Repurposing Drugs for Synergistic Combination Therapies to Counteract Monkeypox Virus Tecovirimat Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Viruses
2.2. Compounds
2.3. Determination of Compound EC50
2.4. Determination of Compound CC50
2.5. Drug Combination Assays in VACV-Infected Cells
2.6. Drug Combination Assays in MPXV-Infected Cells
3. Results
3.1. Dose–Response Inhibitory Effect of Selected Drugs on VACV Multiplication
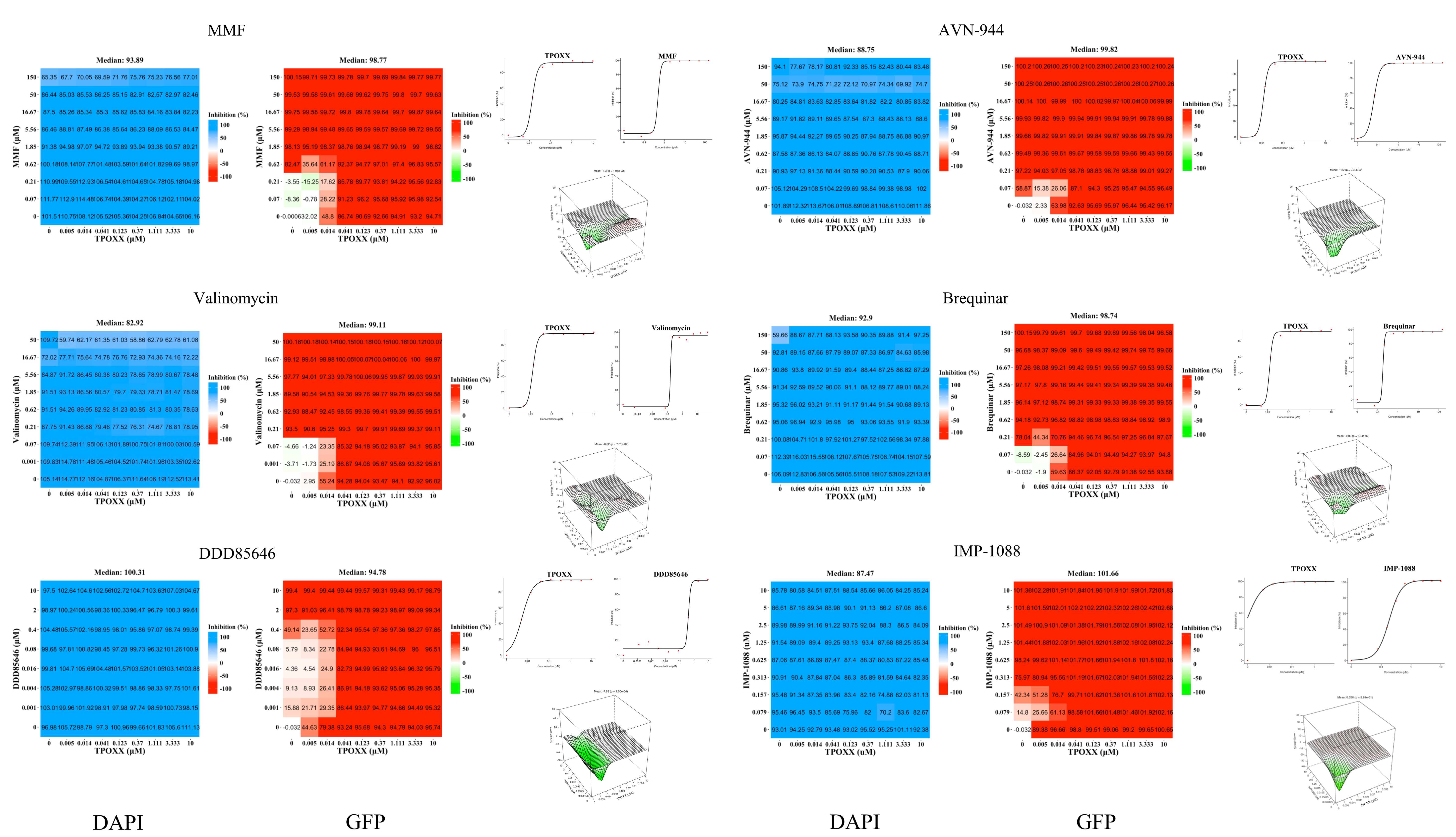
3.2. Assessment of Synergistic Anti-VACV Activity of Selected Drugs in Combination with TPOXX
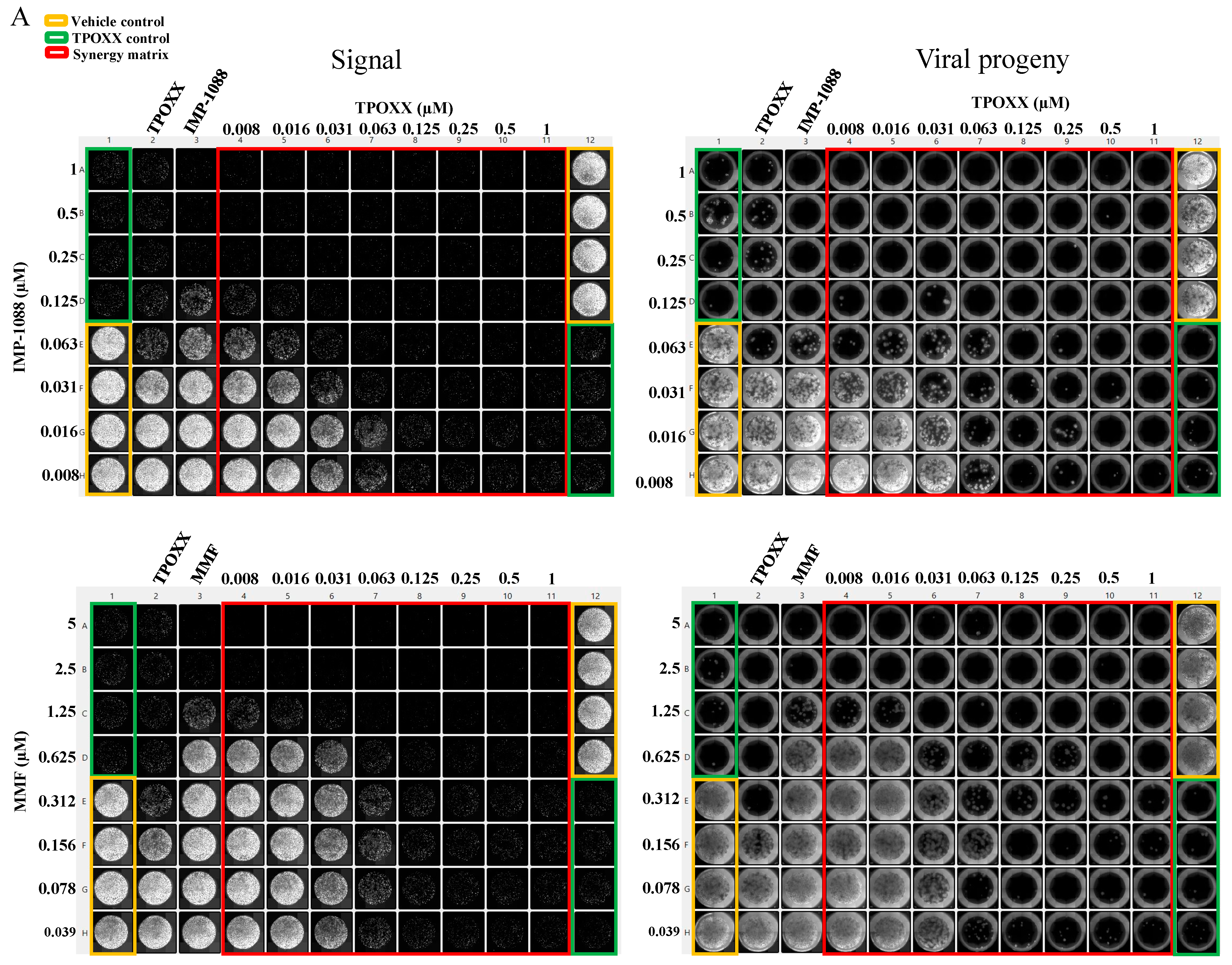
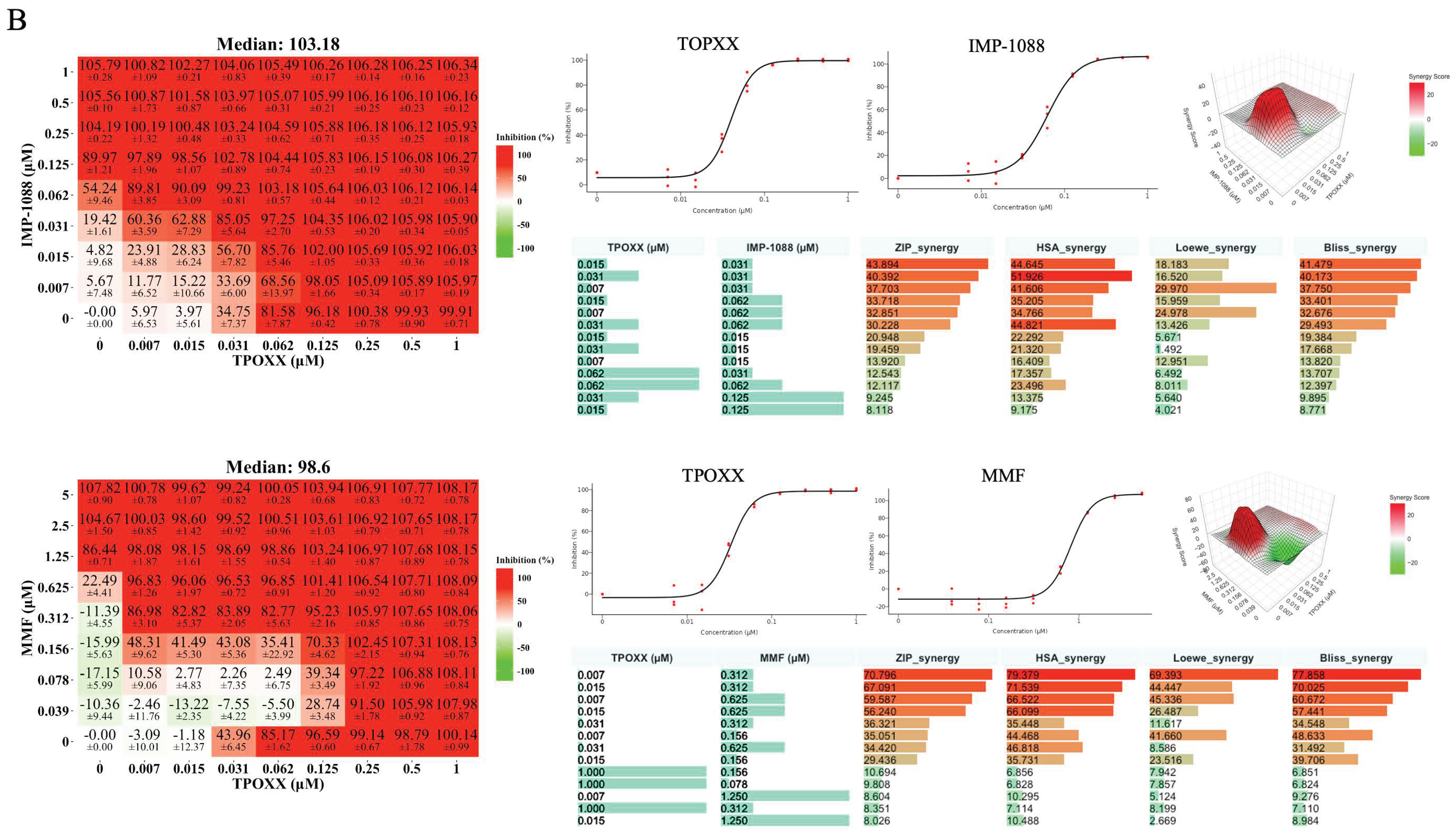
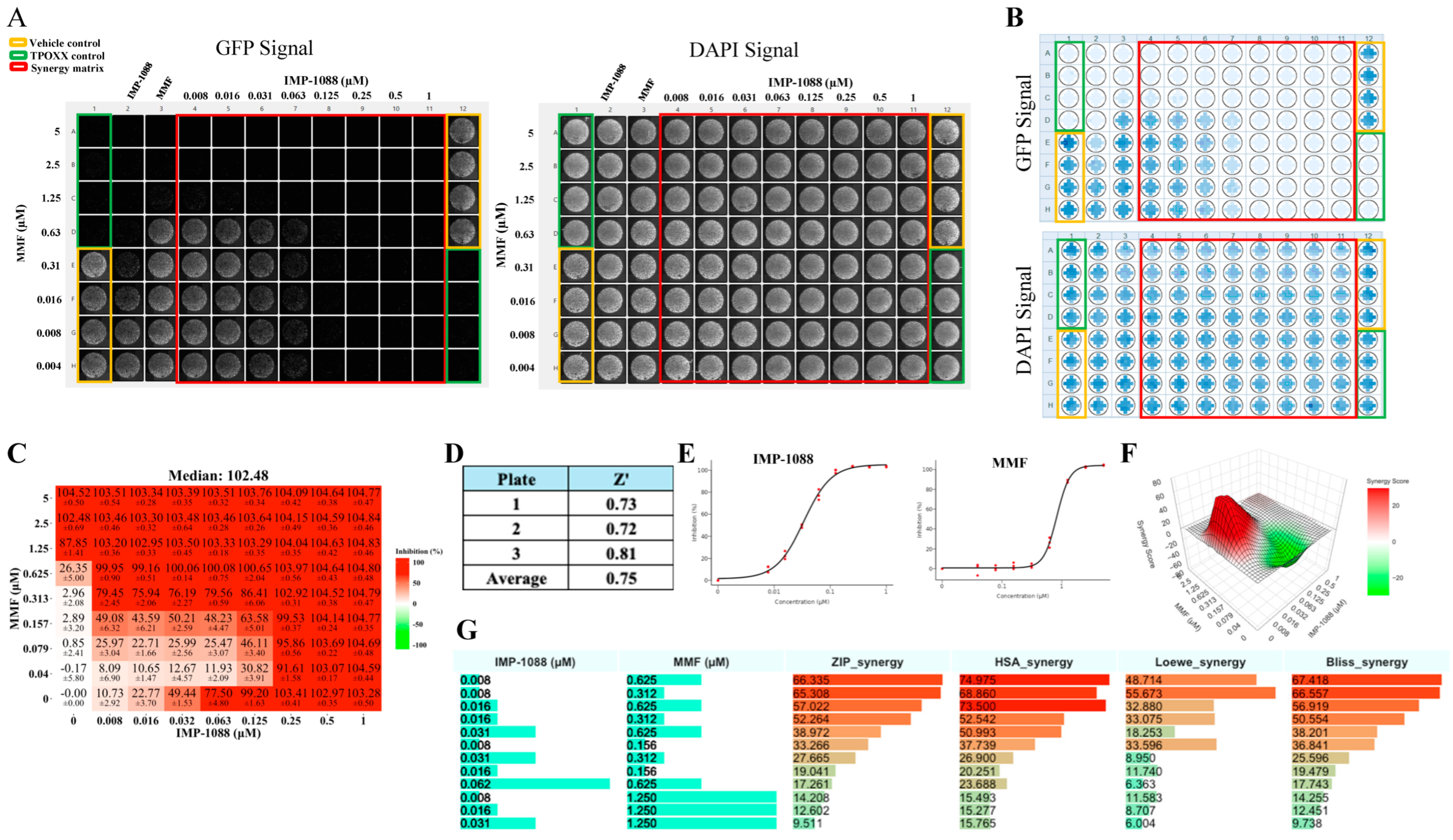
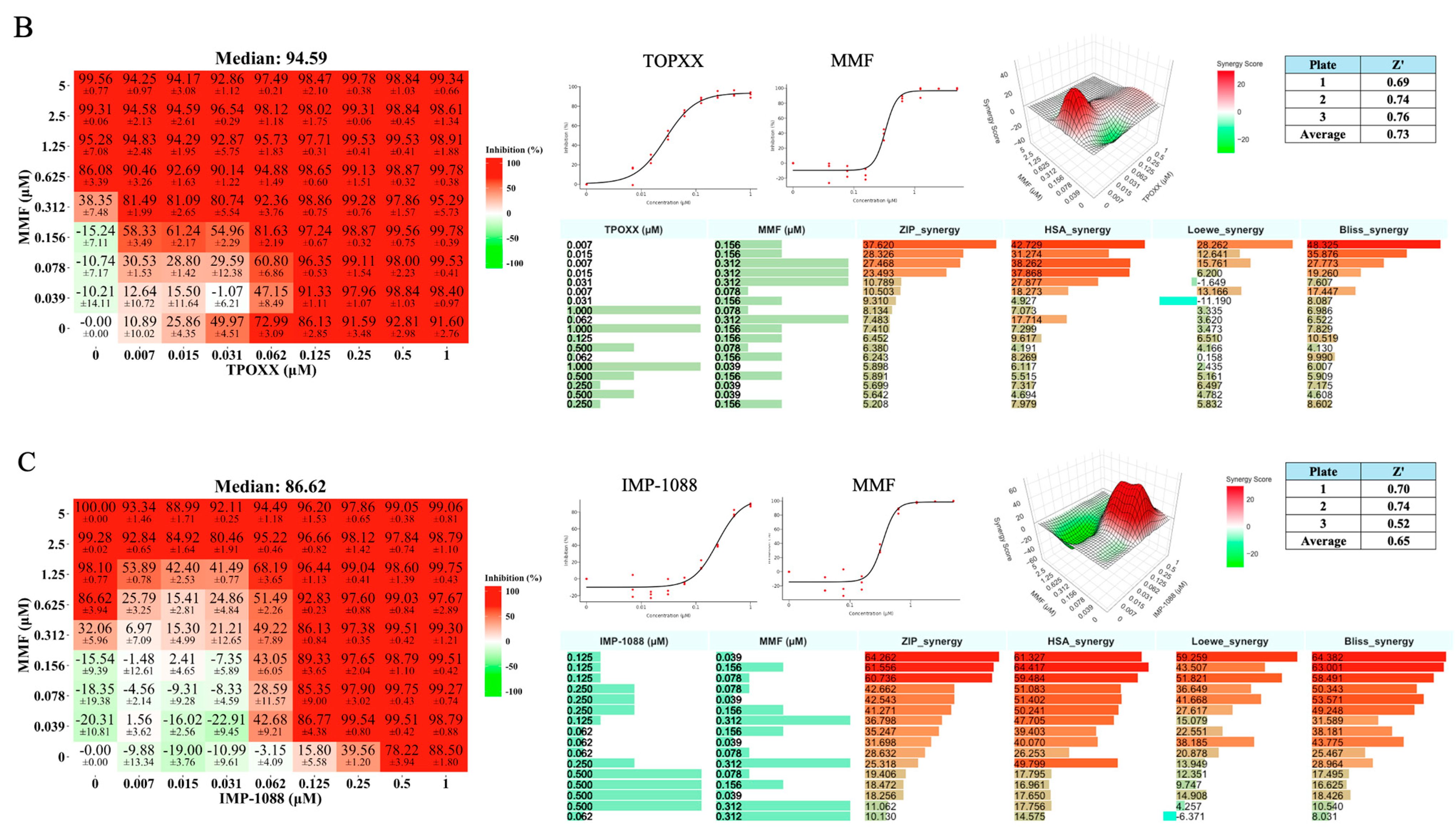
3.3. Detailed Assessment of the Synergistic Anti-VACV Activity of MMF and IMP-1088 in Combination with TPOXX
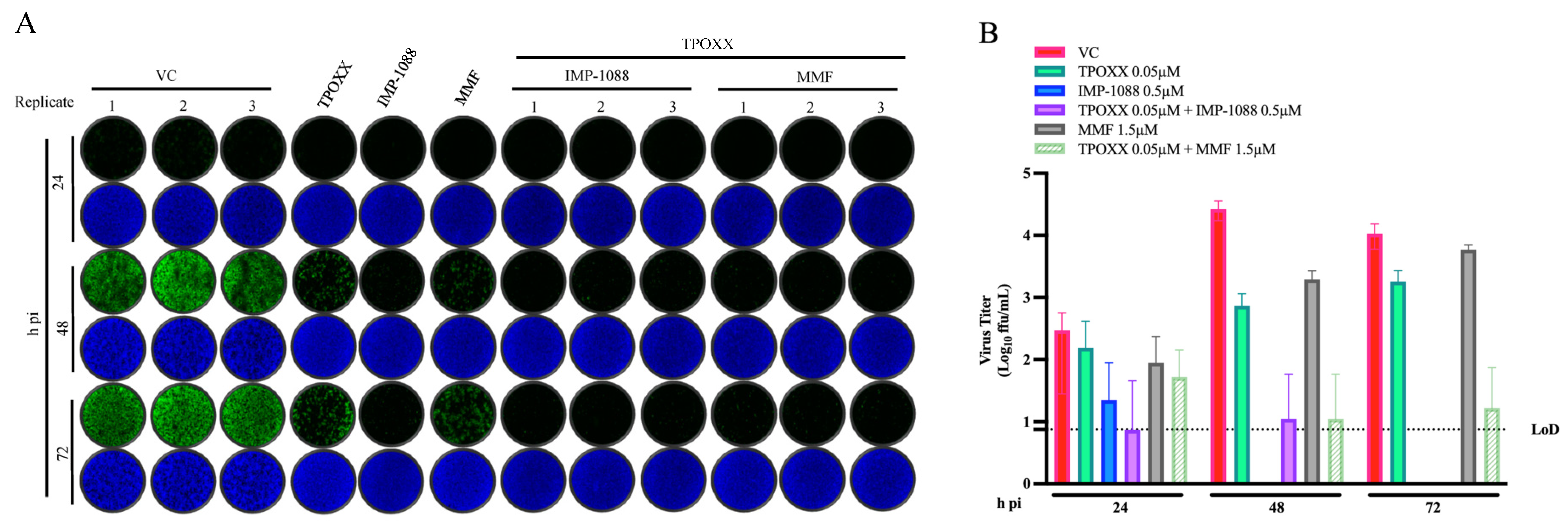
3.4. Effect of Combination Therapy on VACV Multi-Step Growth Kinetics
3.5. Effect of IMP-1088 and MMF Combination Therapy on VACV Infection
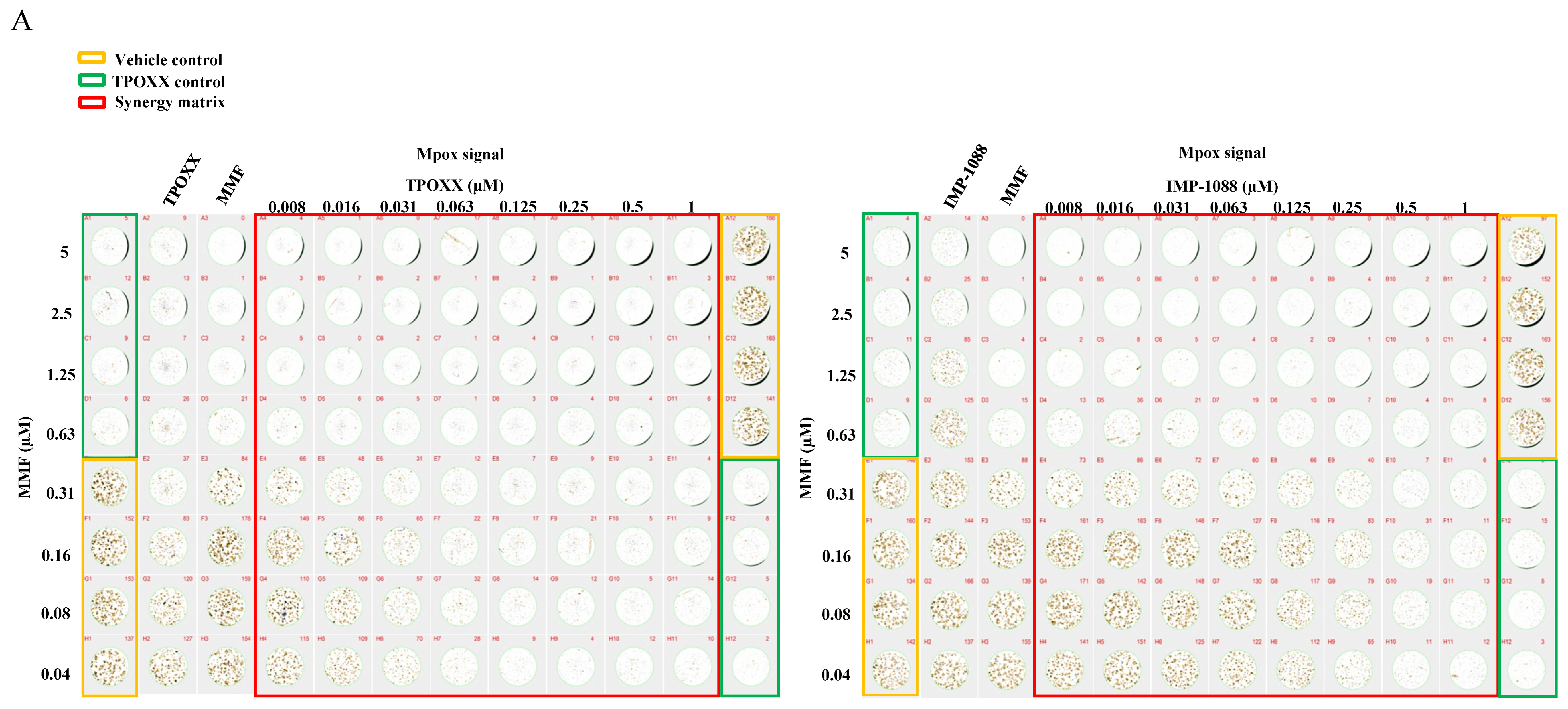
3.6. Effect of Combination Therapy on MPXV Infection
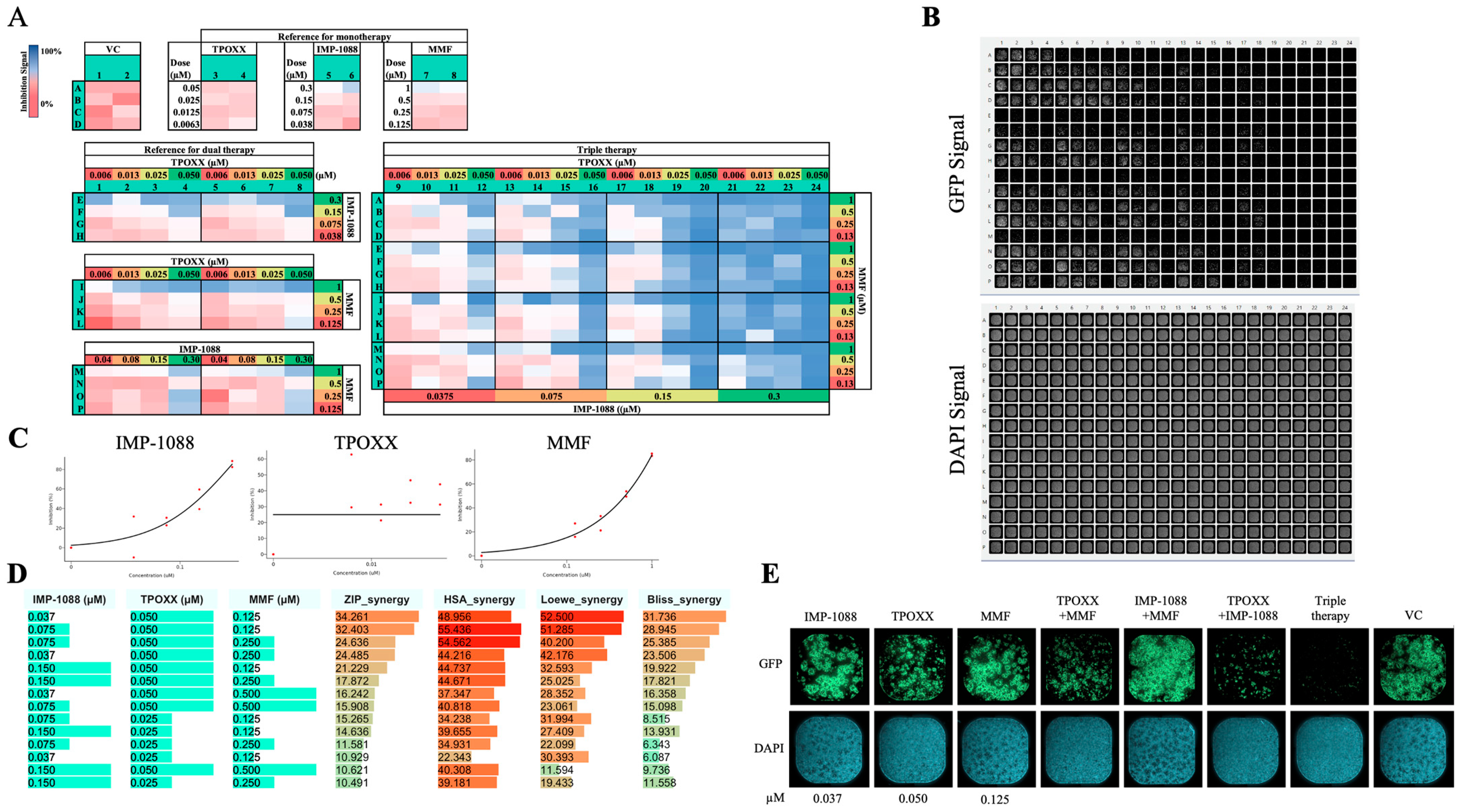
3.7. Triple Therapy in VACV
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The Changing Epidemiology of Human Monkeypox—A Potential Threat? A Systematic Review. PLoS Neglected Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 2022-24 Mpox Outbreak: Global