Enhanced Immunogenicity and Affinity with A35R-Fc-Based Chimeric Protein Compared to MPXV A35R Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cells and Viruses
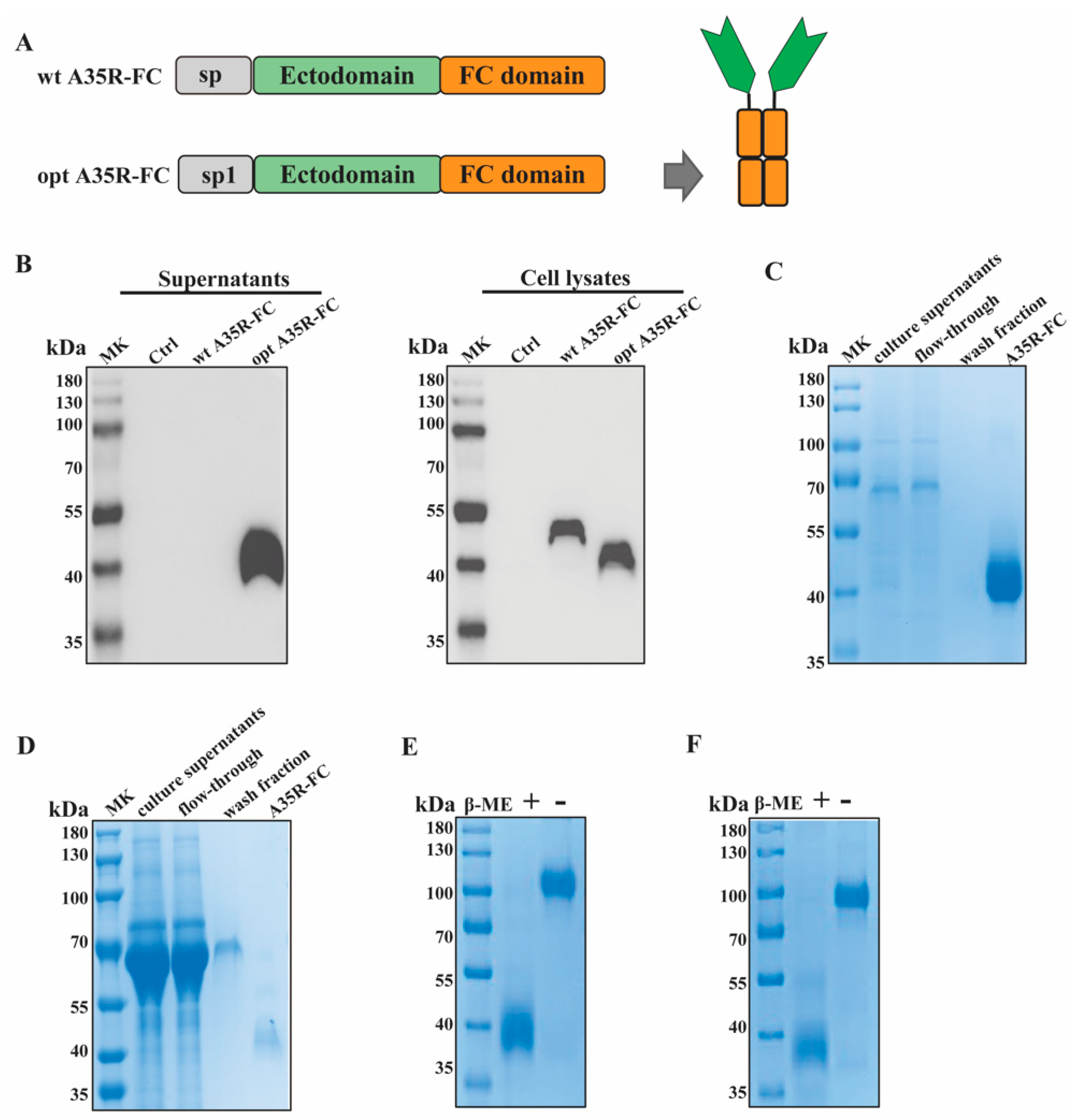
2.3. Construction, Expression, and Purification of the A35R-Fc Fusion Protein
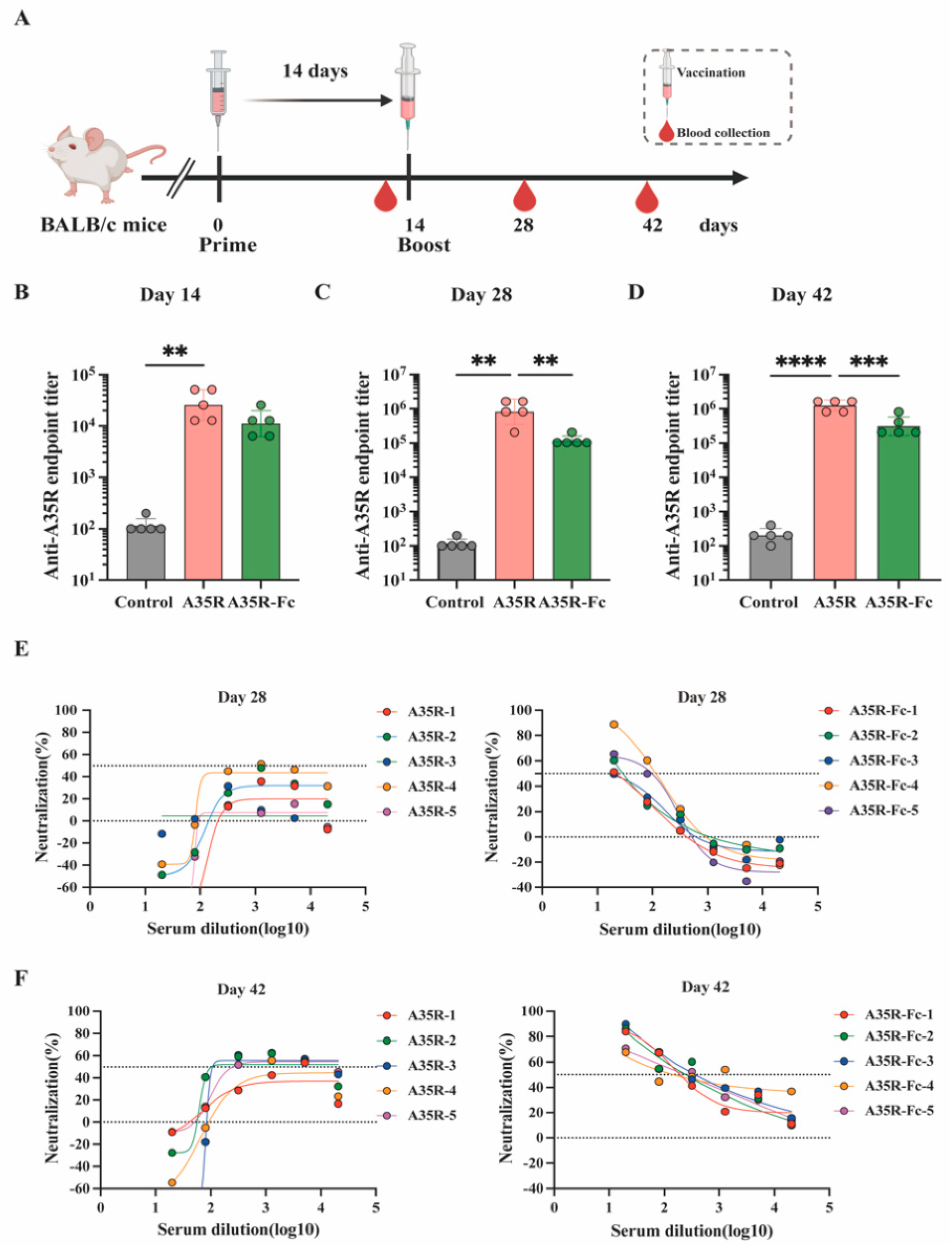
2.4. Mouse Experiments
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Surface Plasmon Resonance
2.7. MPXV Neutralization Assay
2.8. Statistical Analysis
3. Results
3.1. Construction and Production of Recombinant A35R-Fc Fusion Protein
3.2. A35R-Fc Chimeric Protein Showed High Binding Affinity to Different A35R-Specific Antibodies
3.3. A35R-Fc Chimeric Protein Could Be Used for Serological Assays of MPXV Infection
3.4. A35R-Fc Protein Potently Enhances the Induction of Neutralizing Antibodies than A35R Protein in the Presence of Alum Plus CPG Adjuvants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okwor, T.; Mbala, P.K.; Evans, D.H.; Kindrachuk, J. A contemporary review of clade-specific virological differences in monkeypox viruses. Clin. Microbiol. Infect. 2023, 29, 1502–1507. [Google Scholar] [CrossRef]
- World Health Organization. 2022–24 Mpox (Monkeypox) Outbreak: Global Trends. Available online: https://worldhealthorg.shinyapps.io/mpx_global/ (accessed on 1 December 2024).
