenter, C.; Nerurkar, V.R.; Yates, T.L.; Yanagihara, R. Phylogenetically distinct hantaviruses in the masked shrew (Sorex cinereus) and dusky shrew (Sorex monticolus) in the United States. Am. J. Trop. Med. Hyg. 2008, 78, 348–351. [Google Scholar] [CrossRef]
- Arai, S.; Song, J.W.; Sumibcay, L.; Bennett, S.N.; Nerurkar, V.R.; Parmenter, C.; Cook, J.A.; Yates, T.L.; Yanagihara, R. Hantavirus in northern short-tailed shrew, United States. Emerg. Infect. Dis. 2007, 13, 1420–1423. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.H.; Markowski, J.; Kang, H.J.; Hejduk, J.; Sikorska, B.; Liberski, P.P.; Yanagihara, R. Boginia virus, a newfound hantavirus harbored by the Eurasian water shrew (Neomys fodiens) in Poland. Virol. J. 2013, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Kadjo, B.; Dubey, S.; Jacquet, F.; Yanagihara, R. Molecular evolution of Azagny virus, a newfound hantavirus harbored by the West African pygmy shrew (Crocidura obscurior) in Côte d’Ivoire. Virol. J. 2011, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.Q.; Gong, Z.D.; Fang, L.Q.; Jiang, J.F.; Zhang, J.S.; Zhao, Q.M.; Cao, W.C. A new hantavirus from the stripe-backed shrew (Sorex cylindricauda) in the People’s Republic of China. Virus Res. 2014, 184, 82–86. [Google Scholar] [CrossRef]
- Song, J.W.; Kang, H.J.; Gu, S.H.; Moon, S.S.; Bennett, S.N.; Song, K.J.; Baek, L.J.; Kim, H.C.; O’Guinn, M.L.; Chong, S.T.; et al. Characterization of Imjin virus, a newly isolated hantavirus from the Ussuri white-toothed shrew (Crocidura lasiura). J. Virol. 2009, 83, 6184–6191. [Google Scholar] [CrossRef]
- Carey, D.E.; Reuben, R.; Panicker, K.N.; Shope, R.E.; Myers, R.M. Thottapalayam virus: A presumptive arbovirus isolated from a shrew in India. Indian J. Med. Res. 1971, 59, 1758–1760. [Google Scholar]
- Kang, H.J.; Kosoy, M.Y.; Shrestha, S.K.; Shrestha, M.P.; Pavlin, J.A.; Gibbons, R.V.; Yanagihara, R. Short report: Genetic diversity of Thottapalayam virus, a Hantavirus harbored by the Asian house shrew (Suncus murinus) in Nepal. Am. J. Trop. Med. Hyg. 2011, 85, 540–545. [Google Scholar] [CrossRef]
- Kang, H.J.; Stanley, W.T.; Esselstyn, J.A.; Gu, S.H.; Yanagihara, R. Expanded host diversity and geographic distribution of hantaviruses in sub-Saharan Africa. J. Virol. 2014, 88, 7663–7667. [Google Scholar] [CrossRef]
- Chastel, C.; Main, A.J.; Richard, P.; Le Lay, G.; Legrand-Quillien, M.C.; Beaucournu, J.C. Erve virus, a probable member of Bunyaviridae family isolated from shrews (Crocidura russula) in France. Acta Virol. 1989, 33, 270–280. [Google Scholar]
- Ozeki, T.; Abe, H.; Ushijima, Y.; Nze-Nkogue, C.; Akomo-Okoue, E.F.; Ella, G.W.E.; Koumba, L.B.M.; Nso, B.; Mintsa-Nguema, R.; Makouloutou-Nzassi, P.; et al. Identification of novel orthonairoviruses from rodents and shrews in Gabon, Central Africa. J. Gen. Virol. 2022, 103, 001796. [Google Scholar] [CrossRef]