Trends; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Wensing, A.M.; Calvez, V.; Ceccherini-Silberstein, F.; Charpentier, C.; Günthard, H.F.; Paredes, R.; Shafer, R.W.; Richman, D.D. 2022 Update of the Drug Resistance Mutations in HIV-1. Top. Antivir. Med. 2022, 30, 559–574. [Google Scholar] [PubMed]
- Boden, D.; Hurley, A.; Zhang, L.; Cao, Y.; Guo, Y.; Jones, E.; Tsay, J.; Ip, J.; Farthing, C.; Limoli, K.; et al. HIV-1 Drug Resistance in Newly Infected Individuals. JAMA 1999, 282, 1135–1141. [Google Scholar] [CrossRef]
- Duan, Y.; Zhou, H.; Liu, X.; Iketani, S.; Lin, M.; Zhang, X.; Bian, Q.; Wang, H.; Sun, H.; Hong, S.J.; et al. Molecular Mechanisms of SARS-CoV-2 Resistance to Nirmatrelvir. Nature 2023, 622, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Wyles, D.L.; Luetkemeyer, A.F. Understanding Hepatitis C Virus Drug Resistance: Clinical Implications for Current and Future Regimens. Top. Antivir. Med. 2017, 25, 103–109. [Google Scholar]
- Duan, S.; Govorkova, E.A.; Bahl, J.; Zaraket, H.; Baranovich, T.; Seiler, P.; Prevost, K.; Webster, R.G.; Webby, R.J. Epistatic Interactions between Neuraminidase Mutations Facilitated the Emergence of the Oseltamivir-Resistant H1N1 Influenza Viruses. Nat. Commun. 2014, 5, 5029. [Google Scholar] [CrossRef]
- Staff, G.B. Disappointing Results for Tecovirimat Mpox Trial in the DRC. Global Biodefense. 2024. Available online: https://globalbiodefense.com/2024/08/15/disappointing-results-for-tecovirimat-mpox-trial-in-the-drc/#:~:text=SIGA's%20antiviral%20drug%20tecovirimat%20did,randomized%2C%20placebo%2Dcontrolled%20trial (accessed on 2024).