- Isidro, J.; Borges, V.; Pinto, M.; Sobral, D.; Santos, J.D.; Nunes, A.; Mixao, V.; Ferreira, R.; Santos, D.; Duarte, S.; et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat. Med. 2022, 28, 1569–1572. [Google Scholar] [CrossRef]
- Rao, A.K.; Petersen, B.W.; Whitehill, F.; Razeq, J.H.; Isaacs, S.N.; Merchlinsky, M.J.; Campos-Outcalt, D.; Morgan, R.L.; Damon, I.; Sánchez, P.J.; et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 734–742. [Google Scholar] [CrossRef]
- Xiang, Y.; White, A. Monkeypox virus emerges from the shadow of its more infamous cousin: Family biology matters. Emerg. Microbes Infect. 2022, 11, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kwon, S.L.; Park, J.; Bae, H.; Lee, H.; Kwon, G.Y. JYNNEOS vaccine safety monitoring in the Republic of Korea, 2022: A cross-sectional study. Osong Public Health Res. Perspect 2023, 14, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Lopez, L.K.; Glover, C.; Cashman, P.; Reynolds, R.; Macartney, K.; Wood, N. Short-term Adverse Events Following Immunization With Modified Vaccinia Ankara-Bavarian Nordic (MVA-BN) Vaccine for Mpox. JAMA 2023, 329, 2091–2094. [Google Scholar] [CrossRef]
- Lim, S.Y.; Jung, Y.M.; Kim, Y.; Kim, G.; Jeon, J.; Chin, B.; Kim, M.K. Adverse Reactions After Intradermal Vaccination With JYNNEOS for Mpox in Korea. J. Korean Med. Sci. 2024, 39, e100. [Google Scholar] [CrossRef]
- Thy, M.; Peiffer-Smadja, N.; Mailhe, M.; Kramer, L.; Ferre, V.M.; Houhou, N.; Tarhini, H.; Bertin, C.; Beaumont, A.L.; Gare, M.; et al. Breakthrough Infections after Postexposure Vaccination against Mpox. N. Engl. J. Med. 2022, 387, 2477–2479. [Google Scholar] [CrossRef]
- Moss, B. Smallpox vaccines: Targets of protective immunity. Immunol. Rev. 2011, 239, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Sagdat, K.; Batyrkhan, A.; Kanayeva, D. Exploring monkeypox virus proteins and rapid detection techniques. Front. Cell. Infect. Microbiol. 2024, 14, 1414224. [Google Scholar] [CrossRef] [PubMed]
- Perdiguero, B.; Blasco, R. Interaction between vaccinia virus extracellular virus envelope A33 and B5 glycoproteins. J. Virol. 2006, 80, 8763–8777. [Google Scholar] [CrossRef]
- Roper, R.L.; Payne, L.G.; Moss, B. Extracellular vaccinia virus envelope glycoprotein encoded by the A33R gene. J. Virol. 1996, 70, 3753–3762. [Google Scholar] [CrossRef]
