Abstract
Different types of feline papillomaviruses (PVs) are associated with a variety of skin lesions and neoplasia, such as papillomas and cell carcinomas, but the virus can also be found in healthy skin. In this review, the European Advisory Board on Cat Diseases (ABCD), a scientifically independent board of veterinary experts on feline infectious diseases from 11 European Countries, discusses the current knowledge of feline PV infections. Cats most likely become infected through lesions or abrasions of the skin. Most PV infections remain asymptomatic. Besides cat-specific PVs, DNA sequences most closely related to human and bovine PVs have been detected in feline skin lesions. Diagnosis is supported by the histological detection of PV-induced cell changes and intralesional detection of viral antigen (immunostaining) or viral DNA (in situ hybridization). Immunostaining of p16CDKN2A protein (p16) can be performed as a proxy marker for PV-induced neoplasms. There is no specific treatment for PV-induced skin lesions. Spontaneous regression commonly occurs. In the case of invasive squamous cell carcinoma (ISCC), complete excision should be considered, if possible.
1. Introduction
Papillomaviruses (PVs) are epitheliotropic infectious agents that cause infections in humans as well as many different animal species, including fish. In general, PVs are host-species-specific with tropism for mucosal and cutaneous epithelia and a preference for specific locations on the body [1,2]. Most PVs cause asymptomatic or harmless infections, but some PV types can induce neoplasia. In each host, different PV types exist. In humans, more than 200 types have been identified [3]. Presently, eight different PV types with complete genome sequences have been identified in cats, seven feline PVs (Felis catus papillomaviruses 1-7; FcaPV1-7) and bovine PV14, which is a bovine-specific PV (BPV) found in cats with sarcoids [4,5,6]. In addition, several putative PVs, based on sequence similarities with known feline PVs, have been detected from lesions in cats [7,8]. This review presents an update on the current state of knowledge on infections with FcaPV.
2. Virus
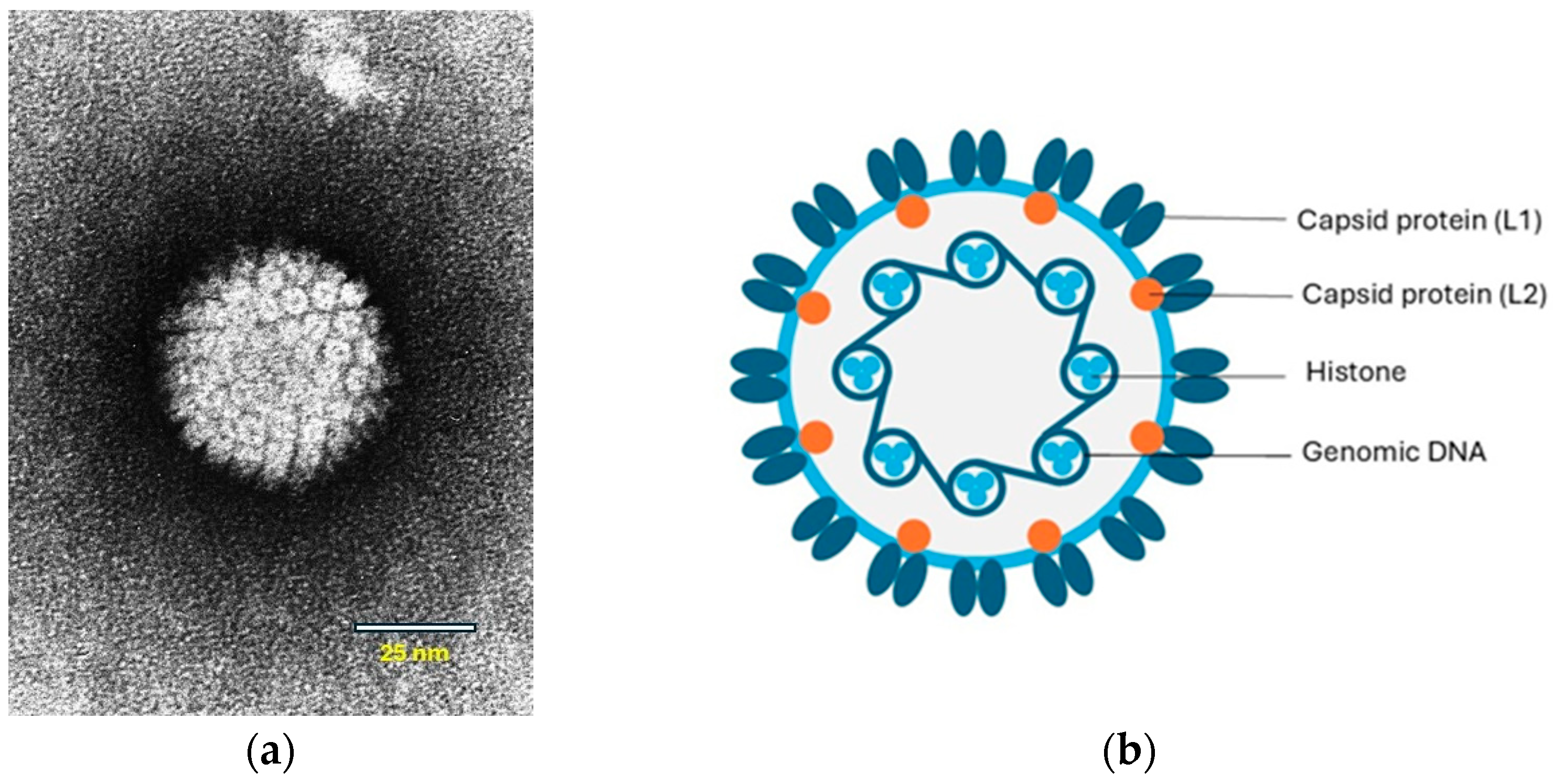
Papillomaviruses are small viruses containing circular double-stranded DNA that belong to the family Papillomaviridae (Figure 1a,b).
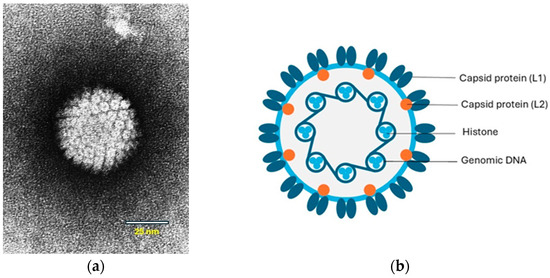
Figure 1.
(a) Papilloma virion, negative contrast electron microscopic (EM) image. Source: Laboratory of Tumor Virus Biology, NIH-Visuals Online# AV-8610-3067. (b) Cross-sectional model of a papillomavirus. The circular dsDNA is associated with cellular histones and surrounded by a capsid consisting of the L1 and L2 proteins. © Karin de Lange (Aksent Veterinary Communications) for the ABCD.
The viral genome contains early (E) genes, which encode several E proteins, and two late (L) genes. Some of the E proteins play roles in viral transcription and replication. The E6 and E7 proteins are oncogenes that interfere with cell regulation. The L genes encode two structural proteins, L1 and L2, that form the capsid during virus assembly (Figure 1b). A third gene, L3, of which the function is unknown has only been identified in some papillomaviruses [9]. In human PV-induced neoplasms, the viral DNA is integrated into the host-cell DNA. However, in papillomas (warts), the viral DNA is not integrated and persists as an autonomously replicating episome. Viral DNA integration also appears to be uncommon within PV-induced neoplasms in animals [10]. However, in a recent study, genomic integration and expression of feline PV oncogenes were shown in feline Merkel cell carcinoma (MCC) in cats [11].
Two subfamilies of PVs are recognized: firstpapillomavirinae (containing 52 genera and several hundred species) and secondpapillomavirinae (currently one genus). The conserved L1 gene is used for the classification of PVs. If two PVs have less than 90% sequence similarity in the L gene, they are considered to be different types, and if less than 60% similarity, to be within different genera [12,13].