- Lenharo, M. Hopes Dashed for Drug Aimed at Monkeypox Virus Spreading in Africa. Nature 2024, 632, 965. [Google Scholar] [CrossRef]
- NIH Study Finds Tecovirimat Was Safe but Did Not Improve Mpox Resolution or Pain. Available online: https://www.nih.gov/news-events/news-releases/nih-study-finds-tecovirimat-was-safe-did-not-improve-mpox-resolution-or-pain (accessed on 10 December 2024).
- Smith, T.G.; Gigante, C.M.; Wynn, N.T.; Matheny, A.; Davidson, W.; Yang, Y.; Condori, R.E.; O’Connell, K.; Kovar, L.; Williams, T.L.; et al. Tecovirimat Resistance in Mpox Patients, United States, 2022–2023. Emerg. Infect. Dis. J. CDC 2023, 29, 2426–2432. [Google Scholar] [CrossRef]
- Moss, B. Poxvirus DNA Replication. Cold Spring Harb. Perspect. Biol. 2013, 5, a010199. [Google Scholar] [CrossRef]
- Smith, G.L.; Vanderplasschen, A.; Law, M. The Formation and Function of Extracellular Enveloped Vaccinia Virus. J. Gen. Virol. 2002, 83, 2915–2931. [Google Scholar] [CrossRef] [PubMed]
- Honeychurch, K.M.; Yang, G.; Jordan, R.; Hruby, D.E. The Vaccinia Virus F13L YPPL Motif Is Required for Efficient Release of Extracellular Enveloped Virus. J. Virol. 2007, 81, 7310–7315. [Google Scholar] [CrossRef]
- Yang, G.; Pevear, D.C.; Davies, M.H.; Collett, M.S.; Bailey, T.; Rippen, S.; Barone, L.; Burns, C.; Rhodes, G.; Tohan, S.; et al. An Orally Bioavailable Antipoxvirus Compound (ST-246) Inhibits Extracellular Virus Formation and Protects Mice from Lethal Orthopoxvirus Challenge. J. Virol. 2005, 79, 13139–13149. [Google Scholar] [CrossRef] [PubMed]