- Duncan, S.A.; Smith, G.L. Identification and characterization of an extracellular envelope glycoprotein affecting vaccinia virus egress. J. Virol. 1992, 66, 1610–1621. [Google Scholar] [CrossRef] [PubMed]
- Matho, M.H.; Schlossman, A.; Meng, X.; Benhnia, M.R.; Kaever, T.; Buller, M.; Doronin, K.; Parker, S.; Peters, B.; Crotty, S.; et al. Structural and Functional Characterization of Anti-A33 Antibodies Reveal a Potent Cross-Species Orthopoxviruses Neutralizer. PLoS Pathog. 2015, 11, e1005148. [Google Scholar] [CrossRef]
- Su, H.P.; Singh, K.; Gittis, A.G.; Garboczi, D.N. The structure of the poxvirus A33 protein reveals a dimer of unique C-type lectin-like domains. J. Virol. 2010, 84, 2502–2510. [Google Scholar] [CrossRef]
- Fang, Z.; Monteiro, V.S.; Renauer, P.A.; Shang, X.; Suzuki, K.; Ling, X.; Bai, M.; Xiang, Y.; Levchenko, A.; Booth, C.J.; et al. Polyvalent mRNA vaccination elicited potent immune response to monkeypox virus surface antigens. Cell Res. 2023, 33, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Yefet, R.; Friedel, N.; Tamir, H.; Polonsky, K.; Mor, M.; Hagin, D.; Sprecher, E.; Israely, T.; Freund, N.T. A35R and H3L are Serological and B Cell Markers for Monkeypox Infection. medRxiv 2022. [Google Scholar] [CrossRef]
- Song, S.; Ren, Z.; Chen, J.; Li, M.; Jiang, Y.; Liu, Y.; Zhang, B.; Lu, H.; Zhao, W.; Shen, C.; et al. Analysis of binding and authentic virus-neutralizing activities of immune sera induced by various monkeypox virus antigens. Immunol. Res. 2024, 72, 902–907. [Google Scholar] [CrossRef]
- Galmiche, M.C.; Goenaga, J.; Wittek, R.; Rindisbacher, L. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology 1999, 254, 71–80. [Google Scholar] [CrossRef]
- Freyn, A.W.; Atyeo, C.; Earl, P.L.; Americo, J.L.; Chuang, G.Y.; Natarajan, H.; Frey, T.R.; Gall, J.G.; Moliva, J.I.; Hunegnaw, R.; et al. An mpox virus mRNA-lipid nanoparticle vaccine confers protection against lethal orthopoxviral challenge. Sci. Transl. Med. 2023, 15, eadg3540. [Google Scholar] [CrossRef] [PubMed]
