Bacteriophage and Phage-Encoded Depolymerase Exhibit Antibacterial Activity Against K9-Type Acinetobacter baumannii in Mouse Sepsis and Burn Skin Infection Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial and Bacteriophage Growth Conditions
2.2. Preparation of Phage AM24 and the Recombinant Protein DepAPK09 for In Vivo Experiments
2.3. Phage AM24 Infection Inhibition Assay
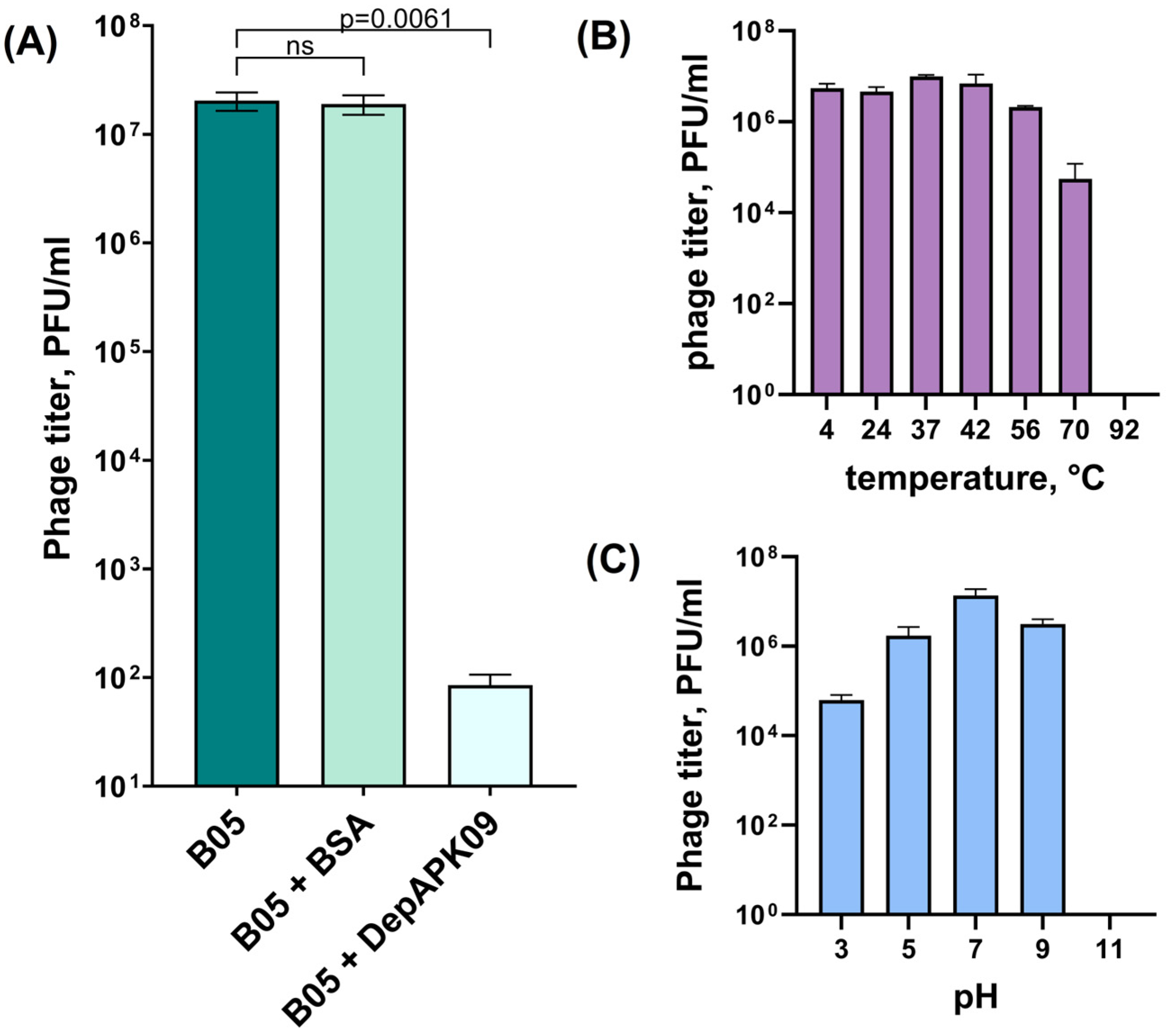
2.4. Phage AM24 and Recombinant Depolymerase Stability
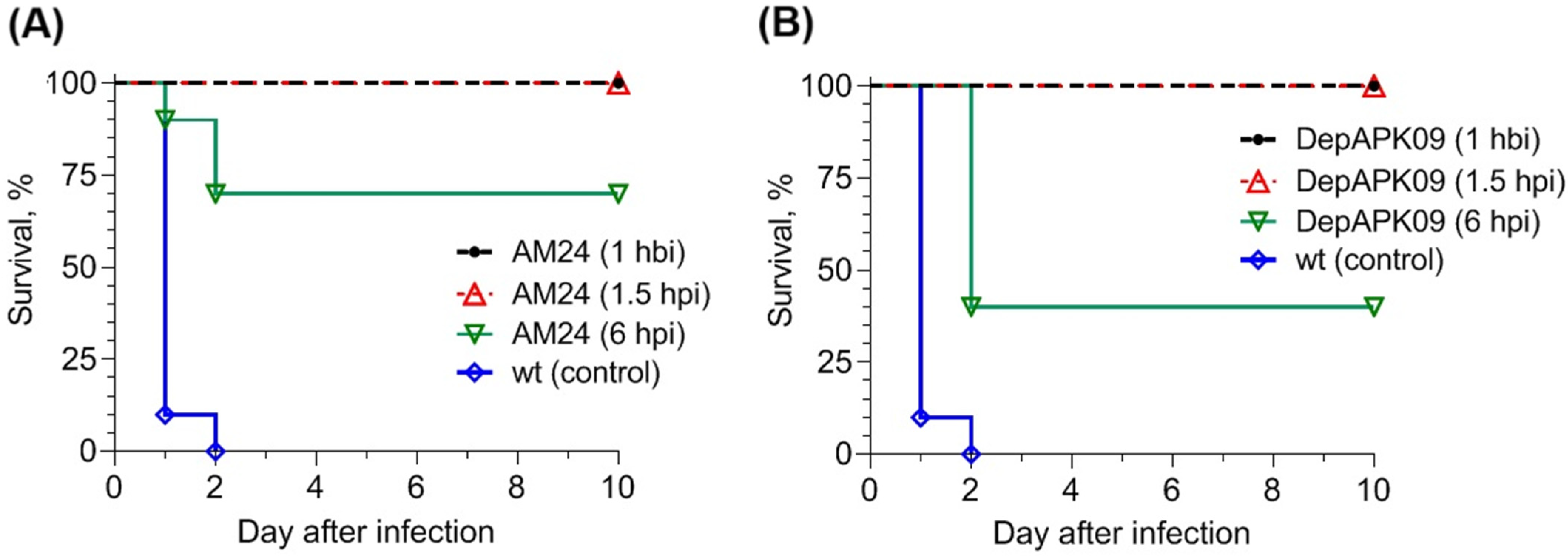
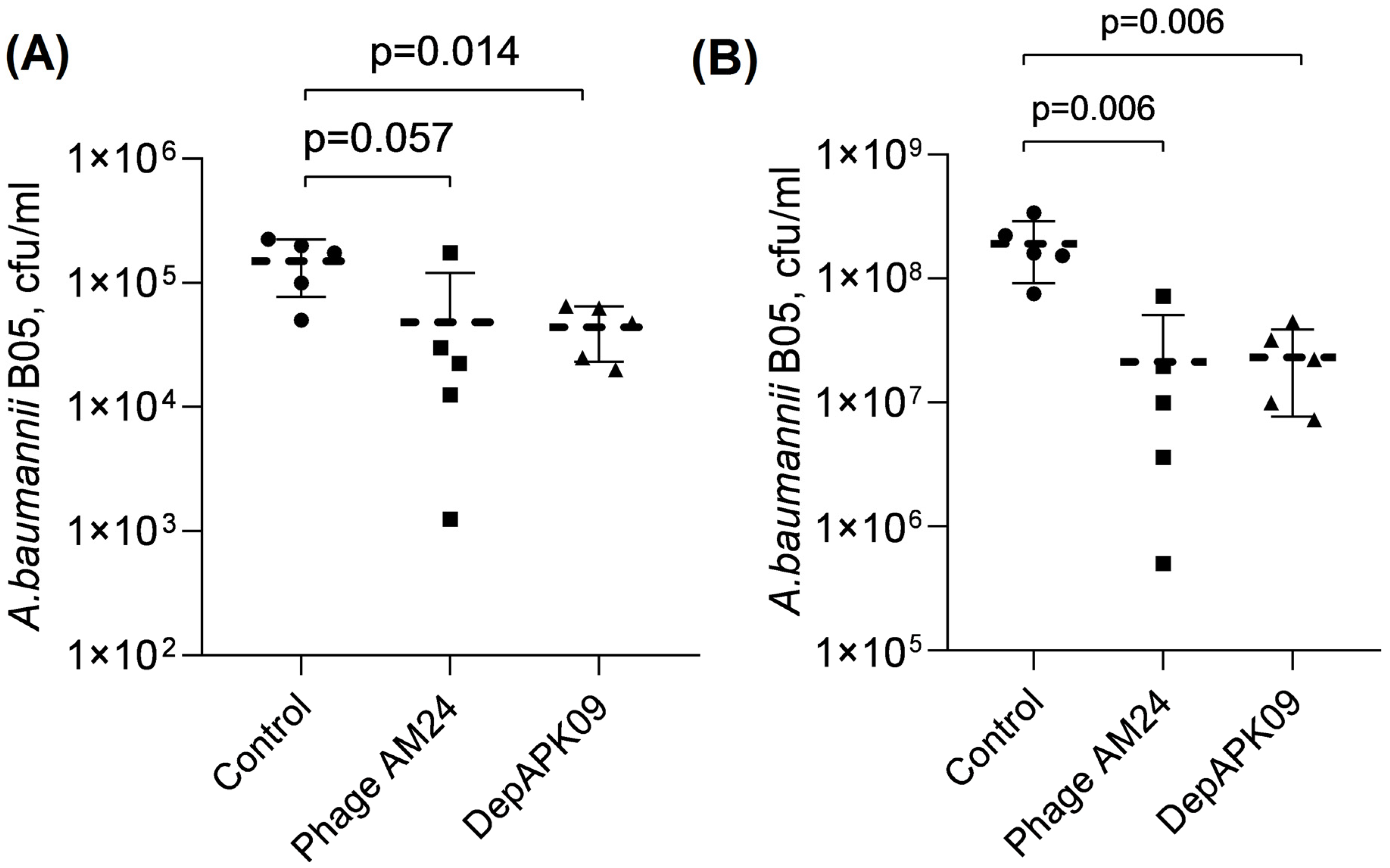
2.5. In Vivo A. baumannii Infection Models
2.6. Statistical Analysis
3. Results
3.1. Overall Characteristics of the K9-Specific Phage AM24 and Depolymerase DepAPK09 Used in In Vivo Experiments
3.2. Therapeutic Potential of the Phage AM24 and DepAPK09 in Mouse Models of A. baumannii Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Microbiol. 2018, 16, 91–102. [Google Scholar] [CrossRef]
- Ayoub Moubareck, C.; Hammoudi Halat, D. Insights into Acinetobacter baumannii: A review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef]
- Sarshar, M.; Behzadi, P.; Scribano, D.; Palamara, A.T.; Ambrosi, C. Acinetobacter baumannii: An ancient commensal with weapons of a pathogen. Pathogens 2021, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Santajit, S.; Indrawattana, N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef] [PubMed]
- Denissen, J.; Reyneke, B.; Waso-Reyneke, M.; Havenga, B.; Barnard, T.; Khan, S.; Khan, W. Prevalence of ESKAPE pathogens in the environment: Antibiotic resistance status, community-acquired infection and risk to human health. Int. J. Hyg. Environ. Health 2022, 244, 114006. [Google Scholar] [CrossRef]