- Blasco, R.; Moss, B. Role of Cell-Associated Enveloped Vaccinia Virus in Cell-to-Cell Spread. J. Virol. 1992, 66, 4170–4179. [Google Scholar] [CrossRef] [PubMed]
- Bryk, P.; Brewer, M.G.; Ward, B.M. Vaccinia Virus Phospholipase Protein F13 Promotes Rapid Entry of Extracellular Virions into Cells. J. Virol. 2018, 92, e02154-17. [Google Scholar] [CrossRef]
- Blasco, R.; Moss, B. Extracellular Vaccinia Virus Formation and Cell-to-Cell Virus Transmission Are Prevented by Deletion of the Gene Encoding the 37,000-Dalton Outer Envelope Protein. J. Virol. 1991, 65, 5910–5920. [Google Scholar] [CrossRef]
- Verardi, P.H.; Titong, A.; Hagen, C.J. A Vaccinia Virus Renaissance. Hum. Vaccines Immunother. 2012, 8, 961–970. [Google Scholar] [CrossRef]
- Chiem, K.; Nogales, A.; Lorenzo, M.; Morales Vasquez, D.; Xiang, Y.; Gupta, Y.K.; Blasco, R.; de la Torre, J.C.; Martínez-Sobrido, L. Identification of In Vitro Inhibitors of Monkeypox Replication. Microbiol. Spectr. 2023, 11, e04745-22. [Google Scholar] [CrossRef]
- Duraffour, S.; Lorenzo, M.M.; Zöller, G.; Topalis, D.; Grosenbach, D.; Hruby, D.E.; Andrei, G.; Blasco, R.; Meyer, H.; Snoeck, R. ST-246 Is a Key Antiviral to Inhibit the Viral F13L Phospholipase, One of the Essential Proteins for Orthopoxvirus Wrapping. J. Antimicrob. Chemother. 2015, 70, 1367–1380. [Google Scholar] [CrossRef]