Feline PVs were previously designated as Felis domesticus PVs (FdPVs), but a more correct taxonomic name, Felis catus PVs (FcaPVs), has been proposed [14]. The feline PVs are classified within three genera: Lambdapapillomavirus (FcaPV1), Dyothetapapillomavirus (FcaPV2) and the Taupapillomavirus genus (FcaPV 3-7) [15]. The BPV, classified within the genus Deltapapillomavirus, is an exception to the rule that PVs are highly species-specific [4]. A novel FcaPV type was suggested in a cat with a cutaneous papilloma, based on a low level of sequence similarity with known FcaPVs [7] and another putative novel FcaPV type was determined in a feline osteoinductive squamous cell carcinoma [8].
3. Epidemiology
Papillomaviruses have been detected in several animal species and in humans as a cause of cutaneous lesions [16]. Although the viruses tend to be host-species-specific, sequences related to bovine and human PVs have been found in cats, suggesting cross-species transmission [17,18]. However, it is not excluded that the detection of human PVs might be due to cross-contamination during the sampling or processing of samples [4]. PV infection has also been detected in other felids, including the Florida panther subspecies of cougar (Puma concolor coryi), Bobcat (Lynx rufus), Asiatic lion (Panthera leo persica), Snow leopard (Panthera unica), clouded leopard (Neofelis nebulosa), cheetah (Acinonyx jubatus) and caracals (Caracal caracal) [19,20,21,22,23,24].
4. Pathogenesis
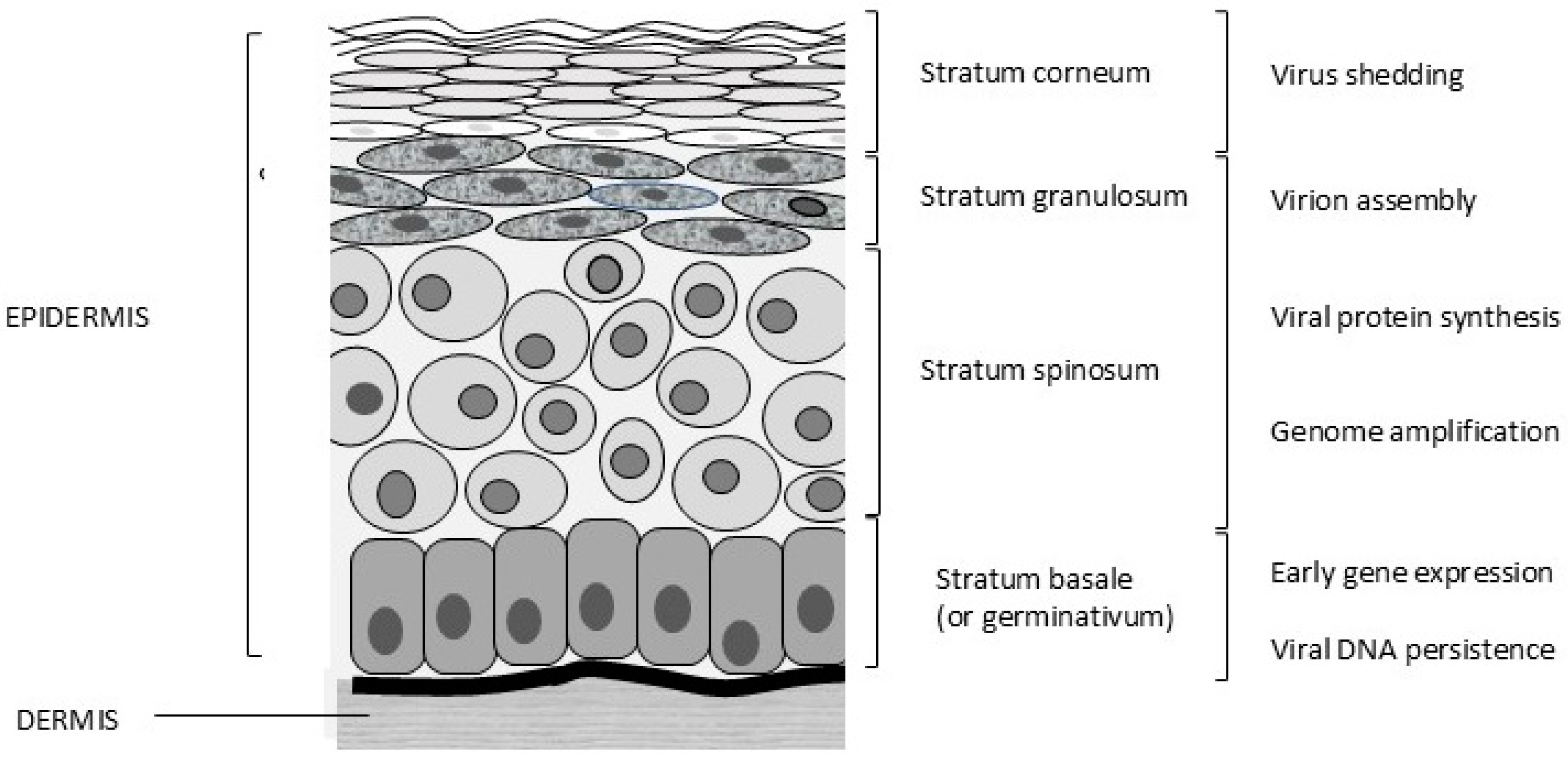
Papillomaviruses are epitheliotropic infectious agents. Infections usually occur through lesions or abrasions of the skin. Initially, the basal cells of the stratum germinativum (stratum basale, Figure 2) are infected, which leads to hyperplasia and delayed maturation of cells in the stratum spinosum and stratum granulosum. In the basal cells, only early gene expression occurs, and these cells allow the persistence of PV infection. Viral protein (L1 and L2) synthesis and virion assembly occur in terminally differentiated cells of the stratum spinosum and, more specifically, the stratum granulosum. The virus is present in the differentiated keratinized cells and is shed with exfoliated cells of the stratum corneum (Figure 2).
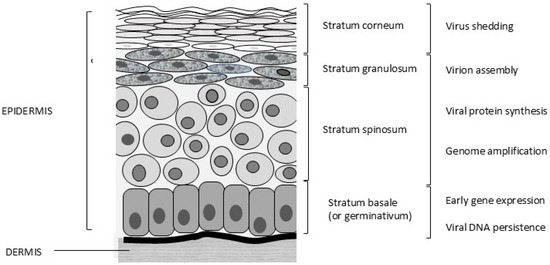
Figure 2.
Different layers of feline skin and stages of the viral life cycle. © Karin de Lange (Aksent Veterinary Communications) for the ABCD.
Due to their ability to alter cell regulation, PVs can also induce tumours. PV is one of the most common causes of viral neoplasia in humans and has also been associated with neoplasia in dogs, horses, ruminants and pigs. Both E6 and E7 are considered oncogenes, expressing proteins that inhibit the retinoblastoma (pRB) and p53 protein, respectively, resulting in interference with normal cell regulation leading to malignant lesions [25]. PV is, however, commonly found in the healthy skin of different animals, including the cat [26]; this makes definitive proof of a causal relationship between the presence of PV sequences and skin lesions difficult.
5. Immunity
Papillomaviruses tend to stimulate a mild immune response, due to viral replication in the superficial layers of the epithelium. If an effective immune response is induced, it is based on cell-mediated immunity [4,27]. This leads to the lysis of infected cells and the resolution of the hyperplastic lesions. Antibodies can be induced but do not appear to play a role in lesion resolution. However, antibodies can play a role in the type-specific protection against (re)infection [28].
6. Clinical Signs
The majority of cats are infected by PVs, but the disease seems to occur less frequently compared to other domestic animals [4,29]. Nevertheless, several diseases have been associated with different types of PVs (Table 1). Lesions reported to be associated with PVs in domestic cats include hyperplastic plaques, skin papillomas, oral papillomas, Bowenoid in situ carcinomas (BISCs), cutaneous and oral squamous cell carcinomas ((O)SCCs), feline basal cell carcinomas and feline sarcoids. Also, there is evidence for the involvement of FcaPV2 in the development of MCC in cats (Table 2) [30].