- Cureño-Díaz, M.A.; Plascencia-Nieto, E.S.; Loyola-Cruz, M.Á.; Cruz-Cruz, C.; Nolasco-Rojas, A.E.; Durán-Manuel, E.M.; Ibáñez-Cervantes, G.; Gómez-Zamora, E.; Tamayo-Ordóñez, M.C.; Tamayo-Ordóñez, Y.J.; et al. Gram-negative ESKAPE bacteria surveillance in COVID-19 pandemic exposes high-risk sequence types of Acinetobacter baumannii MDR in a tertiary care hospital. Pathogens 2024, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Halim, J.; Carr, R.A.; Fliorent, R.; Jonnalagadda, K.; Kurbonnazarova, M.; Kaur, M.; Millstein, I.; Carabetta, V.J. Combinations of antibiotics effective against extensively- and pandrug-resistant Acinetobacter baumannii patient isolates. Microorganisms 2024, 12, 1353. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the infectious diseases society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Gupta, N.; Angadi, K.; Jadhav, S. Molecular characterization of carbapenem-resistant Acinetobacter baumannii with special reference to carbapenemases: A systematic review. Infect. Drug Resist. 2022, 15, 7631–7650. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024; WHO Press: Geneva, Switzerland, 2024; Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 1 November 2024).
- Knecht, L.E.; Veljkovic, M.; Fieseler, L. Diversity and Function of Phage Encoded Depolymerases. Front. Microbiol. 2020, 10, 2949. [Google Scholar] [CrossRef] [PubMed]
- Latka, A.; Maciejewska, B.; Majkowska-Skrobek, G.; Briers, Y.; Drulis-Kawa, Z. Bacteriophage-encoded virion-associated enzymes to overcome the carbohydrate barriers during the infection process. Appl. Microbiol. Biotechnol. 2017, 101, 3103–3119. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Costa, A.R.; Konstantinidis, N.; Ferreira, A.; Akturk, E.; Sillankorva, S.; Nemec, A.; Shneider, M.; Dötsch, A.; Azeredo, J. Ability of phages to infect Acinetobacter calcoaceticus-Acinetobacter baumannii complex species through acquisition of different pectate lyase depolymerase domains: Specific genomic pattern variation of phages. Environ. Microbiol. 2017, 19, 5060–5077. [Google Scholar] [CrossRef] [PubMed]