- Kontermann, R.E. Strategies for extended serum half-life of protein therapeutics. Curr. Opin. Biotechnol. 2011, 22, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Mekhaiel, D.N.; Czajkowsky, D.M.; Andersen, J.T.; Shi, J.; El-Faham, M.; Doenhoff, M.; McIntosh, R.S.; Sandlie, I.; He, J.; Hu, J.; et al. Polymeric human Fc-fusion proteins with modified effector functions. Sci. Rep. 2011, 1, 124. [Google Scholar] [CrossRef]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef]
- Loureiro, S.; Ren, J.; Phapugrangkul, P.; Colaco, C.A.; Bailey, C.R.; Shelton, H.; Molesti, E.; Temperton, N.J.; Barclay, W.S.; Jones, I.M. Adjuvant-free immunization with hemagglutinin-Fc fusion proteins as an approach to influenza vaccines. J. Virol. 2011, 85, 3010–3014. [Google Scholar] [CrossRef]
- Cheng, L.; Yang, L.; Wang, M.; Peng, Y.; Wang, H.; Yang, X.; Zhao, J.; Zhang, M.; Wang, F.; Zhang, Z. Isolation and characterization of mpox virus from the first mpox case in Shenzhen, China. Virol. Sin. 2024, 39, 335–337. [Google Scholar] [CrossRef]
- Ju, B.; Zhang, Q.; Ge, J.; Wang, R.; Sun, J.; Ge, X.; Yu, J.; Shan, S.; Zhou, B.; Song, S.; et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020, 584, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Moraes-Cardoso, I.; Benet, S.; Carabelli, J.; Perez-Zsolt, D.; Mendoza, A.; Rivero, A.; Alemany, A.; Descalzo, V.; Alarcón-Soto, Y.; Grifoni, A.; et al. Immune responses associated with mpox viral clearance in men with and without HIV in Spain: A multisite, observational, prospective cohort study. Lancet Microbe 2024, 5, 100859. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Earl, P.; Americo, J.; Damon, I.; Smith, S.K.; Yu, F.; Sebrell, A.; Emerson, S.; Cohen, G.; Eisenberg, R.J.; et al. Characterization of chimpanzee/human monoclonal antibodies to vaccinia virus A33 glycoprotein and its variola virus homolog in vitro and in a vaccinia virus mouse protection model. J. Virol. 2007, 81, 8989–8995. [Google Scholar] [CrossRef] [PubMed]
- Lustig, S.; Fogg, C.; Whitbeck, J.C.; Eisenberg, R.J.; Cohen, G.H.; Moss, B. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J. Virol. 2005, 79, 13454–13462. [Google Scholar] [CrossRef]
- Golden, J.W.; Hooper, J.W. Heterogeneity in the A33 protein impacts the cross-protective efficacy of a candidate smallpox DNA vaccine. Virology 2008, 377, 19–29. [Google Scholar] [CrossRef]
- Cohen, M.E.; Xiao, Y.; Eisenberg, R.J.; Cohen, G.H.; Isaacs, S.N. Antibody against extracellular vaccinia virus (EV) protects mice through complement and Fc receptors. PLoS ONE 2011, 6, e20597. [Google Scholar] [CrossRef]