6.1. Hyperkeratotic Plaques
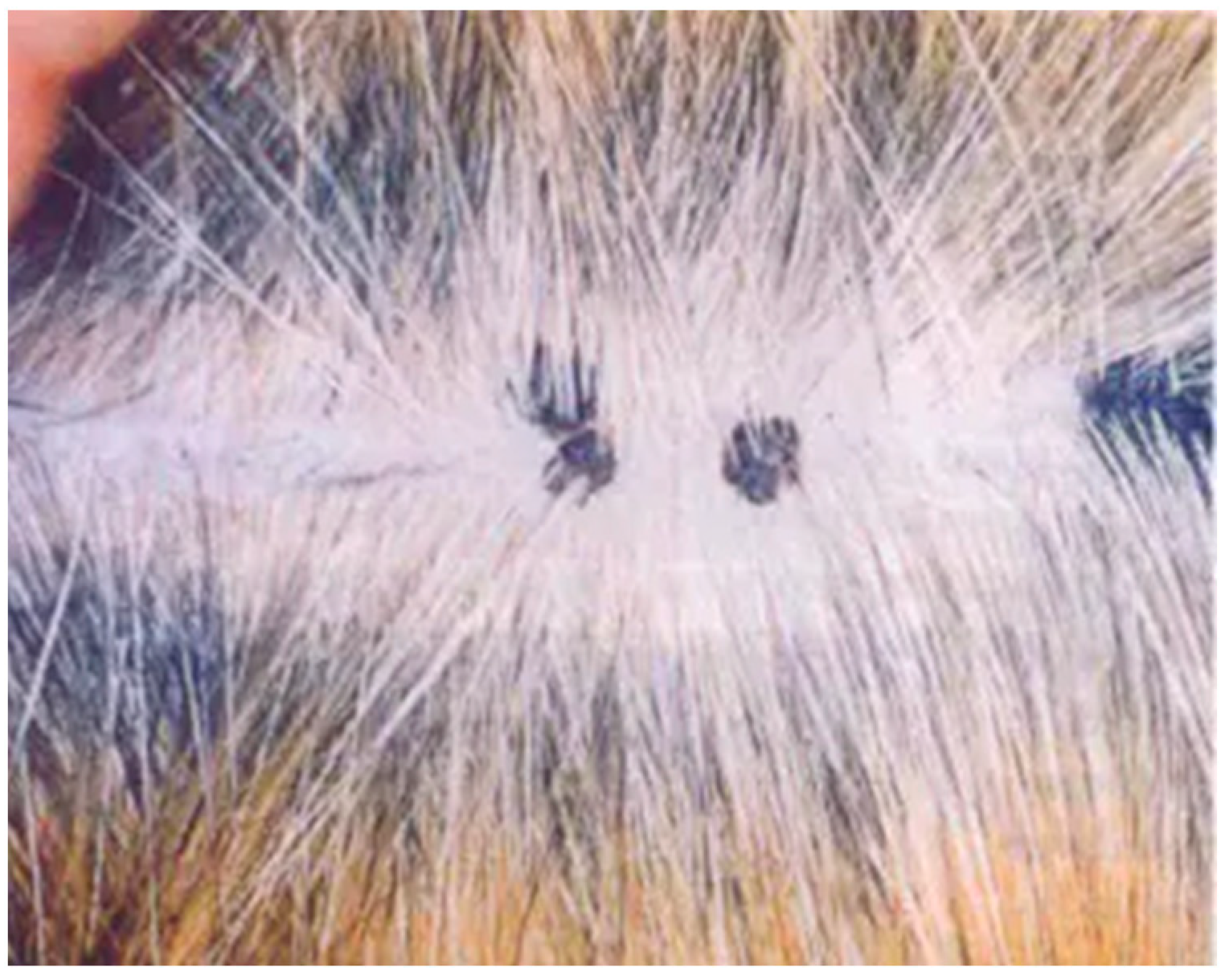
Cutaneous hyperkeratotic plaques seem to be most common in older and immunosuppressed cats, e.g., FIV-infected animals [31,32]. However, plaques have also been reported in cats without any recognised immunodeficiency [33]. The plaques appear as flat, slightly raised, scaly and variably pigmented lesions and are generally less than 1 cm in diameter (Figure 3).
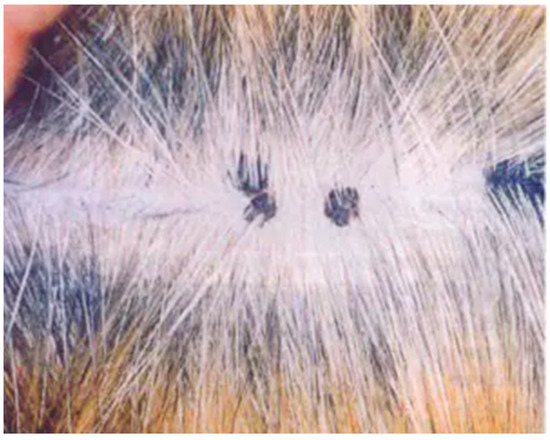
Figure 3.
Pigmented flat cutaneous papillomas (photo Herman Egberink, Ph.D thesis Utrecht).
Feline catus PVs 2, 3 and 5 have been associated with viral plaques [34,35]. The lesions share many histological features with BISCs, and are thought to be precursors of BISC, representing different severities of the same disease [33].
6.2. Skin Papillomas (Warts)
Papillomas of the skin, characterised by marked thickening and folding of the epidermis, seem to be rare in cats, in contrast to dogs and other domestic animals. To date only three cases of PV-induced cutaneous papillomas have been reported in domestic cats. In one cat with a focal area of hyperplasia on the eyelid, PV L1 was detected in the skin lesion by immunostaining [36]. In a cat with a papilloma that developed on the nasal planum only, sequences from human PV type 9 could be amplified [37]. PVs are usually highly species-specific, and detection of these human PV-type sequences might be due to cross-contamination and not cross-infection. Humans are ubiquitously and asymptomatically infected, which can lead to sample contamination. The third case was a 4.5-year-old male domestic shorthair cat with a papilloma on the nasal planum. The PV DNA sequence amplified from the lesion had a low similarity to other feline PV types, suggestive of a novel PV genus [7].
6.3. Oral Papillomas
Only a few cases of oral papillomas have been described in cats; they seem to occur less frequently than in other species [38]. However, because these papillomas seem to be restricted to the ventral surface of the tongue, it was suggested that they might occur more frequently but remain undetected [4]. The lesions appear as clusters of exophytic lesions on the ventral surface of the tongue. They are characterised histologically by foci of markedly hyperplastic folded epithelium with PV-induced cell changes. In a report describing two cats, oral papillomas were associated with FcaPV1 [38]. This feline PV type is the only feline type classified within the genus Lambdapapillomavirus, in which the PV types that cause oral papillomas in dogs and some other species also belong [4].
6.4. Bowenoid In Situ Carcinomas (BISCs)
Feline BISCs are superficial lesions that are confined to the epidermis and occur often in pigmented-haired skin as crusting, de- or hyperpigmented, and roughly circular lesions [33]. BISCs tend to be larger than viral plaques and are more markedly raised [39]. The lesions are typically found in middle-aged or older cats [33]. Spontaneous regression can occur, although many BISCs persist, and some progress to an invasive squamous cell carcinoma (ISCC). Devon Rex and Sphynx breeds seem to be more prone to developing BISC; lesions develop at an earlier age and tend to be more aggressive, rapidly progressing to ISCC and metastatic SCC [40,41]. A clear association between PV DNA (the then-called Felis domesticus papillomavirus 2; FdPV-2) and feline SCCs has been found; viral DNA was detected in all 20 BISCs examined and in 17 out of 20 cases of SCCs [42]. However, FdPV-2 DNA was also detected in 52% of healthy skin swabs [26]. Although FdPV-2 has been detected most frequently in BISCs and SCCs, other PV types and FcaPV3, FcaPV4 and FcaPV5 were identified as well [14,43,44].