- Garrigues, J.M.; Hemarajata, P.; Espinosa, A.; Hacker, J.K.; Wynn, N.T.; Smith, T.G.; Gigante, C.M.; Davidson, W.; Vega, J.; Edmondson, H.; et al. Community Spread of a Human Monkeypox Virus Variant with a Tecovirimat Resistance-Associated Mutation. Antimicrob. Agents Chemother. 2023, 67, e00972-23. [Google Scholar] [CrossRef]
- Garrigues, J.M.; Hemarajata, P.; Karan, A.; Shah, N.K.; Alarcón, J.; Marutani, A.N.; Finn, L.; Smith, T.G.; Gigante, C.M.; Davidson, W.; et al. Identification of Tecovirimat Resistance-Associated Mutations in Human Monkeypox Virus—Los Angeles County. Antimicrob. Agents Chemother. 2023, 67, e00568-23. [Google Scholar] [CrossRef] [PubMed]
- Vakaniaki, E.H.; Kacita, C.; Kinganda-Lusamaki, E.; O’Toole, Á.; Wawina-Bokalanga, T.; Mukadi-Bamuleka, D.; Amuri-Aziza, A.; Malyamungu-Bubala, N.; Mweshi-Kumbana, F.; Mutimbwa-Mambo, L.; et al. Sustained Human Outbreak of a New MPXV Clade I Lineage in Eastern Democratic Republic of the Congo. Nat. Med. 2024, 30, 2791–2795. [Google Scholar] [CrossRef] [PubMed]
- Masirika, L.M.; Udahemuka, J.C.; Schuele, L.; Ndishimye, P.; Otani, S.; Mbiribindi, J.B.; Marekani, J.M.; Mambo, L.M.; Bubala, N.M.; Boter, M.; et al. Ongoing Mpox Outbreak in Kamituga, South Kivu Province, Associated with Monkeypox Virus of a Novel Clade I Sub-Lineage, Democratic Republic of the Congo, 2024. Eurosurveillance 2024, 29, 2400106. [Google Scholar] [CrossRef]
- Mpox—Sweden. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON531 (accessed on 31 August 2024).