- Mucker, E.M.; Freyn, A.W.; Bixler, S.L.; Cizmeci, D.; Atyeo, C.; Earl, P.L.; Natarajan, H.; Santos, G.; Frey, T.R.; Levin, R.H.; et al. Comparison of protection against mpox following mRNA or modified vaccinia Ankara vaccination in nonhuman primates. Cell 2024, 187, 5540–5553.e5510. [Google Scholar] [CrossRef] [PubMed]
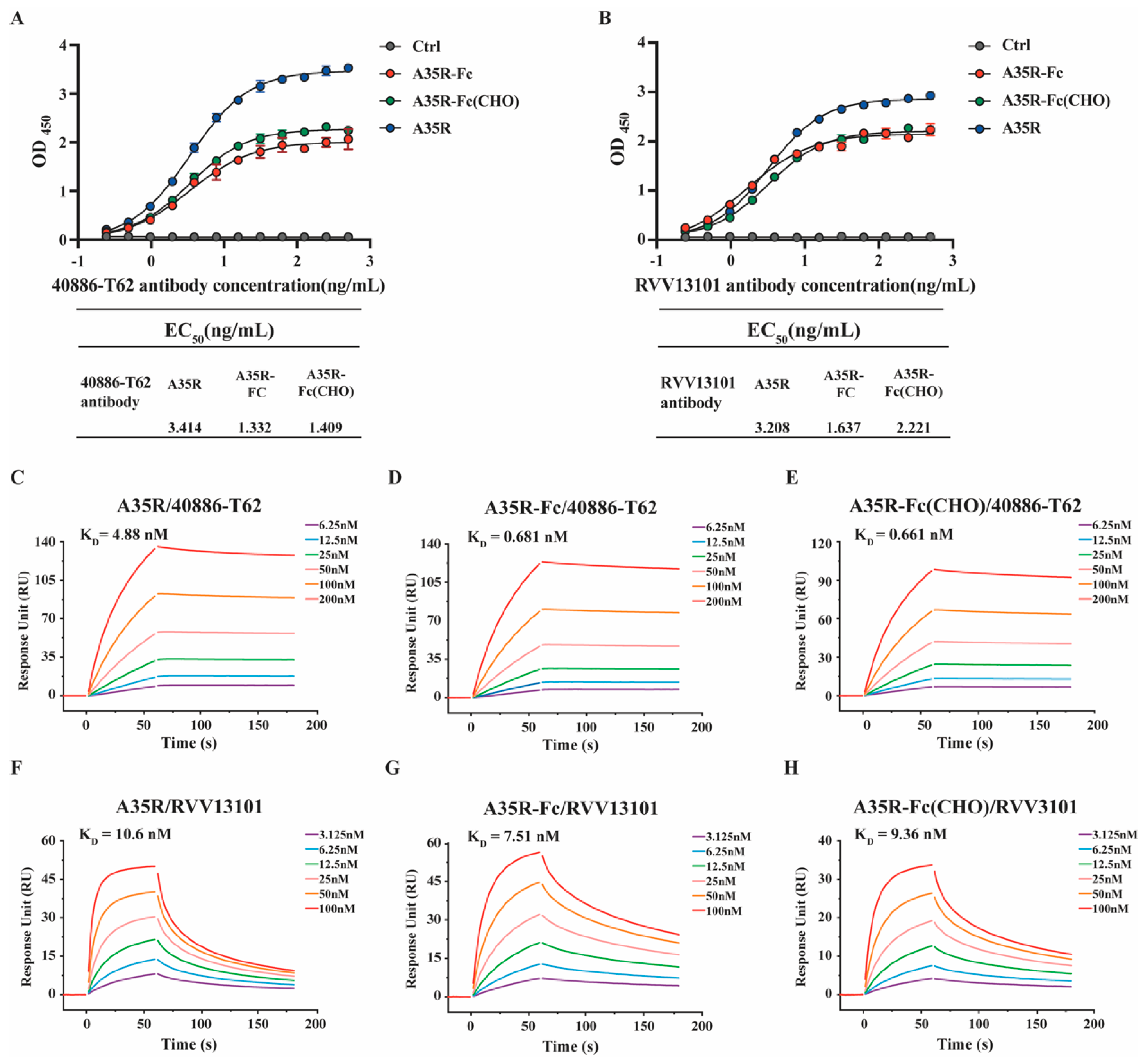

| KD (M) | Ka (1/Ms) | Kd (1/s) | ꭓ2 | |
|---|---|---|---|---|
| A35R | ||||
| 40886-T62 | 4.88 × 10−9 | 1.66 × 10−5 | 8.10 × 10−4 | 1.890 |
| RVV13101 | 1.06 × 10−8 | 5.81 × 10−6 | 6.15 × 10−2 | 3.150 |
| A35R-Fc | ||||
| 40886-T62 | 6.81 × 10−10 | 1.25 × 10−5 | 8.54 × 10−5 | 0.949 |
| RVV13101 | 7.51 × 10−9 | 9.91 × 10−5 | 7.45 × 10−3 | 2.140 |
| A35R-Fc (CHO) | ||||
| 40886-T62 | 6.61 × 10−10 | 1.62 × 10−5 | 1.07 × 10−4 | 1.410 |
| RVV13101 | 9.36 × 10−9 | 2.01 × 10−6 | 1.88 × 10−2 | 1.210 |
| RU (Mean, ±SD) | |
|---|---|
| A35R | |
| MPXV | 74.61, 44.79 |
| MPXV + HIV | 46.01, 34.13 |
| Negative | 0, 0 |
| A35R-Fc | |
| MPXV | 73.86, 43.74 |
| MPXV + HIV | 46.11, 32.81 |
| Negative | 0, 0 |
| A35R-Fc (CHO) | |
| MPXV | 42.45, 27.74 |
| MPXV + HIV | 22.52, 18.58 |
| Negative | 0, 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, S.; Cui, Y.; Liao, Q.; Yi, H.; Liao, Z.; Zhang, G.; Wu, F.; Lu, H. Enhanced Immunogenicity and Affinity with A35R-Fc-Based Chimeric Protein Compared to MPXV A35R Protein. Viruses 2025, 17, 116. https://doi.org/10.3390/v17010116
Bai S, Cui Y, Liao Q, Yi H, Liao Z, Zhang G, Wu F, Lu H. Enhanced Immunogenicity and Affinity with A35R-Fc-Based Chimeric Protein Compared to MPXV A35R Protein. Viruses. 2025; 17(1):116. https://doi.org/10.3390/v17010116
Chicago/Turabian StyleBai, Shimeng, Yanxin Cui, Qibin Liao, Hongyang Yi, Zhonghui Liao, Gengwei Zhang, Fenfang Wu, and Hongzhou Lu. 2025. "Enhanced Immunogenicity and Affinity with A35R-Fc-Based Chimeric Protein Compared to MPXV A35R Protein" Viruses 17, no. 1: 116. https://doi.org/10.3390/v17010116
APA StyleBai, S., Cui, Y., Liao, Q., Yi, H., Liao, Z., Zhang, G., Wu, F., & Lu, H. (2025). Enhanced Immunogenicity and Affinity with A35R-Fc-Based Chimeric Protein Compared to MPXV A35R Protein. Viruses, 17(1), 116. https://doi.org/10.3390/v17010116