6.5. Feline Cutaneous Squamous Cell Carcinomas (SCCs)
Cutaneous SCCs are common skin cancers in cats. Sunlight plays a role in their development. Lesions tend to be found in sparsely haired areas such as the eyelids, nose, and pinnae and typically occur in non-pigmented skin. A contributory role for FcaPV is suggested by the more frequent detection of FcaPV2 DNA in cutaneous SCCs than in the healthy skin of cats [42,45]. The relative roles of ultraviolet (UV) light, other co-factors, or FcaPV infection in the development of SCCs are uncertain [4]. Studies suggest that FcaPVs could have an aetiological role in a quarter to a third of all cutaneous SCCs [45,46,47]. Besides the more frequent detection of FcaPV2 in SCC lesions, other studies support its role in the development of SCCs. Firstly, higher viral loads have been detected in BISCs and in a subset of SCCs than in healthy skin [48]. Secondly, FcaPV2 RNA, as evidence of gene expression, has been detected in a proportion of SCCs but not in healthy skin [25,48]. Also, staining of the p16CDKN2A protein can be visualised in SCCs that also contain PV DNA (see “diagnosis” section later) [49,50]. Feline PV type 2 is the most common type of PV detected in cutaneous SCCs, but in some cases of SCCs, FcaPV3, FcaPV4 or FcaPV6 DNA has been identified [44,51,52]. In one study, 50% of the sequenced PV DNA was most closely related to human PV DNA [18].
6.6. Oral Squamous Cell Carcinomas (OSCCs)
Feline oral SCCs are aggressive invasive neoplasms and an important cause of mortality [4,46]. They are most commonly located in the sublingual/lingual region, maxilla, mandible, buccal mucosa, lip and caudal pharynx/tonsillar region [53]. In humans, PV is an established cause of a proportion of the OSCCs. Consequently, several studies on the aetiology of feline OSCCs have focused on the detection of PV in OSCC lesions. Results of these studies show significant differences in the detection of PV DNA within feline OSCCs, as described below.
Papillomavirus DNA could not be detected in any of the 30 cases of OSCC from cats in New Zealand nor in seven OSCC samples screened in cats from Japan [52,54]. In cats in New Zealand, FcaPV1 DNA was found in only one of 36 samples, and also in one of 16 samples from cats with non-neoplastic inflammatory lesions [55]. Recently, using a metagenomic approach for DNA viruses, the virome was sequenced and FcaPV3 was detected in only one of 20 OSCC samples and none of 9 samples of healthy gingiva [56]. These findings contrast with the results of other studies. In a study performed in Italian cats, a higher number of samples from cats with OSCC and non-neoplastic ulcerative oral lesions were positive for FcaPV2 DNA, namely 10 of 32 (31%) and 4 of 11 (36%), respectively [57]. The presence of viral gene expression suggested active viral replication, but no significant differences in viral loads were detected between the samples of OSCC and ulcerative oral lesions. In a recent study, 11 of 19 (57.9%) OSCC samples of cats from Taiwan and one of 9 (11.1%) OSCC samples of cats from Japan contained FcaPV2 DNA, but no oral samples from cats without OSCC samples were included [58]. In conclusion, the markedly different results for the detection of numbers of PV-positive OSCC samples together with similar detection rates in non-neoplastic samples seem, so far, not to support a role for PV in the development of OSCCs although it is acknowledged that FcaPV2 is most commonly identified.
6.7. Feline Basal Cell Carcinomas (BCC)
Basal cell carcinomas are rare feline skin neoplasms presenting mostly as single ulcerated raised plaques or nodules; keratinization is generally absent [46]. An association between PV infection and feline BCC was suggested by the presence of PV cytopathic changes in cells within BCCs [4]. The association was further supported by the detection of FcaPV3 DNA in a cat with multiple BCCs [43] and the amplification of sequences from a feline BCC of a probably novel type of PV that was not further classified [59]. A novel type was amplified from a BCC, and was designated FcaPV7 and considered to be associated with skin cancer. The histological lesions were different from those reported with other subtypes of FcaPV [15].
6.8. Feline Cutaneous Fibropapillomas or Feline Sarcoids
Feline sarcoids are rare, presenting as skin neoplasms; solitary or multiple nodular masses are found most commonly on the head (nasal philtrum, lips), neck, ventral abdomen and limbs.
Lesions are characterised by the proliferation of mesenchymal rather than epithelial cells. Metastasis does not occur [60]. The finding of a PV similar to BPV type 14 (BPV14) and the higher prevalence in cats with known exposure to cattle suggest an association with the bovine virus [16,61,62]. Also, PV DNA could be localised in neoplastic mesenchymal cells by in situ hybridisation (ISH) [62]. This hypothesis is in line with the known association between BPV and equine sarcoids [63].
6.9. Merkel Cell Carcinoma (MCC)
Merkel cell carcinoma is a rare and aggressive neuroendocrine carcinoma of the skin. In humans, Merkel cell polyomavirus (MCPyV) is involved in approximately 80% of Merkel cell carcinomas, and in 20% of cases, UV light exposure plays a role [64]. Tumorigenesis is caused by the integration of the MCPyV DNA into the host genome and the induction of persistent expression of viral oncogenes or through the occurrence of DNA mutations induced by UV exposure [64,65]. Feline MCCs are rare skin tumours, located in the dermis and/or subcutis and present as firm, red, dome-shaped, solitary skin nodules. The overlying skin is frequently ulcerated [66]. The tumours are characterised by the expression of some epithelial markers (cytokeratin 18 and 20) and neuroendocrine markers, such as synaptophysin and CD56 [66]. Cats with MCC often have other proliferative skin lesions such as SCC and BCC, and a role for FcaPV2 in feline MCC has also been suggested [30]. FcaPV DNA could be detected in 95% of feline MCC cases and the presence of FcaPV DNA in the tumour cells was confirmed by ISH [30]. The hybridisation signal pattern suggested the integration of the DNA into the genome, which was further evidenced by whole genome sequencing of two FcaPV-positive cases [11].

Table 1.
Papillomavirus lesions and their associated papillomavirus type.
Table 1.
Papillomavirus lesions and their associated papillomavirus type.
| Papillomavirus Lesion | Papillomavirus Type | Reference |
|---|---|---|
| Hyperkeratotic plaques | FcaPV2, * FcaPV3, FcaPV5 | [35] [40] [67] |
| Skin papillomas | HPV9 ? ** FcaPV, type not classified | [37] [7] |
| Oral papillomas | FcaPV1 | [38] |
| Bowenoid in situ carcinomas | FcaPV2, FcaPV3, FcaPV4, FcaPV5 | [42] [14] [44] |
| Feline cutaneous squamous cell carcinomas | FcaPV2, FcaPV3, FcaPV4, FcaPV6 | [42] [52] [51,58] |
| Oral squamous cell carcinomas | FcaPV2 ? | [57] |
| Feline basal cell carcinoma | FcaPV3, FcaPV7 ? | [43] [15] |
| Feline sarcoids | BPV14 *** | [26] |
| Merkel cell carcinoma | FcaPV2 | [11,30] |
* FcaPV indicates Feline catus papillomavirus; ** HPV indicates human papillomavirus; *** BPV indicates bovine papillomavirus; “?” indicates results of studies vary or are unclear.

Table 2.
Papillomavirus lesion and clinical presentation.
Table 2.