- Ett fall av mpox klad 1 rapporterat i Sverige. Available online: https://www.folkhalsomyndigheten.se/nyheter-och-press/nyhetsarkiv/2024/augusti/ett-fall-av-mpox-klad-i-rapporterat-i-sverige/ (accessed on 31 August 2024).
- Ramljak, I.C.; Stanger, J.; Real-Hohn, A.; Dreier, D.; Wimmer, L.; Redlberger-Fritz, M.; Fischl, W.; Klingel, K.; Mihovilovic, M.D.; Blaas, D.; et al. Cellular N-Myristoyltransferases Play a Crucial Picornavirus Genus-Specific Role in Viral Assembly, Virion Maturation, and Infectivity. PLoS Pathog. 2018, 14, e1007203. [Google Scholar] [CrossRef]
- Witwit, H.; Betancourt, C.A.; Cubitt, B.; Khafaji, R.; Kowalski, H.; Jackson, N.; Ye, C.; Martinez-Sobrido, L.; de la Torre, J.C. Cellular N-Myristoyl Transferases Are Required for Mammarenavirus Multiplication. Viruses 2024, 16, 1362. [Google Scholar] [CrossRef]
- Priyamvada, L.; Kallemeijn, W.W.; Faronato, M.; Wilkins, K.; Goldsmith, C.S.; Cotter, C.A.; Ojeda, S.; Solari, R.; Moss, B.; Tate, E.W.; et al. Inhibition of Vaccinia Virus L1 N-Myristoylation by the Host N-Myristoyltransferase Inhibitor IMP-1088 Generates Non-Infectious Virions Defective in Cell Entry. PLoS Pathog. 2022, 18, e1010662. [Google Scholar] [CrossRef]
- Chiem, K.; Lorenzo, M.M.; Rangel-Moreno, J.; Garcia-Hernandez, M.D.L.L.; Park, J.-G.; Nogales, A.; Blasco, R.; Martínez-Sobrido, L. Bi-Reporter Vaccinia Virus for Tracking Viral Infections In Vitro and In Vivo. Microbiol. Spectr. 2021, 9, e01601-21. [Google Scholar] [CrossRef]
- Lorenzo, M.M.; Sánchez-Puig, J.M.; Blasco, R. Genes A27L and F13L as Genetic Markers for the Isolation of Recombinant Vaccinia Virus. Sci. Rep. 2019, 9, 15684. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Cubitt, B.; Chen, E.; Hull, M.V.; Chatterjee, A.K.; Cai, Y.; Kuhn, J.H.; de la Torre, J.C. The ReFRAME Library as a Comprehensive Drug Repurposing Library to Identify Mammarenavirus Inhibitors. Antivir. Res. 2019, 169, 104558. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, W.; Aldahdooh, J.; Malyutina, A.; Shadbahr, T.; Tanoli, Z.; Pessia, A.; Tang, J. SynergyFinder Plus: Toward Better Interpretation and Annotation of Drug Combination Screening Datasets. Genom. Proteom. Bioinform. 2022, 20, 587–596. [Google Scholar] [CrossRef]
- Allison, A.C.; Eugui, E.M. Mechanisms of Action of Mycophenolate Mofetil in Preventing Acute and Chronic Allograft Rejection. Transplantation 2005, 80, S181. [Google Scholar] [CrossRef] [PubMed]
- Sajgure, A.; Kulkarni, A.; Joshi, A.; Sajgure, V.; Pathak, V.; Melinkeri, R.; Pathak, S.; Agrawal, S.; Naik, M.; Rajurkar, M.; et al. Safety and Efficacy of Mycophenolate in COVID-19: A Nonrandomised Prospective Study in Western India. Lancet Reg. Health Southeast Asia 2023, 11, 100154. [Google Scholar] [CrossRef] [PubMed]
- Neyts, J.; Andrei, G.; De Clercq, E. The Novel Immunosuppressive Agent Mycophenolate Mofetil Markedly Potentiates the Antiherpesvirus Activities of Acyclovir, Ganciclovir, and Penciclovir In Vitro and In Vivo. Antimicrob. Agents Chemother. 1998, 42, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Yi, H.; Jang, E.Y.; Lee, M.-S.; Lee, J.-Y.; Kang, C.; Lee, C.H.; Kim, K. Mycophenolic Mofetil, an Alternative Antiviral and Immunomodulator for the Highly Pathogenic Avian Influenza H5N1 Virus Infection. Biochem. Biophys. Res. Commun. 2017, 494, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Boyle, D.B.; Coupar, B.E.H. A Dominant Selectable Marker for the Construction of Recombinant Poxviruses. Gene 1988, 65, 123–128. [Google Scholar] [CrossRef]
- Falkner, F.G.; Moss, B. Escherichia Coli Gpt Gene Provides Dominant Selection for Vaccinia Virus Open Reading Frame Expression Vectors. J. Virol. 1988, 62, 1849–1854. [Google Scholar] [CrossRef]
- Strovel, J.W.; Jain, J.; Natarajan, P.; Lawrence, T.; Castaneda, J.; Chakiath, M.; Zuck, K.; Harding, M.W.; Kelliher, K.; Shames, B.; et al. Global Gene Expression Effects of AVN-944, a Novel Small Molecule Inhibitor of Inosine Monophosphate Dehydrogenase (IMPDH). Cancer Res. 2006, 66, 736. [Google Scholar]
- Cuthbertson, C.R.; Guo, H.; Kyani, A.; Madak, J.T.; Arabzada, Z.; Neamati, N. The Dihydroorotate Dehydrogenase Inhibitor Brequinar Is Synergistic with ENT1/2 Inhibitors. ACS Pharmacol. Transl. Sci. 2020, 3, 1242–1252. [Google Scholar] [CrossRef]