- Walker, P.J.; Widen, S.G.; Firth, C.; Blasdell, K.R.; Wood, T.G.; Travassos da Rosa, A.P.; Guzman, H.; Tesh, R.B.; Vasilakis, N. Genomic characterization of Yogue, Kasokero, Issyk-Kul, Keterah, Gossas, and Thiafora viruses: Nairoviruses naturally infecting bats, shrews, and ticks. Am. J. Trop. Med. Hyg. 2015, 93, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Low, D.H.W.; Ch’ng, L.; Su, Y.C.F.; Linster, M.; Zhang, R.; Zhuang, Y.; Kwak, M.L.; Borthwick, S.A.; Hitch, A.T.; Smith, G.J.D.; et al. Cencurut virus: A novel Orthonairovirus from Asian house shrews (Suncus murinus) in Singapore. One Health 2023, 16, 100529. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, K.; Kim, J.; No, J.S.; Park, K.; Budhathoki, S.; Lee, S.H.; Lee, J.; Cho, S.H.; Cho, S.; et al. Discovery and genetic characterization of novel paramyxoviruses related to the genus Henipavirus in Crocidura species in the republic of Korea. Viruses 2021, 13, 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.A.; Li, H.; Jiang, F.C.; Zhu, F.; Zhang, Y.F.; Chen, J.J.; Tan, C.W.; Anderson, D.E.; Fan, H.; Dong, L.Y.; et al. A zoonotic henipavirus in febrile patients in China. N. Engl. J. Med. 2022, 387, 470–472. [Google Scholar] [CrossRef]
- Vanmechelen, B.; Meurs, S.; Horemans, M.; Loosen, A.; Joly Maes, T.; Laenen, L.; Vergote, V.; Koundouno, F.R.; Magassouba, N.; Konde, M.K.; et al. The characterization of multiple novel paramyxoviruses highlights the diverse nature of the subfamily Orthoparamyxovirinae. Virus Evol. 2022, 8, veac061. [Google Scholar] [CrossRef]
- Horemans, M.; Van Bets, J.; Joly Maes, T.; Maes, P.; Vanmechelen, B. Discovery and genome characterization of six new orthoparamyxoviruses in small Belgian mammals. Virus Evol. 2023, 9, vead065. [Google Scholar] [CrossRef]
- Chen, J.J.; Zhang, X.A.; Fan, H.; Jiang, F.C.; Jin, M.Z.; Dai, K.; Wang, N.; Zhang, P.H.; Li, X.K.; Li, H.; et al. Distribution and characteristics of Beilong virus among wild rodents and shrews in China. Infect. Genet. Evol. 2020, 85, 104454. [Google Scholar] [CrossRef]
- Liu, J.W.; Wen, H.L.; Fang, L.Z.; Zhang, Z.T.; He, S.T.; Xue, Z.F.; Ma, D.Q.; Zhang, X.S.; Wang, T.; Yu, H.; et al. Prevalence of SFTSV among Asian house shrews and rodents, China, January–August 2013. Emerg. Infect. Dis. 2014, 20, 2126–2128. [Google Scholar] [CrossRef]
- Shope, R.E.; Murphy, F.A.; Harrison, A.K.; Causey, O.R.; Kemp, G.E.; Simpson, D.I.; Moore, D.L. Two African viruses serologically and morphologically related to rabies virus. J. Virol. 1970, 6, 690–692. [Google Scholar] [CrossRef]
- Radoshitzky, S.R.; Buchmeier, M.J.; Charrel, R.N.; Gonzalez, J.J.; Günther, S.; Hepojoki, J.; Kuhn, J.H.; Lukashevich, I.S.; Romanowski, V.; Salvato, M.S.; et al. ICTV Virus Taxonomy Profile: Arenaviridae 2023. J Gen Virol 2023, 104, 001891. [Google Scholar] [CrossRef]
- Radoshitzky, S.R.; Buchmeier, M.J.; Charrel, R.N.; Clegg, J.C.S.; Gonzalez, J.J.; Günther, S.; Hepojoki, J.; Kuhn, J.H.; Lukashevich, I.S.; Romanowski, V.; et al. ICTV Virus Taxonomy Profile: Arenaviridae. J. Gen. Virol. 2019, 100, 1200–1201. [Google Scholar] [CrossRef] [PubMed]
- Rubbenstroth, D.; Briese, T.; Dürrwald, R.; Horie, M.; Hyndman, T.H.; Kuhn, J.H.; Nowotny, N.; Payne, S.; Stenglein, M.D.; Tomonaga, K.; et al. ICTV Virus Taxonomy Profile: Bornaviridae. J. Gen. Virol. 2021, 102, 001613. [Google Scholar] [CrossRef] [PubMed]