Papillomavirus lesion and clinical presentation.
| Lesion | Clinical Signs | Prognosis/Treatment |
|---|---|---|
| Hyperkeratotic plaques | Flat, slightly raised, scaly and variably pigmented lesions. | Spontaneous regression possible but some might persist. No specific treatment. |
| Skin papillomas | Localised lesions: thickening and folding of the epidermis. | No specific treatment. Lesions can be removed surgically. Recurrence is possible (as in dogs with canine papillomas). |
| Oral papillomas | Exophytic lesions on the ventral surface of the tongue. | Incidental occurrence. No clinical signs. Most probably resolve spontaneously. |
| Bowenoid in situ carcinomas | Crusting, mostly hyperpigmented and roughly circular lesions, often in pigmented, haired skin. | Spontaneous regression can occur. But also progression to ISCC (metastasis especially reported in Devon Rex and Sphynx cats). Surgical excision, cryo-surgery or carbon dioxide laser surgery can be considered. Efficacy and safety of imiquimod (used in humans) needs additional controlled studies. |
| Feline cutaneous squamous cell carcinomas | Majority present in non-haired, non-pigmented areas of the body. | PV-associated SCCs have a more favourable prognosis than non-PV induced. |
| Oral squamous cell carcinomas | Exophytic filiform masses on the ventral surface of the tongue. | Almost all invariably fatal. |
| Feline basal cell carcinoma | Mostly single, ulcerated raised lesions, with keratinization generally absent. | Lesions can be removed surgically. |
| Feline sarcoids | Solitary or multiple nodular masses found most commonly on the head (nasal philtrum, lips), neck, ventral abdomen and limbs. | Often recur after surgical removal, but metastasis does not occur. |
| Merkel cell carcinoma | Firm, red, dome-shaped, solitary skin nodules; overlying skin is frequently ulcerated. | Poor prognosis. Lesions can be removed surgically but often recur, and tendency for metastasis (despite margin-negative surgery). |
ISCC; invasive squamous cell carcinoma.
7. Diagnosis
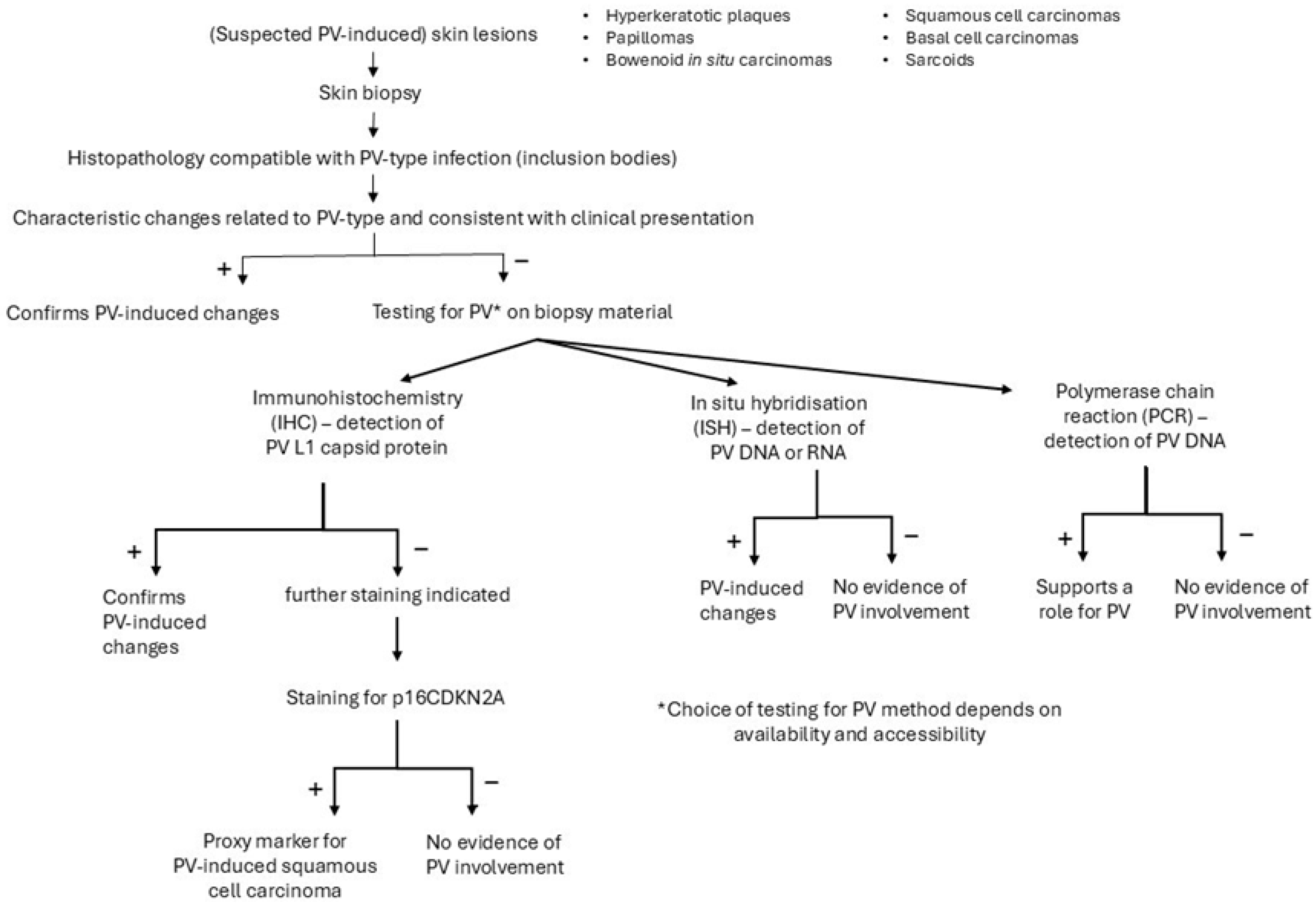
Several methods can support the diagnosis of a viral-induced hyperplastic lesion (Figure 4). A biopsy from a skin lesion can be taken for histopathologic examination and immunohistochemical staining of PV group-specific antigens [4]. The different PV-induced lesions of virally-induced oral papillomas, BISCs and feline sarcoids all show characteristic histological changes, often related to the PV type involved [4]. Infection of cells with FcaPV1 can result in eosinophilic intracytoplasmic bodies [38], infection with FcaPV2, in expanded cytoplasm by clear or slightly granular grey-blue material, and with FcaPV3, slender elongated perinuclear basophilic bodies [68]. In cells infected with FcaPV4 or FcaPV5, the cytoplasm may be expanded by dark blue-grey material [67,69]. These changes become less visible in advanced neoplastic lesions where there is less, or no, viral replication. In lesions with PV-induced cell changes, antibodies against the L1 protein can be used to stain the PV protein within cells. However, feline PV-type specific antibodies are not commercially available and antibodies against PV types of other species (including humans) might not cross-react; if the cross-reactivity with feline-type PVs is unknown, a negative result will be difficult to interpret [4]. Immunostaining is of no use in PV-induced cancers since viral replication in these lesions is rare. However, in these lesions, immunostaining of p16CDKN2A protein (p16) can be performed as a proxy marker for PV-induced SCCs [70]. The p16 inhibits the retinoblastoma protein (pRB) to prevent cell division. In PV-induced neoplasms, the pRB is degraded, which leads to the loss of pRB and increased amounts of p16 [4]. A strong association between the presence of PV-DNA and p16 immunostaining has been shown [45,48]. Also, PCR can be used to demonstrate PV DNA in the lesions and to identify the viral strain by further sequencing. However, the presence of PV DNA in the healthy skin of cats makes interpretation of positive PCR results of skin lesions difficult. Therefore, a positive PCR result is not definitive proof that the lesion is caused by PV if DNA is extracted from the whole sample. In contrast to PCR, ISH detects PV DNA or RNA within the cells of a lesion, and the presence of PV DNA or RNA in the basal and suprabasal epithelial layers of the lesion supports the role of the virus in the pathogenesis [4].
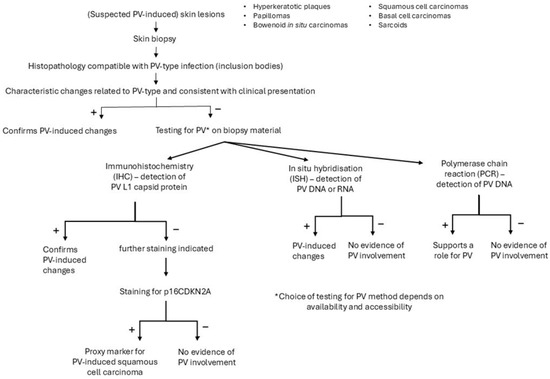
Figure 4.