- Huang, S.; Liu, Y.; Liu, W.-Q.; Neubauer, P.; Li, J. The Nonribosomal Peptide Valinomycin: From Discovery to Bioactivity and Biosynthesis. Microorganisms 2021, 9, 780. [Google Scholar] [CrossRef]
- Lee, T.X.; Packer, M.D.; Huang, J.; Akhmametyeva, E.M.; Kulp, S.K.; Chen, C.-S.; Giovannini, M.; Jacob, A.; Welling, D.B.; Chang, L.-S. Growth Inhibitory and Anti-Tumour Activities of OSU-03012, a Novel PDK-1 Inhibitor, on Vestibular Schwannoma and Malignant Schwannoma Cells. Eur. J. Cancer 2009, 45, 1709–1720. [Google Scholar] [CrossRef]
- Zhang, S.; Suvannasankha, A.; Crean, C.D.; White, V.L.; Johnson, A.; Chen, C.-S.; Farag, S.S. OSU-03012, a Novel Celecoxib Derivative, Is Cytotoxic to Myeloma Cells and Acts through Multiple Mechanisms. Clin. Cancer Res. 2007, 13, 4750–4758. [Google Scholar] [CrossRef] [PubMed]
- Rayner, J.O.; Roberts, R.A.; Kim, J.; Poklepovic, A.; Roberts, J.L.; Booth, L.; Dent, P. AR12 (OSU-03012) Suppresses GRP78 Expression and Inhibits SARS-CoV-2 Replication. Biochem. Pharmacol. 2020, 182, 114227. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Aguado-Martínez, A.; Manser, V.; Wong, H.N.; Haynes, R.K.; Hemphill, A. Repurposing of Antiparasitic Drugs: The Hydroxy-Naphthoquinone Buparvaquone Inhibits Vertical Transmission in the Pregnant Neosporosis Mouse Model. Vet. Res. 2016, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.M.; Löbenberg, R.; Barbosa, E.J.; de Araujo, G.L.B.; Sato, P.K.; Kanashiro, E.; de Araujo Eliodoro, R.H.; Rocha, M.; de Freitas, V.L.T.; Fotaki, N.; et al. Oral Administration of Buparvaquone Nanostructured Lipid Carrier Enables in Vivo Activity against Leishmania infantum. Eur. J. Pharm. Sci. 2022, 169, 106097. [Google Scholar] [CrossRef]
- Pacylex Pharmaceuticals Announces First Patient Dosed in a Phase 2a Study of PCLX-001 in Patients with Relapsed/Refractory Non-Hodgkin Lymphoma. Available online: https://www.pacylex.com/post/pacylex-pharmaceuticals-announces-first-patient-dosed-in-a-phase-2a-study-of-pclx-001-in-patients-with-relapsed-refractory-non-hodgkin-lymphoma (accessed on 5 November 2024).
- Sangha, R.S.; Jamal, R.; Spratlin, J.L.; Kuruvilla, J.; Sehn, L.H.; Weickert, M.; Berthiaume, L.G.; Mackey, J.R. A First-in-Human, Open-Label, Phase I Trial of Daily Oral PCLX-001, an NMT Inhibitor, in Patients with Relapsed/Refractory B-Cell Lymphomas and Advanced Solid Tumors. JCO 2023, 41, e15094. [Google Scholar] [CrossRef]
- Sangha, R.; Davies, N.M.; Namdar, A.; Chu, M.; Spratlin, J.; Beauchamp, E.; Berthiaume, L.G.; Mackey, J.R. Novel, First-in-Human, Oral PCLX-001 Treatment in a Patient with Relapsed Diffuse Large B-Cell Lymphoma. Curr. Oncol. 2022, 29, 1939–1946. [Google Scholar] [CrossRef]
- Hishiki, T.; Morita, T.; Akazawa, D.; Ohashi, H.; Park, E.-S.; Kataoka, M.; Mifune, J.; Shionoya, K.; Tsuchimoto, K.; Ojima, S.; et al. Identification of IMP Dehydrogenase as a Potential Target for Anti-Mpox Virus Agents. Microbiol. Spectr. 2023, 11, e00566-23. [Google Scholar] [CrossRef]
- Cellcept. 2024. Available online: https://www.gene.com/download/pdf/cellcept_prescribing.pdf (accessed on 2024).
- Prussick, L.; Plotnikova, N.; Gottlieb, A. Mycophenolate Mofetil in Severe Atopic Dermatitis: A Review. JDDonline J. Drugs Dermatol. 2016, 15, 715–718. [Google Scholar]
- Mycophenolate. Available online: https://www.versusarthritis.org/about-arthritis/treatments/drugs/mycophenolate/ (accessed on 12 November 2024).
- He, X.; Smeets, R.L.; Koenen, H.J.P.M.; Vink, P.M.; Wagenaars, J.; Boots, A.M.H.; Joosten, I. Mycophenolic Acid-Mediated Suppression of Human CD4+ T Cells: More than Mere Guanine Nucleotide Deprivation. Am. J. Transplant. 2011, 11, 439–449. [Google Scholar] [CrossRef]
- Eugui, E.M.; Almquist, S.J.; Muller, C.D.; Allison, A.C. Lymphocyte-selective cytostatic and immunosuppressive effects of mycophenolic acid in vitro: Role of deoxyguanosine nucleotide depletion. Scand. J. Immunol. 1991, 33, 161–173. [Google Scholar] [CrossRef]
- Bologna, G.; Yvon, C.; Duvaud, S.; Veuthey, A.-L. N-Terminal Myristoylation Predictions by Ensembles of Neural Networks. Proteomics 2004, 4, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- NMT—The Predictor. Available online: https://mendel.imp.ac.at/myristate/SUPLpredictor.htm (accessed on 20 December 2024).