- Sandvik, T.; Tryland, M.; Hansen, H.; Mehl, R.; Moens, U.; Olsvik, O.; Traavik, T. Naturally occurring orthopoxviruses: Potential for recombination with vaccine vectors. J. Clin. Microbiol. 1998, 36, 2542–2547. [Google Scholar] [CrossRef] [PubMed]
- Nobach, D.; Bourg, M.; Herzog, S.; Lange-Herbst, H.; Encarnação, J.A.; Eickmann, M.; Herden, C. Shedding of Infectious Borna Disease Virus-1 in Living Bicolored White-Toothed Shrews. PLoS ONE 2015, 10, e0137018. [Google Scholar] [CrossRef]
- Kuhn, J.H.; Dheilly, N.M.; Junglen, S.; Paraskevopoulou, S.; Shi, M.; Di Paola, N. ICTV Virus Taxonomy Profile: Jingchuvirales 2023. J. Gen. Virol. 2023, 104, 001924. [Google Scholar] [CrossRef]
- Biedenkopf, N.; Bukreyev, A.; Chandran, K.; Di Paola, N.; Formenty, P.B.H.; Griffiths, A.; Hume, A.J.; Mühlberger, E.; Netesov, S.V.; Palacios, G.; et al. ICTV Virus Taxonomy Profile: Filoviridae 2024. J. Gen. Virol. 2024, 105, 001955. [Google Scholar] [CrossRef]
- Hussein, H.A. Brief review on ebola virus disease and one health approach. Heliyon 2023, 9, e19036. [Google Scholar] [CrossRef]
- Bradfute, S.B.; Calisher, C.H.; Klempa, B.; Klingström, J.; Kuhn, J.H.; Laenen, L.; Tischler, N.D.; Maes, P. ICTV Virus Taxonomy Profile: Hantaviridae 2024. J. Gen. Virol. 2024, 105, 001975. [Google Scholar] [CrossRef]
- Song, J.W.; Kang, H.J.; Song, K.J.; Truong, T.T.; Bennett, S.N.; Arai, S.; Truong, N.U.; Yanagihara, R. Newfound hantavirus in Chinese mole shrew, Vietnam. Emerg. Infect. Dis. 2007, 13, 1784–1787. [Google Scholar] [CrossRef]
- Kuhn, J.H.; Alkhovsky, S.V.; Avšič-Županc, T.; Bergeron, É.; Burt, F.; Ergünay, K.; Garrison, A.R.; Marklewitz, M.; Mirazimi, A.; Papa, A.; et al. ICTV Virus Taxonomy Profile: Nairoviridae 2024. J. Gen. Virol. 2024, 105, 001974. [Google Scholar] [CrossRef]
- Uribe, M.; Rodríguez-Posada, M.E.; Ramirez-Nieto, G.C. Molecular Evidence of Orthomyxovirus Presence in Colombian Neotropical Bats. Front. Microbiol. 2022, 13, 845546. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhuang, Q.; Wang, S.; Jiang, W.; Jin, J.; Peng, C.; Hou, G.; Li, J.; Yu, J.; Yu, X.; et al. Control of avian influenza in China: Strategies and lessons. Transbound. Emerg. Dis. 2020, 67, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.H.; Abe, J.; Adkins, S.; Alkhovsky, S.V.; Avšič-Županc, T.; Ayllón, M.A.; Bahl, J.; Balkema-Buschmann, A.; Ballinger, M.J.; Kumar Baranwal, V.; et al. Annual (2023) taxonomic update of RNA-directed RNA polymerase-encoding negative-sense RNA viruses (realm Riboviria: Kingdom Orthornavirae: Phylum Negarnaviricota). J. Gen. Virol. 2023, 104, 001864. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; Hu, B.; Ding, Q.; Zhang, N.; Wei, J.; Nie, T.; Cai, K.; Zheng, Z. Discovery and characterization of novel jeilongviruses in wild rodents from Hubei, China. Virol. J. 2024, 21, 146. [Google Scholar] [CrossRef]
- Sasaya, T.; Palacios, G.; Briese, T.; Di Serio, F.; Groschup, M.H.; Neriya, Y.; Song, J.W.; Tomitaka, Y. ICTV Virus Taxonomy Profile: Phenuiviridae 2023. J. Gen. Virol. 2023, 104, 001893. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Yang, L.; Cao, P.; Lu, J. Severe fever with thrombocytopenia syndrome virus: A highly lethal bunyavirus. Crit. Rev. Microbiol. 2021, 47, 112–125. [Google Scholar] [CrossRef]