Diagnostic procedure to support the role of a papillomavirus (PV) infection in case of a clinical suspicion of a PV-induced lesion.
8. Treatment
No specific treatment for feline PV-induced skin lesions is known. In immunocompetent cats, spontaneous regression can be expected, similar to dogs, but it might take up to several months. The treatment of superficial layers of the skin can be sufficient for lesions that are confined to the epidermis, like BISCs. Imiquimod cream stimulates inflammation and is used for the topical treatment of Bowen’s disease in humans. The cream has never been thoroughly evaluated in cats with this condition; although a response was noted, no conclusion with respect to the efficacy of the drug in cats was reached in one study [71]. In this study, the SSC lesions were also PV antigen-negative. Although anecdotal evidence supports the use of this medication, more controlled studies are required to evaluate the safety and efficacy of this treatment. If only a few superficial lesions are present, surgical excision can be considered assuming that all of the affected epidermis can be removed [4]. Carbon dioxide laser therapy, which has been used in other species, can also be considered [72], particularly in areas where wound closure is difficult. Cryotherapy can also be used to treat the superficial lesions [4]. Feline ISCCs tend to slowly metastasise. Therefore, if anatomical location allows, complete excision might be curative.
9. Prognosis
Most PV infections remain asymptomatic with limited replication in epithelial cells. Cutaneous and oral papillomas often resolve spontaneously. The prognosis of PV-induced SCCs depends on the number, location and invasiveness of the lesions. PV-induced SCCs tend to have a more favourable prognosis than non-PV-associated SCCs [60]. If successfully treated, e.g., by excision of the lesion, new additional lesions might develop in the same cat. Sphinx and Devon Rex cats are predisposed to BISCs and have a higher risk of developing invasive and metastatic SCCs [40]. Therefore, all viral plaques/BISCs should be carefully monitored for progression to SCC. Feline OSCCs are almost invariably fatal, but no association of feline OSCCs with PV infection has been proven.
10. Vaccination
Although vaccination against PV infections can be effective, as evidenced by the use of successful vaccines in humans, no vaccines are available for papillomatosis in cats. In one study, an experimental FcaPV2 virus-like particle vaccine was shown to be safe and immunogenic [73], but vaccination-induced high antibody titres had no effect on the viral loads in cats that were already infected [73]. The majority of PV-induced lesions in cats are caused by FcaPV2, which infects cats within the first few days of life [74]. To prevent disease development, a FcaPV2 vaccination would need to be given before infection, which is not feasible due to early infection. Vaccines against other PV types are unlikely to be developed, considering the development costs and the relatively rare occurrence of PV-induced disease by these virus types [4].
Author Contributions
All authors contributed to the discussions and preparation of the ABCD guideline on which this review article was based. All authors have read and agreed to the published version of the manuscript.
Funding
The European ABCD gratefully acknowledges the support of Boehringer Ingelheim (the founding sponsor of the ABCD), Virbac, MSD Animal Health and Vetoquinol.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors are members of the European Advisory Board on Cat Diseases (ABCD), a scientifically independent board of experts in feline medicine. Some individual authors have been consultants for or have received research funding and honoraria from animal health companies and other organisations, but all authors declare no conflicts of interest for the writing of the independent review. All authors declare no conflicts of interest. The ABCD sponsors had no role in the writing nor in the decision to publish this review.
References
- Doorbar, J. The papillomavirus life cycle. J. Clin. Virol. 2005, 32, 7–15. [Google Scholar] [CrossRef]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The Biology and Life-Cycle of Human Papillomaviruses. Vaccine 2012, 30, F55–F70. [Google Scholar] [CrossRef] [PubMed]
- Arroyo Mühr, L.S.; Lagheden, C.; Hassan, S.S.; Eklund, C.; Dillner, J. The International Human Papillomavirus Reference Center: Standardization, collaboration, and quality assurance in HPV research and diagnostics. J. Med. Virol. 2023, 95, e29332. [Google Scholar] [CrossRef]
- Munday, J.S.; Thomson, N.A. Papillomaviruses in Domestic Cats. Viruses 2021, 13, 1664. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Thomson, N.A.; Luff, J.A. Papillomaviruses in dogs and cats. Vet. J. 2017, 225, 23–31. [Google Scholar] [CrossRef]
- Medeiros-Fonseca, B.; Faustino-Rocha, A.I.; Medeiros, R.; Oliveira, P.A.; Gil da Costa, R.M. Canine and feline papillomaviruses: An update. Front. Vet. Sci. 2023, 10, 1174673. [Google Scholar] [CrossRef]
- Munday, J.S.; Wong, A.K.; Julian, A.F. Cutaneous papilloma associated with a novel papillomavirus sequence in a cat. J. Vet. Diagnost Investig. 2022, 34, 902–904. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Dunbar, M.E.; Wightman, P.; Piripi, S. Osteoinductive squamous cell carcinoma associated with a putative novel papillomavirus on the digit of a cat. N. Z. Vet. J. 2024, 72, 112–117. [Google Scholar] [CrossRef]
- Eriksson, A.; Ahola, H.; Pettersson, U.; Moreno-Lopez, J. Genome of the European elk papillomavirus (EEPV). Virus Genes 1988, 1, 123–133. [Google Scholar] [CrossRef]
- MacLachlan, N.J.; Dubovi, E.J.; Barthold, S.W.; Swayne, D.E.; Winton, J.R. Fenner’s Veterinary Virology, 5th ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 1–581. [Google Scholar]
- Ito, S.; Chambers, J.K.; Sumi, A.; Omachi, T.; Haritani, M.; Nakayama, H.; Uchida, K. Genomic integration and expression of Felis catus papillomavirus type 2 oncogenes in feline Merkel cell carcinoma. Vet. Pathol. 2023, 60, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Bernard, H.U.; Burk, R.D.; Chen, Z.; van Doorslaer, K.; Hausen, H.Z.; de Villiers, E.M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 2010, 401, 70–79. [Google Scholar] [CrossRef] [PubMed]
- de Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.U.; zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef]
- Munday, J.S.; Dunowska, M.; Hills, S.F.; Laurie, R.E. Genomic characterization of Felis catus papillomavirus-3: A novel papillomavirus detected in a feline Bowenoid in situ carcinoma. Vet. Microbiol. 2013, 165, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Gedye, K.; Knox, M.A.; Pfeffer, H.; Lin, X. Genetic characterisation of Felis catus papillomavirus type 7, a rare infection of cats that may be associated with skin cancer. Vet. Microbiol. 2023, 284, 109813. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Kiupel, M. Papillomavirus-associated cutaneous neoplasia in mammals. Vet. Pathol. 2010, 47, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Anis, E.A.; O’Neill, S.H.; Newkirk, K.M.; Brahmbhatt, R.A.; Abd-Eldaim, M.; Frank, L.A.; Kania, S.A. Molecular characterization of the L1 gene of papillomaviruses in epithelial lesions of cats and comparative analysis with corresponding gene sequences of human and feline papillomaviruses. Am. J. Vet. Res. 2010, 71, 1457–1461. [Google Scholar] [CrossRef]
