Tenofovir and Doravirine Are Potential Reverse-Transcriptase Analogs in Combination with the New Reverse-Transcriptase Translocation Inhibitor (Islatravir) Among Treatment-Experienced Patients in Cameroon: Designing Future Treatment Strategies for Low- and Middle-Income Countries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Used for Analysis
2.2. Subtyping and Drug Resistance Determination
2.3. Mutation’s Prevalence
2.4. Mutation’s Covariation
2.5. Deduction of Potential ARV in Combination
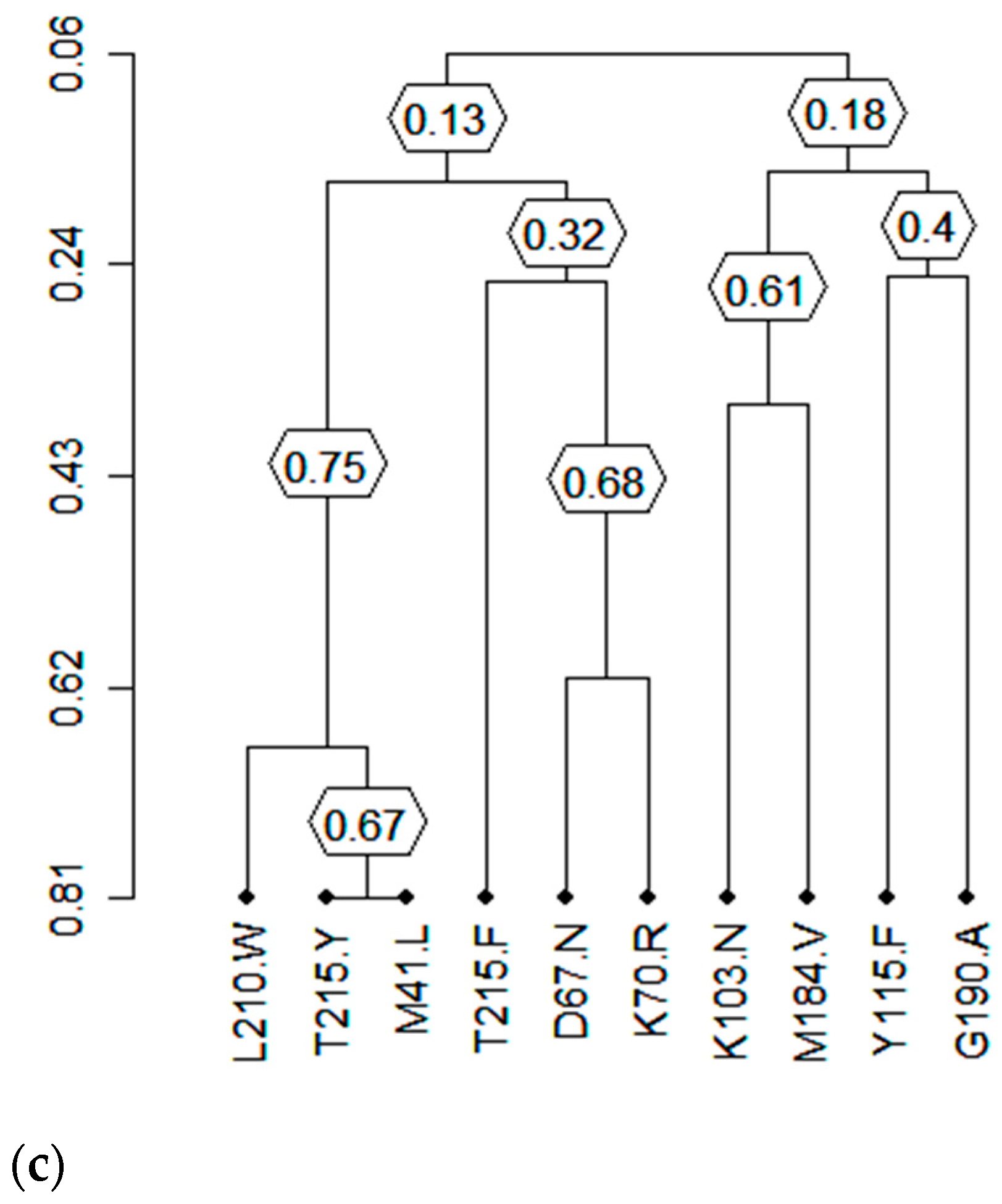
2.6. Cluster Analysis