- Walker, P.J.; Freitas-Astúa, J.; Bejerman, N.; Blasdell, K.R.; Breyta, R.; Dietzgen, R.G.; Fooks, A.R.; Kondo, H.; Kurath, G.; Kuzmin, I.V.; et al. ICTV Virus Taxonomy Profile: Rhabdoviridae 2022. J. Gen. Virol. 2022, 103, 001689. [Google Scholar] [CrossRef]
- Delmas, O.; Holmes, E.C.; Talbi, C.; Larrous, F.; Dacheux, L.; Bouchier, C.; Bourhy, H. Genomic diversity and evolution of the lyssaviruses. PLoS ONE 2008, 3, e2057. [Google Scholar] [CrossRef]
- Vanmechelen, B.; Vergote, V.; Laenen, L.; Koundouno, F.R.; Bore, J.A.; Wada, J.; Kuhn, J.H.; Carroll, M.W.; Maes, P. Expanding the arterivirus host spectrum: Olivier’s shrew virus 1, a novel arterivirus discovered in African giant shrews. Sci. Rep. 2018, 8, 11171. [Google Scholar] [CrossRef]
- Hu, B.; Chmura, A.A.; Li, J.; Zhu, G.; Desmond, J.S.; Zhang, Y.; Zhang, W.; Epstein, J.H.; Daszak, P.; Shi, Z. Detection of diverse novel astroviruses from small mammals in China. J. Gen. Virol. 2014, 95, 2442–2449. [Google Scholar] [CrossRef]
- Calvete, C.; Mendoza, M.; Sarto, M.P.; Bagüés, M.P.J.; Luján, L.; Molín, J.; Calvo, A.J.; Monroy, F.; Calvo, J.H. Detection of rabbit hemorrhagic disease virus GI.2/RHDV2/b in the Mediterranean pine vole ( Microtus duodecimcostatus) and White-Toothed Shrew ( Crocidura russula). J. Wildl. Dis. 2019, 55, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Tsoleridis, T.; Onianwa, O.; Horncastle, E.; Dayman, E.; Zhu, M.; Danjittrong, T.; Wachtl, M.; Behnke, J.M.; Chapman, S.; Strong, V.; et al. Discovery of novel Alphacoronaviruses in European rodents and shrews. Viruses 2016, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Ntumvi, N.F.; Mbala Kingebeni, P.; Tamoufe, U.; Kumakamba, C.; Ndze, V.; Ngay Lukusa, I.; LeBreton, M.; Atibu Losoma, J.; Le Doux Diffo, J.; N’Kawa, F.; et al. High herpesvirus diversity in wild rodent and shrew species in Central Africa. Intervirology 2018, 61, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Lu, L.; Du, J.; Yang, L.; Ren, X.; Liu, B.; Jiang, J.; Yang, J.; Dong, J.; Sun, L.; et al. Comparative analysis of rodent and small mammal viromes to better understand the wildlife origin of emerging infectious diseases. Microbiome 2018, 6, 178. [Google Scholar] [CrossRef]
- Nosek, J.; Grulich, I. The relationship between the tick-borne encephalitis virus and the ticks and mammals of the Tribec mountain range. Bull. World Health Organ. 1967, 36, 31–47. [Google Scholar]
- Bakhvalova, V.N.; Chicherina, G.S.; Potapova, O.F.; Panov, V.V.; Glupov, V.V.; Potapov, M.A.; Seligman, S.J.; Morozova, O.V. Tick-Borne Encephalitis Virus Diversity in Ixodid Ticks and Small Mammals in South-Western Siberia, Russia. Vector Borne Zoonotic Dis. 2016, 16, 541–549. [Google Scholar] [CrossRef]
- Goethert, H.K.; Mather, T.N.; Johnson, R.W.; Telford, S.R., 3rd. Incrimination of shrews as a reservoir for Powassan virus. Commun. Biol. 2021, 4, 1319. [Google Scholar] [CrossRef]