- O’Neill, S.H.; Newkirk, K.M.; Anis, E.A.; Brahmbhatt, R.; Frank, L.A.; Kania, S.A. Detection of human papillomavirus DNA in feline premalignant and invasive squamous cell carcinoma. Vet. Dermatol. 2011, 22, 68–74. [Google Scholar] [CrossRef]
- Mitsouras, K.; Faulhaber, E.A.; Hui, G.; Joslin, J.O.; Eng, C.; Barr, M.C.; Irizarry, K.J.L. Development of a PCR Assay to detect Papillomavirus Infection in the Snow Leopard. BMC Vet. Res. 2011, 7, 38. [Google Scholar] [CrossRef]
- Orbell, G.M.B.; Young, S.; Munday, J.S. Cutaneous sarcoids in captive African lions associated with feline sarcoid-associated papillomavirus infection. Vet. Pathol. 2011, 48, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Steenkamp, G.; Tordiffe, A.S.W.; Suleman, E.; Oosthuizen, A.; Brettschneider, H.; Boy, S.C. Sublingual papillomas of cheetahs in southern Africa. Vet. Pathol. 2022, 59, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, J.P.; Van Ranst, M.; Montali, R.; Homer, B.L.; Miller, W.H.; Rowland, P.H.; Scott, D.W.; England, J.J.; Dunstan, R.W.; Mikaelian, I.; et al. Feline papillomas and papillomaviruses. Vet. Pathol. 2000, 37, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, L.L.; Spraker, T.R. Oral papillomatosis in Canada lynx (Lynx canadensis). J. Wildl. Dis. 2007, 43, 731–733. [Google Scholar] [CrossRef]
- Kraberger, S.; Serieys, L.E.K.; Leighton, G.R.M.; De Koch, M.D.; Munday, J.S.; Bishop, J.M.; Varsani, A. Two Lineages of Papillomaviruses Identified from Caracals (Caracal caracal) in South Africa. Viruses 2024, 16, 701. [Google Scholar] [CrossRef]
- Altamura, G.; Corteggio, A.; Pacini, L.; Conte, A.; Pierantoni, G.M.; Tommasino, M.; Accardi, R.; Borzacchiello, G. Transforming properties of Felis catus papillomavirus type 2 E6 and E7 putative oncogenes in vitro and their transcriptional activity in feline squamous cell carcinoma in vivo. Virology 2016, 496, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Witham, A.I. Frequent detection of papillomavirus DNA in clinically normal skin of cats infected and noninfected with feline immunodeficiency virus. Vet. Dermatol. 2010, 21, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Moore, R.A.; Anderson, D.M.; Gough, G.W.; Stanley, M.A. Cell-mediated immune responses to COPV early proteins. Virology 2006, 356, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Ghim, S.J.; Newsome, J.; Bell, J.; Sundberg, J.P.; Schlegel, R.; Jenson, A.B. Spontaneously regressing oral papillomas induce systemic antibodies that neutralize canine oral papillomavirus. Exp. Mol. Pathol. 2000, 68, 147–151. [Google Scholar] [CrossRef]
- Munday, J.S. Papillomaviruses in felids. Vet. J. 2014, 199, 340–347. [Google Scholar] [CrossRef]
- Ito, S.; Chambers, J.K.; Sumi, A.; Yamashita-Kawanishi, N.; Omachi, T.; Haga, T.; Nakayama, H.; Uchida, K. Involvement of Felis catus papillomavirus type 2 in the tumorigenesis of feline Merkel cell carcinoma. Vet. Pathol. 2022, 59, 63–74. [Google Scholar] [CrossRef]
- Carney, H.C.; England, J.J.; Hodgin, E.C.; Whiteley, H.E.; Adkison, D.L.; Sundberg, J.P. Papillomavirus infection of aged Persian cats. J. Vet. Diagn. Investig. 1990, 2, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Egberink, H.F.; Berrocal, A.; Bax, H.A.; van den Ingh, T.S.; Walter, J.H.; Horzinek, M.C. Papillomavirus associated skin lesions in a cat seropositive for feline immunodeficiency virus. Vet. Microbiol. 1992, 31, 117–125. [Google Scholar] [CrossRef]
- Wilhelm, S.; Degorce-Rubiales, F.; Godson, D.; Favrot, C. Clinical, histological and immunohistochemical study of feline viral plaques and bowenoid in situ carcinomas. Vet. Dermatol. 2006, 17, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Dittmer, K.E.; Thomson, N.A.; Hills, S.F.; Laurie, R.E. Genomic characterisation of Felis catus papillomavirus type 5 with proposed classification within a new papillomavirus genus. Vet. Microbiol. 2017, 207, 50–55. [Google Scholar] [CrossRef]
- Munday, J.S.; Peters-Kennedy, J. Consistent detection of Felis domesticus papillomavirus 2 DNA sequences within feline viral plaques. J. Vet. Diagnost Investig. 2010, 22, 946–948. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.L.; Kreider, J.W.; Alroy, J.; Schmidt, G.M. Cutaneous Xanthogranuloma and Viral Papilloma on an Eyelid of a Cat. Vet. Dermatol. 1992, 3, 187–190. [Google Scholar] [CrossRef]
- Munday, J.S.; Hanlon, E.M.; Howe, L.; Squires, R.A.; French, A.F. Feline cutaneous viral papilloma associated with human papillomavirus type 9. Vet. Pathol. 2007, 44, 924–927. [Google Scholar] [CrossRef]
- Munday, J.S.; Fairley, R.A.; Mills, H.; Kiupel, M.; Vaatstra, B.L. Oral Papillomas Associated With Felis catus Papillomavirus Type 1 in 2 Domestic Cats. Vet. Pathol. 2015, 52, 1187–1190. [Google Scholar] [CrossRef]
- Munday, J.S.; Wilhelm, S. Viral Diseases. In Feline Dermatology; Noli, C., Colombo, S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 359–385. [Google Scholar]
- Munday, J.S.; Benfell, M.W.; French, A.; Orbell, G.M.B.; Thomson, N. Bowenoid in situ carcinomas in two Devon Rex cats: Evidence of unusually aggressive neoplasm behaviour in this breed and detection of papillomaviral gene expression in primary and metastatic lesions. Vet. Dermatol. 2016, 27, 215-e55. [Google Scholar] [CrossRef] [PubMed]
- Ravens, P.A.; Vogelnest, L.J.; Tong, L.J.; Demos, L.E.; Bennett, M.D. Papillomavirus-associated multicentric squamous cell carcinoma in situ in a cat: An unusually extensive and progressive case with subsequent metastasis. Vet. Dermatol. 2013, 24, 642-e162. [Google Scholar] [CrossRef]
- Munday, J.S.; Kiupel, M.; French, A.F.; Howe, L. Amplification of papillomaviral DNA sequences from a high proportion of feline cutaneous in situ and invasive squamous cell carcinomas using a nested polymerase chain reaction. Vet. Dermatol. 2008, 19, 259–263. [Google Scholar] [CrossRef]
- Munday, J.S.; Thomson, N.A.; Henderson, G.; Fairley, R.; Orbell, G.M. Identification of Felis catus papillomavirus 3 in skin neoplasms from four cats. J. Vet. Diagnost Investig. 2018, 30, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Vascellari, M.; Mazzei, M.; Zanardello, C.; Melchiotti, E.; Albanese, F.; Forzan, M.; Croce, M.F.; Alberti, A.; Abramo, F. Felis catus Papillomavirus Types 1, 2, 3, 4, and 5 in Feline Bowenoid in Situ Carcinoma: An In Situ Hybridization Study. Vet. Pathol. 2019, 56, 818–825. [Google Scholar] [CrossRef]
- Munday, J.S.; Gibson, I.; French, A.F. Papillomaviral DNA and increased p16CDKN2A protein are frequently present within feline cutaneous squamous cell carcinomas in ultraviolet-protected skin. Vet. Dermatol. 2011, 22, 360–366. [Google Scholar] [CrossRef]