- Popova, A.V.; Shneider, M.M.; Arbatsky, N.P.; Kasimova, A.A.; Senchenkova, S.N.; Shashkov, A.S.; Dmitrenok, A.S.; Chizhov, A.O.; Mikhailova, Y.V.; Shagin, D.A.; et al. Specific interaction of novel Friunavirus phages encoding tailspike depolymerases with corresponding Acinetobacter baumannii capsular types. J. Virol. 2021, 95, e01714-20. [Google Scholar] [CrossRef]
- Timoshina, O.Y.; Kasimova, A.A.; Shneider, M.M.; Matyuta, I.O.; Nikolaeva, A.Y.; Evseev, P.V.; Arbatsky, N.P.; Shashkov, A.S.; Chizhov, A.O.; Shelenkov, A.A.; et al. Friunavirus phage-encoded depolymerases specific to different capsular types of Acinetobacter baumannii. Int. J. Mol. Sci. 2023, 24, 9100. [Google Scholar] [CrossRef] [PubMed]
- Cahill, S.M.; Hall, R.M.; Kenyon, J.J. An update to the database for Acinetobacter baumannii capsular polysaccharide locus typing extends the extensive and diverse repertoire of genes found at and outside the K locus. Microb. Genom. 2022, 8, mgen000878. [Google Scholar] [CrossRef] [PubMed]
- Evseev, P.V.; Sukhova, A.S.; Tkachenko, N.A.; Skryabin, Y.P.; Popova, A.V. Lytic Capsule-Specific Acinetobacter Bacteriophages Encoding Polysaccharide-Degrading Enzymes. Viruses 2024, 16, 771. [Google Scholar] [CrossRef]
- Taylor, C.M.; Roberts, I.S. Capsular polysaccharides and their role in virulence. Contrib. Microbiol. 2005, 12, 55–66. [Google Scholar] [CrossRef]
- Russo, T.A.; Luke, N.R.; Beanan, J.M.; Olson, R.; Sauberan, S.L.; MacDonald, U.; Schultz, L.W.; Umland, T.C.; Campagnari, A.A. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect. Immun. 2010, 78, 3993–4000. [Google Scholar] [CrossRef]