- Diagne, M.M.; Ndione, M.H.D.; Di Paola, N.; Fall, G.; Bedekelabou, A.P.; Sembène, P.M.; Faye, O.; Zanotto, P.M.A.; Sall, A.A. Usutu virus isolated from rodents in Senegal. Viruses 2019, 11, 181. [Google Scholar] [CrossRef]
- Gao, Y.W.; Wan, Z.W.; Wu, Y.; Li, X.F.; Tang, S.X. PCR-based screening and phylogenetic analysis of rat pegivirus (RPgV) carried by rodents in China. J. Vet. Med. Sci. 2020, 82, 1464–1471. [Google Scholar] [CrossRef]
- Guan, D.; Li, W.; Su, J.; Fang, L.; Takeda, N.; Wakita, T.; Li, T.C.; Ke, C. Asian musk shrew as a reservoir of rat hepatitis E virus, China. Emerg. Infect. Dis. 2013, 19, 1341–1343. [Google Scholar] [CrossRef]
- Drexler, J.F.; Corman, V.M.; Lukashev, A.N.; van den Brand, J.M.; Gmyl, A.P.; Brünink, S.; Rasche, A.; Seggewiβ, N.; Feng, H.; Leijten, L.M.; et al. Evolutionary origins of hepatitis A virus in small mammals. Proc. Natl. Acad. Sci. USA 2015, 112, 15190–15195. [Google Scholar] [CrossRef] [PubMed]
- Fevola, C.; Rossi, C.; Rosso, F.; Girardi, M.; Rosà, R.; Manica, M.; Delucchi, L.; Rocchini, D.; Garzon-Lopez, C.X.; Arnoldi, D.; et al. Geographical distribution of Ljungan virus in small mammals in Europe. Vector Borne Zoonotic Dis. 2020, 20, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Brinton, M.A.; Gulyaeva, A.A.; Balasuriya, U.B.R.; Dunowska, M.; Faaberg, K.S.; Goldberg, T.; Leung, F.C.C.; Nauwynck, H.J.; Snijder, E.J.; Stadejek, T.; et al. ICTV Virus Taxonomy Profile: Arteriviridae 2021. J. Gen. Virol. 2021, 102, 001632. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.; Rosenstengle, C.; Spak, C. Underrecognized cause diarrhea in solid organ transplant: A report of astroviridae enteritis in liver transplant. Transpl. Infect. Dis. 2024, 26, e14257. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; Hargest, V.; Cortez, V.; Meliopoulos, V.A.; Schultz-Cherry, S. Astrovirus Pathogenesis. Viruses 2017, 9, 22. [Google Scholar] [CrossRef]
- Vinjé, J.; Estes, M.K.; Esteves, P.; Green, K.Y.; Katayama, K.; Knowles, N.J.; L’Homme, Y.; Martella, V.; Vennema, H.; White, P.A.; et al. ICTV Virus Taxonomy Profile: Caliciviridae. J. Gen. Virol. 2019, 100, 1469–1470. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; de Groot, R.J.; Haagmans, B.; Lau, S.K.P.; Neuman, B.W.; Perlman, S.; Sola, I.; van der Hoek, L.; Wong, A.C.P.; Yeh, S.H. ICTV Virus Taxonomy Profile: Coronaviridae 2023. J. Gen. Virol. 2023, 104, 001843. [Google Scholar] [CrossRef]
- Postler, T.S.; Beer, M.; Blitvich, B.J.; Bukh, J.; de Lamballerie, X.; Drexler, J.F.; Imrie, A.; Kapoor, A.; Karganova, G.G.; Lemey, P.; et al. Renaming of the genus Flavivirus to Orthoflavivirus and extension of binomial species names within the family Flaviviridae. Arch. Virol. 2023, 168, 224. [Google Scholar] [CrossRef]
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3. [Google Scholar] [CrossRef]
- Guo, H.; Cai, C.; Wang, B.; Zhuo, F.; Jiang, R.; Wang, N.; Li, B.; Zhang, W.; Zhu, Y.; Fan, Y.; et al. Novel Hepacivirus in Asian house shrew, China. Sci. China Life Sci. 2019, 62, 701–704. [Google Scholar] [CrossRef]