- Munday, J.S.; Sharp, C.R.; Beatty, J.A. Novel viruses: Update on the significance of papillomavirus infections in cats. J. Feline Med. Surg. 2019, 21, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Hoggard, N.; Munday, J.S.; Luff, J. Localization of Felis catus Papillomavirus Type 2 E6 and E7 RNA in Feline Cutaneous Squamous Cell Carcinoma. Vet. Pathol. 2018, 55, 409–416. [Google Scholar] [CrossRef]
- Thomson, N.A.; Munday, J.S.; Dittmer, K.E. Frequent detection of transcriptionally active felis catus papillomavirus 2 in feline cutaneous squamous cell carcinomas. J. Gen. Virol. 2016, 97, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; French, A.F.; Gibson, I.R.; Knight, C.G. The Presence of p16CDKN2A Protein Immunostaining Within Feline Nasal Planum Squamous Cell Carcinomas Is Associated With an Increased Survival Time and the Presence of Papillomaviral DNA. Vet. Pathol. 2013, 50, 269–273. [Google Scholar] [CrossRef]
- Munday, J.S.; French, A.F.; Peters-Kennedy, J.; Orbell, G.M.; Gwynne, K. Increased p16CDKN2A protein within feline cutaneous viral plaques, bowenoid in situ carcinomas, and a subset of invasive squamous cell carcinomas. Vet. Pathol. 2011, 48, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Yamashita-Kawanishi, N.; Gushino, Y.; Chang, C.Y.; Chang, H.W.; Chambers, J.K.; Uchida, K.; Haga, T. Full-genome characterization of a novel Felis catus papillomavirus 4 subtype identified in a cutaneous squamous cell carcinoma of a domestic cat. Virus Genes 2021, 57, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Yamashita-Kawanishi, N.; Sawanobori, R.; Matsumiya, K.; Uema, A.; Chambers, J.K.; Uchida, K.; Shimakura, H.; Tsuzuki, M.; Chang, C.Y.; Chang, H.W.; et al. Detection of felis catus papillomavirus type 3 and 4 DNA from squamous cell carcinoma cases of cats in Japan. J. Vet. Med. Sci. 2018, 80, 1236–1240. [Google Scholar] [CrossRef]
- Bilgic, O.; Duda, L.; Sánchez, M.D.; Lewis, J.R. Feline Oral Squamous Cell Carcinoma: Clinical Manifestations and Literature Review. J. Vet. Dent. 2015, 32, 30–40. [Google Scholar] [CrossRef]
- Munday, J.S.; Knight, C.G.; French, A.F. Evaluation of feline oral squamous cell carcinomas for p16CDKN2A protein immunoreactivity and the presence of papillomaviral DNA. Res. Vet. Sci. 2011, 90, 280–283. [Google Scholar] [CrossRef]
- Munday, J.S.; French, A.F. Felis catus papillomavirus types 1 and 4 are rarely present in neoplastic and inflammatory oral lesions of cats. Res. Vet. Sci. 2015, 100, 220–222. [Google Scholar] [CrossRef]
- Chu, S.; Wylie, T.N.; Wylie, K.M.; Johnson, G.C.; Skidmore, Z.L.; Fleer, M.; Griffith, O.L.; Bryan, J.N. A virome sequencing approach to feline oral squamous cell carcinoma to evaluate viral causative factors. Vet. Microbiol. 2020, 240, 108491. [Google Scholar] [CrossRef] [PubMed]
- Altamura, G.; Cardeti, G.; Cersini, A.; Eleni, C.; Cocumelli, C.; Bartolome Del Pino, L.E.; Razzuoli, E.; Martano, M.; Maiolino, P.; Borzacchiello, G. Detection of Felis catus papillomavirus type-2 DNA and viral gene expression suggest active infection in feline oral squamous cell carcinoma. Vet. Comp. Oncol. 2020, 18, 494–501. [Google Scholar] [CrossRef]
- Yamashita-Kawanishi, N.; Chang, C.Y.; Chambers, J.K.; Uchida, K.; Sugiura, K.; Kukimoto, I.; Chang, H.W.; Haga, T. Comparison of prevalence of felis catus papillomavirus type 2 in squamous cell carcinomas in cats between Taiwan and Japan. J. Vet. Med. Sci. 2021, 83, 1229–1233. [Google Scholar] [CrossRef]
- Munday, J.S.; French, A.; Thomson, N. Detection of DNA sequences from a novel papillomavirus in a feline basal cell carcinoma. Vet. Dermatol. 2017, 28, 236-e60. [Google Scholar] [CrossRef] [PubMed]
- Luff, J.A.; Munday, J.S. 40—Papillomavirus Infections. In Greene’s Infectious Diseases of the Dog and Cat, 5th ed.; Sykes, J.E., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2023; pp. 477–488. [Google Scholar]
- Schulman, F.Y.; Krafft, A.E.; Janczewski, T. Feline cutaneous fibropapillomas: Clinicopathologic findings and association with papillomavirus infection. Vet. Pathol. 2001, 38, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Teifke, J.P.; Kidney, B.A.; Löhr, C.V.; Yager, J.A. Detection of papillomavirus-DNA in mesenchymal tumour cells and not in the hyperplastic epithelium of feline sarcoids. Vet. Dermatol. 2003, 14, 47–56. [Google Scholar] [CrossRef]
- Ogłuszka, M.; Starzyński, R.R.; Pierzchała, M.; Otrocka-Domagała, I.; Raś, A. Equine Sarcoids—Causes, Molecular Changes, and Clinicopathologic Features: A Review. Vet. Pathol. 2021, 58, 472–482. [Google Scholar] [CrossRef]
- Dellambra, E.; Carbone, M.L.; Ricci, F.; Ricci, F.; Di Pietro, F.R.; Moretta, G.; Verkoskaia, S.; Feudi, E.; Failla, C.M.; Abeni, D.; et al. Merkel Cell Carcinoma. Biomedicines 2021, 9, 718. [Google Scholar] [CrossRef]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Sumi, A.; Chambers, J.K.; Doi, M.; Kudo, T.; Omachi, T.; Uchida, K. Clinical features and outcomes of Merkel cell carcinoma in 20 cats. Vet. Comp. Oncol. 2018, 16, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Marshall, S.; Thomson, N.A.; Kiupel, M.; Heathcott, R.W.; French, A. Multiple viral plaques with sebaceous differentiation associated with an unclassified papillomavirus type in a cat. N. Z. Vet. J. 2017, 65, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Hunt, H.; Orbell, G.; Pfeffer, H. Detection of a Novel Papillomavirus Type within a Feline Cutaneous Basal Cell Carcinoma. Vet. Sci. 2022, 9, 671. [Google Scholar] [CrossRef]
- Dunowska, M.; Munday, J.S.; Laurie, R.E.; Hills, S.F.K. Genomic characterisation of Felis catus papillomavirus 4, a novel papillomavirus detected in the oral cavity of a domestic cat. Virus Genes 2014, 48, 111–119. [Google Scholar] [CrossRef]
- Cunningham, L.L., Jr.; Pagano, G.M.; Li, M.; Tandon, R.; Holm, S.W.; White, D.K.; Lele, S.M. Overexpression of p16INK4 is a reliable marker of human papillomavirus-induced oral high-grade squamous dysplasia. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radio. Endod. 2006, 102, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Gill, V.L.; Bergman, P.J.; Baer, K.E.; Craft, D.; Leung, C. Use of imiquimod 5% cream (Aldara™) in cats with multicentric squamous cell carcinoma in situ: 12 cases (2002–2005). Vet. Comp. Oncol. 2008, 6, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.C.; Munday, J.S.; Stone, B.M.; Shipstone, M.A. Carbon dioxide laser treatment of extensive pigmented viral plaque lesions in a golden retriever dog. Vet. Dermatol. 2016, 27, 442-e117. [Google Scholar] [CrossRef] [PubMed]
- Thomson, N.A.; Howe, L.; Weidgraaf, K.; Thomas, D.G.; Young, V.; Ward, V.K.; Munday, J.S. Felis catus papillomavirus type 2 virus-like particle vaccine is safe and immunogenic but does not reduce FcaPV-2 viral loads in adult cats. Vet. Immunol. Immunopathol. 2019, 213, 109888. [Google Scholar] [CrossRef]
- Thomson, N.A.; Dunowska, M.; Munday, J.S. The use of quantitative PCR to detect Felis catus papillomavirus type 2 DNA from a high proportion of queens and their kittens. Vet. Microbiol. 2015, 175, 211–217. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).