- Wang, J.L.; Kuo, C.F.; Yeh, C.M.; Chen, J.R.; Cheng, M.F.; Hung, C.H. Efficacy of φkm18p phage therapy in a murine model of extensively drug-resistant Acinetobacter baumannii infection. Infect. Drug Resist. 2018, 32, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Hu, K.; Xie, Y.; Liu, Y.; Mu, D.; Guo, H.; Zhang, Z.; Zhang, Y.; Chang, D.; Shi, Y. A novel phage PD-6A3, and its endolysin Ply6A3, with extended lytic activity against Acinetobacter baumannii. Front. Microbiol. 2019, 9, 3302. [Google Scholar] [CrossRef] [PubMed]
- Leshkasheli, L.; Kutateladze, M.; Balarjishvili, N.; Bolkvadze, D.; Save, J.; Oechslin, F.; Que, Y.A.; Resch, G. Efficacy of newly isolated and highly potent bacteriophages in a mouse model of extensively drug-resistant Acinetobacter baumannii bacteraemia. J. Glob. Antimicrob. Resist. 2019, 19, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, L.; Li, X.; Tan, D.; Cong, C.; Xu, Y. Efficacy of a phage cocktail in controlling phage resistance development in multidrug resistant Acinetobacter baumannii. Virus Res. 2019, 272, 197734. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Leung, S.S.Y.; Guo, Y.; Zhao, L.; Jiang, N.; Mi, L.; Li, P.; Wang, C.; Qin, Y.; Mi, Z.; et al. The Capsule Depolymerase Dpo48 Rescues Galleria mellonella and Mice from Acinetobacter baumannii Systemic Infections. Front. Microbiol. 2019, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Mendes, A.; Fraga, A.G.; Ferreira, A.; Pimenta, A.I.; Mil-Homens, D.; Fialho, A.M.; Pedrosa, J.; Azeredo, J. K2 Capsule Depolymerase Is Highly Stable, Is Refractory to Resistance, and Protects Larvae and Mice from Acinetobacter baumannii Sepsis. Appl. Environ. Microbiol. 2019, 85, e00934-19. [Google Scholar] [CrossRef]