- Purdy, M.A.; Drexler, J.F.; Meng, X.J.; Norder, H.; Okamoto, H.; Van der Poel, W.H.M.; Reuter, G.; de Souza, W.M.; Ulrich, R.G.; Smith, D.B. ICTV Virus Taxonomy Profile: Hepeviridae 2022. J. Gen. Virol. 2022, 103, 001778. [Google Scholar] [CrossRef] [PubMed]
- Sahul Hameed, A.S.; Ninawe, A.S.; Nakai, T.; Chi, S.C.; Johnson, K.L.; Ictv Report, C. ICTV Virus Taxonomy Profile: Nodaviridae. J. Gen. Virol. 2019, 100, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Zell, R.; Delwart, E.; Gorbalenya, A.E.; Hovi, T.; King, A.M.Q.; Knowles, N.J.; Lindberg, A.M.; Pallansch, M.A.; Palmenberg, A.C.; Reuter, G.; et al. ICTV Virus Taxonomy Profile: Picornaviridae. J. Gen. Virol. 2017, 98, 2421–2422. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Qiu, M.; Ke, X.M.; Guan, W.J.; Li, J.M.; Huo, S.T.; Chen, S.W.; Zhong, X.S.; Zhou, W.; Xiong, Y.Q.; et al. Detection of novel adenoviruses in fecal specimens from rodents and shrews in southern China. Virus Genes 2016, 52, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Diffo, J.; Ndze, V.N.; Ntumvi, N.F.; Takuo, J.M.; Mouiche, M.M.M.; Tamoufe, U.; Nwobegahay, J.; LeBreton, M.; Gillis, A.; Schneider, B.S.; et al. DNA of diverse adenoviruses detected in Cameroonian rodent and shrew species. Arch. Virol. 2019, 164, 2359–2366. [Google Scholar] [CrossRef]
- Nie, F.Y.; Tian, J.H.; Lin, X.D.; Yu, B.; Xing, J.G.; Cao, J.H.; Holmes, E.C.; Ma, R.Z.; Zhang, Y.Z. Discovery of a highly divergent hepadnavirus in shrews from China. Virology 2019, 531, 162–170. [Google Scholar] [CrossRef]
- Gedvilaite, A.; Tryland, M.; Ulrich, R.G.; Schneider, J.; Kurmauskaite, V.; Moens, U.; Preugschas, H.; Calvignac-Spencer, S.; Ehlers, B. Novel polyomaviruses in shrews (Soricidae) with close similarity to human polyomavirus 12. J. Gen. Virol. 2017, 98, 3060–3067. [Google Scholar] [CrossRef]
- Orba, Y.; Sasaki, M.; Yamaguchi, H.; Ishii, A.; Thomas, Y.; Ogawa, H.; Hang’ombe, B.M.; Mweene, A.S.; Morikawa, S.; Saijo, M.; et al. Orthopoxvirus infection among wildlife in Zambia. J. Gen. Virol. 2015, 96, 390–394. [Google Scholar] [CrossRef]
- Benkő, M.; Aoki, K.; Arnberg, N.; Davison, A.J.; Echavarría, M.; Hess, M.; Jones, M.S.; Kaján, G.L.; Kajon, A.E.; Mittal, S.K.; et al. ICTV Virus Taxonomy Profile: Adenoviridae 2022. J. Gen. Virol. 2022, 103, 001721. [Google Scholar] [CrossRef]
- Magnius, L.; Mason, W.S.; Taylor, J.; Kann, M.; Glebe, D.; Dény, P.; Sureau, C.; Norder, H.; Ictv Report, C. ICTV Virus Taxonomy Profile: Hepadnaviridae. J. Gen. Virol. 2020, 101, 571–572. [Google Scholar] [CrossRef]
- Moens, U.; Calvignac-Spencer, S.; Lauber, C.; Ramqvist, T.; Feltkamp, M.C.W.; Daugherty, M.D.; Verschoor, E.J.; Ehlers, B.; Ictv Report, C. ICTV Virus Taxonomy Profile: Polyomaviridae. J. Gen. Virol. 2017, 98, 1159–1160. [Google Scholar] [CrossRef] [PubMed]