- Martin, K.H.; Grosenbach, D.W.; Franke, C.A.; Hruby, D.E. Identification and Analysis of Three Myristylated Vaccinia Virus Late Proteins. J. Virol. 1997, 71, 5218–5226. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, S.; Domi, A.; Moss, B. Vaccinia Virus G9 Protein Is an Essential Component of the Poxvirus Entry-Fusion Complex. J. Virol. 2006, 80, 9822–9830. [Google Scholar] [CrossRef]
- Ojeda, S.; Senkevich, T.G.; Moss, B. Entry of Vaccinia Virus and Cell-Cell Fusion Require a Highly Conserved Cysteine-Rich Membrane Protein Encoded by the A16L Gene. J. Virol. 2006, 80, 51–61. [Google Scholar] [CrossRef]
- Ravanello, M.P.; Hruby, D.E. Conditional Lethal Expression of the Vaccinia Virus L1R Myristylated Protein Reveals a Role in Virion Assembly. J. Virol. 1994, 68, 6401–6410. [Google Scholar] [CrossRef]
- Bisht, H.; Weisberg, A.S.; Moss, B. Vaccinia Virus L1 Protein Is Required for Cell Entry and Membrane Fusion. J. Virol. 2008, 82, 8687–8694. [Google Scholar] [CrossRef]
- Rivas, C.; Gil, J.; Mĕlková, Z.; Esteban, M.; Díaz-Guerra, M. Vaccinia Virus E3L Protein Is an Inhibitor of the Interferon (i.f.n.)-Induced 2-5A Synthetase Enzyme. Virology 1998, 243, 406–414. [Google Scholar] [CrossRef]
- Chang, H.W.; Watson, J.C.; Jacobs, B.L. The E3L Gene of Vaccinia Virus Encodes an Inhibitor of the Interferon-Induced, Double-Stranded RNA-Dependent Protein Kinase. Proc. Natl. Acad. Sci. USA 1992, 89, 4825–4829. [Google Scholar] [CrossRef]
- Liu, S.-W.; Katsafanas, G.C.; Liu, R.; Wyatt, L.S.; Moss, B. Poxvirus Decapping Enzymes Enhance Virulence by Preventing the Accumulation of dsRNA and the Induction of Innate Antiviral Responses. Cell Host Microbe 2015, 17, 320–331. [Google Scholar] [CrossRef]
- Gargan, S.; Ahmed, S.; Mahony, R.; Bannan, C.; Napoletano, S.; O’Farrelly, C.; Borrow, P.; Bergin, C.; Stevenson, N.J. HIV-1 Promotes the Degradation of Components of the Type 1 IFN JAK/STAT Pathway and Blocks Anti-Viral ISG Induction. EBioMedicine 2018, 30, 203–216. [Google Scholar] [CrossRef]
- Garaigorta, U.; Chisari, F.V. Hepatitis C Virus Blocks Interferon Effector Function by Inducing Protein Kinase R Phosphorylation. Cell Host Microbe 2009, 6, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Witwit, H.; Khafaji, R.; Salaniwal, A.; Kim, A.S.; Cubitt, B.; Jackson, N.; Ye, C.; Weiss, S.R.; Martinez-Sobrido, L.; de la Torre, J.C. Activation of Protein Kinase Receptor (PKR) Plays a pro-Viral Role in Mammarenavirus-Infected Cells. J. Virol. 2024, 98, e0188323. [Google Scholar] [CrossRef] [PubMed]
- FDA-Approved HIV Medicines | NIH. Available online: https://hivinfo.nih.gov/understanding-hiv/fact-sheets/fda-approved-hiv-medicines (accessed on 10 November 2024).
- Bernstein, D.E.; Aron, J.S.; Kerr, C.A.; Flanigan, C.; Hoffmann, C.J.; Gonzalez, C.J. Treatment of Chronic Hepatitis C Virus Infection in Adults; New York State Department of Health AIDS Institute Clinical Guidelines; Johns Hopkins University: Baltimore, MD, USA, 2023. [Google Scholar]
- Gulick, R.M.; Mellors, J.W.; Havlir, D.; Eron, J.J.; Gonzalez, C.; McMahon, D.; Richman, D.D.; Valentine, F.T.; Jonas, L.; Meibohm, A.; et al. Treatment with Indinavir, Zidovudine, and Lamivudine in Adults with Human Immunodeficiency Virus Infection and Prior Antiretroviral Therapy. N. Engl. J. Med. 1997, 337, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Jilek, B.L.; Zarr, M.; Sampah, M.E.; Rabi, S.A.; Bullen, C.K.; Lai, J.; Shen, L.; Siliciano, R.F. A Quantitative Basis for Antiretroviral Therapy for HIV-1 Infection. Nat. Med. 2012, 18, 446–451. [Google Scholar] [CrossRef]
- Shafran, S.D.; Shaw, D.; Charafeddine, M.; Agarwal, K.; Foster, G.R.; Abunimeh, M.; Pilot-Matias, T.; Pothacamury, R.K.; Fu, B.; Cohen, E.; et al. Efficacy and Safety Results of Patients with HCV Genotype 2 or 3 Infection Treated with Ombitasvir/Paritaprevir/Ritonavir and Sofosbuvir with or without Ribavirin (QUARTZ II-III). J. Viral. Hepat. 2018, 25, 118–125. [Google Scholar] [CrossRef]
- Shen, L.; Rabi, S.A.; Sedaghat, A.R.; Shan, L.; Lai, J.; Xing, S.; Siliciano, R.F. A Critical Subset Model Provides a Conceptual Basis for the High Antiviral Activity of Major HIV Drugs. Sci. Transl. Med. 2011, 3, 91ra63. [Google Scholar] [CrossRef]
- Shen, L.; Peterson, S.; Sedaghat, A.R.; McMahon, M.A.; Callender, M.; Zhang, H.; Zhou, Y.; Pitt, E.; Anderson, K.S.; Acosta, E.P.; et al. Dose-Response Curve Slope Sets Class-Specific Limits on Inhibitory Potential of Anti-HIV Drugs. Nat. Med. 2008, 14, 762–766. [Google Scholar] [CrossRef]
- Lehár, J.; Krueger, A.S.; Avery, W.; Heilbut, A.M.; Johansen, L.M.; Price, E.R.; Rickles, R.J.; Short III, G.F.; Staunton, J.E.; Jin, X.; et al. Synergistic Drug Combinations Tend to Improve Therapeutically Relevant Selectivity. Nat. Biotechnol. 2009, 27, 659–666. [Google Scholar] [CrossRef]
- Cheng, Y.-S.; Williamson, P.R.; Zheng, W. Improving Therapy of Severe Infections through Drug Repurposing of Synergistic Combinations. Curr. Opin. Pharmacol. 2019, 48, 92–98. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witwit, H.; Cubitt, B.; Khafaji, R.; Castro, E.M.; Goicoechea, M.; Lorenzo, M.M.; Blasco, R.; Martinez-Sobrido, L.; de la Torre, J.C. Repurposing Drugs for Synergistic Combination Therapies to Counteract Monkeypox Virus Tecovirimat Resistance. Viruses 2025, 17, 92. https://doi.org/10.3390/v17010092
Witwit H, Cubitt B, Khafaji R, Castro EM, Goicoechea M, Lorenzo MM, Blasco R, Martinez-Sobrido L, de la Torre JC. Repurposing Drugs for Synergistic Combination Therapies to Counteract Monkeypox Virus Tecovirimat Resistance. Viruses. 2025; 17(1):92. https://doi.org/10.3390/v17010092
Chicago/Turabian StyleWitwit, Haydar, Beatrice Cubitt, Roaa Khafaji, Esteban M. Castro, Miguel Goicoechea, Maria M. Lorenzo, Rafael Blasco, Luis Martinez-Sobrido, and Juan C. de la Torre. 2025. "Repurposing Drugs for Synergistic Combination Therapies to Counteract Monkeypox Virus Tecovirimat Resistance" Viruses 17, no. 1: 92. https://doi.org/10.3390/v17010092
APA StyleWitwit, H., Cubitt, B., Khafaji, R., Castro, E. M., Goicoechea, M., Lorenzo, M. M., Blasco, R., Martinez-Sobrido, L., & de la Torre, J. C. (2025). Repurposing Drugs for Synergistic Combination Therapies to Counteract Monkeypox Virus Tecovirimat Resistance. Viruses, 17(1), 92. https://doi.org/10.3390/v17010092