- Jiang, L.; Tan, J.; Hao, Y.; Wang, Q.; Yan, X.; Wang, D.; Tuo, L.; Wei, Z.; Huang, G. Isolation and characterization of a novel myophage Abp9 against pandrug resistant Acinetobacater baumannii. Front. Microbiol. 2020, 11, 506068. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, X.; Wang, H.; Wang, S.; Fang, R.; Li, X.; Xing, J.; Wu, Q.; Li, Z.; Song, N. Characterization and efficacy against carbapenem-resistant Acinetobacter baumannii of a novel Friunavirus phage from sewage. Front. Cell. Infect. Microbiol. 2024, 14, 1382145. [Google Scholar] [CrossRef]
- Popova, A.V.; Shneider, M.M.; Myakinina, V.P.; Bannov, V.A.; Edelstein, M.V.; Rubalskii, E.O.; Aleshkin, A.V.; Fursova, N.K.; Volozhantsev, N.V. Characterization of myophage AM24 infecting Acinetobacter baumannii of the K9 capsular type. Arch. Virol. 2019, 164, 1493–1497. [Google Scholar] [CrossRef]
- Fursova, N.K.; Fursov, M.V.; Astashkin, E.I.; Fursova, A.D.; Novikova, T.S.; Kislichkina, A.A.; Sizova, A.A.; Fedyukina, G.N.; Savin, I.A.; Ershova, O.N. Multidrug-resistant and extensively drug-resistant Acinetobacter baumannii causing nosocomial meningitis in the neurological intensive care unit. Microorganisms 2023, 11, 2020. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular cloning: A laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989; ISBN 0-87969-309-6. [Google Scholar]
- Taylor, N.M.I.; Prokhorov, N.S.; Guerrero-Ferreira, R.C.; Shneider, M.M.; Browning, C.; Goldie, K.N.; Stahlberg, H.; Leiman, P.G. Structure of the T4 baseplate and its function in triggering sheath contraction. Nature 2016, 533, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Shchurova, A.S.; Shneider, M.M.; Arbatsky, N.P.; Shashkov, A.S.; Chizhov, A.O.; Skryabin, Y.P.; Mikhaylova, Y.V.; Sokolova, O.S.; Shelenkov, A.A.; Miroshnikov, K.A.; et al. Novel Acinetobacter baumannii myovirus TaPaz encoding two tailspike depolymerases: Characterization and host-recognition strategy. Viruses 2021, 13, 978. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.D. Bacteriophages; Interscience Publishers, Inc.: New York, NY, USA, 1959. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borzilov, A.I.; Volozhantsev, N.V.; Korobova, O.V.; Kolupaeva, L.V.; Pereskokova, E.S.; Kombarova, T.I.; Shneider, M.M.; Miroshnikov, K.A.; Dyatlov, I.A.; Popova, A.V. Bacteriophage and Phage-Encoded Depolymerase Exhibit Antibacterial Activity Against K9-Type Acinetobacter baumannii in Mouse Sepsis and Burn Skin Infection Models. Viruses 2025, 17, 70. https://doi.org/10.3390/v17010070
Borzilov AI, Volozhantsev NV, Korobova OV, Kolupaeva LV, Pereskokova ES, Kombarova TI, Shneider MM, Miroshnikov KA, Dyatlov IA, Popova AV. Bacteriophage and Phage-Encoded Depolymerase Exhibit Antibacterial Activity Against K9-Type Acinetobacter baumannii in Mouse Sepsis and Burn Skin Infection Models. Viruses. 2025; 17(1):70. https://doi.org/10.3390/v17010070
Chicago/Turabian StyleBorzilov, Alexander I., Nikolay V. Volozhantsev, Olga V. Korobova, Lyubov V. Kolupaeva, Evgenia S. Pereskokova, Tatiana I. Kombarova, Mikhail M. Shneider, Konstantin A. Miroshnikov, Ivan A. Dyatlov, and Anastasia V. Popova. 2025. "Bacteriophage and Phage-Encoded Depolymerase Exhibit Antibacterial Activity Against K9-Type Acinetobacter baumannii in Mouse Sepsis and Burn Skin Infection Models" Viruses 17, no. 1: 70. https://doi.org/10.3390/v17010070
APA StyleBorzilov, A. I., Volozhantsev, N. V., Korobova, O. V., Kolupaeva, L. V., Pereskokova, E. S., Kombarova, T. I., Shneider, M. M., Miroshnikov, K. A., Dyatlov, I. A., & Popova, A. V. (2025). Bacteriophage and Phage-Encoded Depolymerase Exhibit Antibacterial Activity Against K9-Type Acinetobacter baumannii in Mouse Sepsis and Burn Skin Infection Models. Viruses, 17(1), 70. https://doi.org/10.3390/v17010070