- McInnes, C.J.; Damon, I.K.; Smith, G.L.; McFadden, G.; Isaacs, S.N.; Roper, R.L.; Evans, D.H.; Damaso, C.R.; Carulei, O.; Wise, L.M.; et al. ICTV Virus Taxonomy Profile: Poxviridae 2023. J. Gen. Virol. 2023, 104, 001849. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.Q.; Mo, Y.; Chen, M.J.; Cai, W.; He, W.Q.; Chen, Q. Detection and phylogenetic analysis of torque teno virus (TTV) carried by murine rodents and house shrews in China. Virology 2018, 516, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Orba, Y.; Anindita, P.D.; Ishii, A.; Ueno, K.; Hang’ombe, B.M.; Mweene, A.S.; Ito, K.; Sawa, H. Distinct Lineages of Bufavirus in Wild Shrews and Nonhuman Primates. Emerg. Infect. Dis. 2015, 21, 1230–1233. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; You, F.F.; Chen, X.J.; Chen, Y.X.; Wen, Y.Q.; Chen, Q. Detection and phylogenetic analysis of porcine bocaviruses carried by murine rodents and house shrews in China. Transbound. Emerg. Dis. 2019, 66, 259–267. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Zhang, M.Y.; Zhou, J.H.; Li, Y.Z.; You, F.F.; Wen, Y.Q.; He, W.Q.; Chen, Q. A molecular epidemiological investigation of carriage of the Adeno-Associated virus in murine rodents and house shrews in China. Intervirology 2018, 61, 143–148. [Google Scholar] [CrossRef]
- Varsani, A.; Kraberger, S.; Opriessnig, T.; Maggi, F.; Celer, V.; Okamoto, H.; Biagini, P. Anelloviridae taxonomy update 2023. Arch. Virol. 2023, 168, 277. [Google Scholar] [CrossRef]
- Cotmore, S.F.; Agbandje-McKenna, M.; Canuti, M.; Chiorini, J.A.; Eis-Hubinger, A.M.; Hughes, J.; Mietzsch, M.; Modha, S.; Ogliastro, M.; Pénzes, J.J.; et al. ICTV Virus Taxonomy Profile: Parvoviridae. J. Gen. Virol. 2019, 100, 367–368. [Google Scholar] [CrossRef]
- Alexander, K.A.; MacLachlan, N.J.; Kat, P.W.; House, C.; O’Brien, S.J.; Lerche, N.W.; Sawyer, M.; Frank, L.G.; Holekamp, K.; Smale, L.; et al. Evidence of natural bluetongue virus infection among African carnivores. Am. J. Trop. Med. Hyg. 1994, 51, 568–576. [Google Scholar] [CrossRef]
- Li, K.; Lin, X.D.; Huang, K.Y.; Zhang, B.; Shi, M.; Guo, W.P.; Wang, M.R.; Wang, W.; Xing, J.G.; Li, M.H.; et al. Identification of novel and diverse rotaviruses in rodents and insectivores, and evidence of cross-species transmission into humans. Virology 2016, 494, 168–177. [Google Scholar] [CrossRef]
- Delmas, B.; Attoui, H.; Ghosh, S.; Malik, Y.S.; Mundt, E.; Vakharia, V.N.; Ictv Report, C. ICTV virus taxonomy profile: Picobirnaviridae. J. Gen. Virol. 2019, 100, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Attoui, H.; Bányai, K.; Brussaard, C.P.D.; Danthi, P.; Del Vas, M.; Dermody, T.S.; Duncan, R.; Fang, Q.; Johne, R.; et al. ICTV Virus Taxonomy Profile: Sedoreoviridae 2022. J. Gen. Virol. 2022, 103, 001782. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Attoui, H.; Bányai, K.; Brussaard, C.P.D.; Danthi, P.; Del Vas, M.; Dermody, T.S.; Duncan, R.; Fāng, Q.; Johne, R.; et al. ICTV Virus Taxonomy Profile: Spinareoviridae 2022. J. Gen. Virol. 2022, 103, 001781. [Google Scholar] [CrossRef] [PubMed]
- Mihara, T.; Nishimura, Y.; Shimizu, Y.; Nishiyama, H.; Yoshikawa, G.; Uehara, H.; Hingamp, P.; Goto, S.; Ogata, H. Linking Virus Genomes with Host Taxonomy. Viruses 2016, 8, 66. [Google Scholar] [CrossRef]
- Geyer, B.; Erickson, N.A.; Müller, K.; Grübel, S.; Hueber, B.; Hetz, S.K.; Brecht, M. Establishing and maintaining an etruscan shrew colony. J. Am. Assoc. Lab. Anim. Sci. 2022, 61, 52–60. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).