2.7. Ethical Approval and Informed Consent
3. Results
3.1. Demographic and Clinical Characteristics of Study Participants
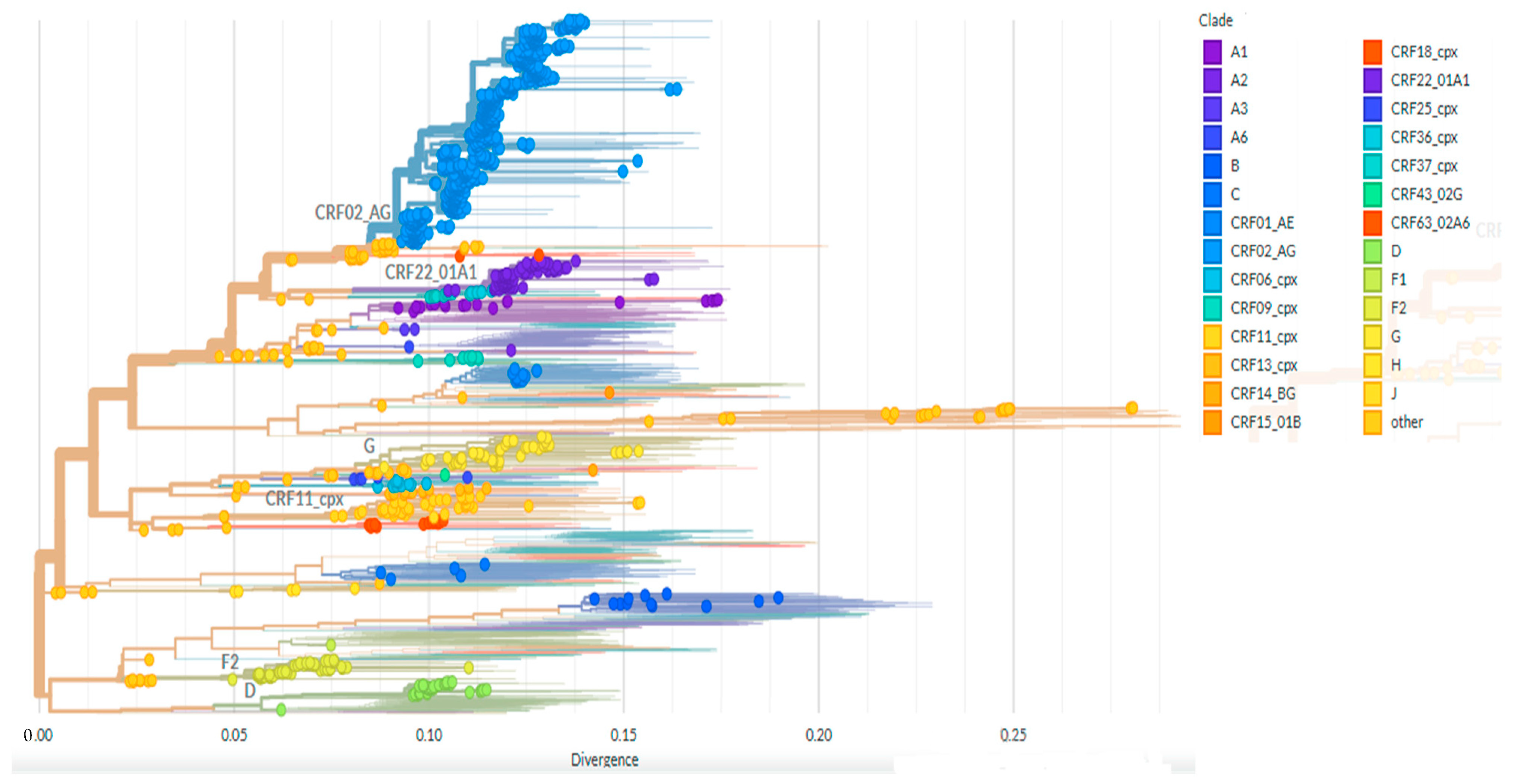
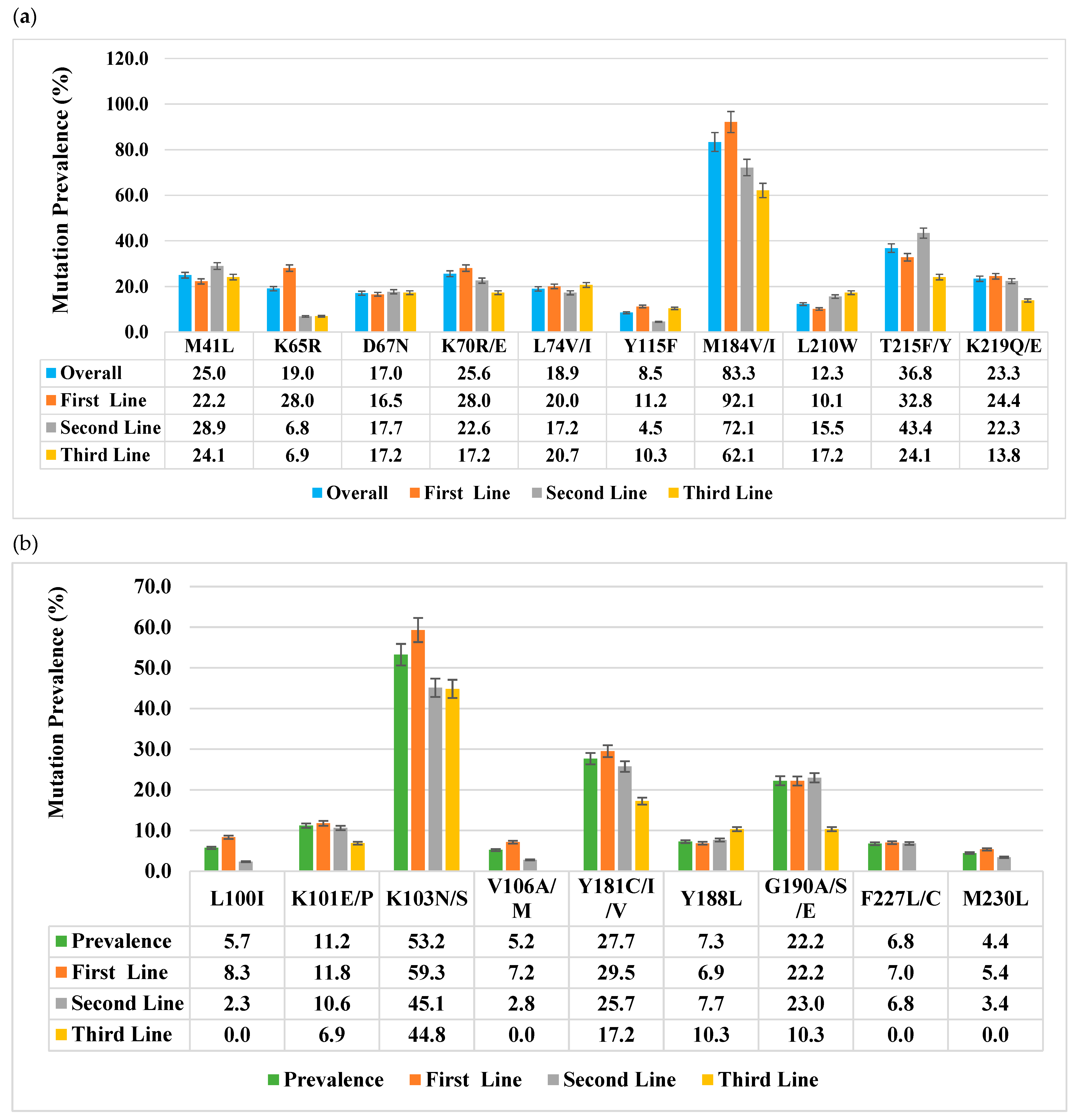
3.2. Viral Subtype Distribution and Reverse Transcriptase Drug Resistance Mutations
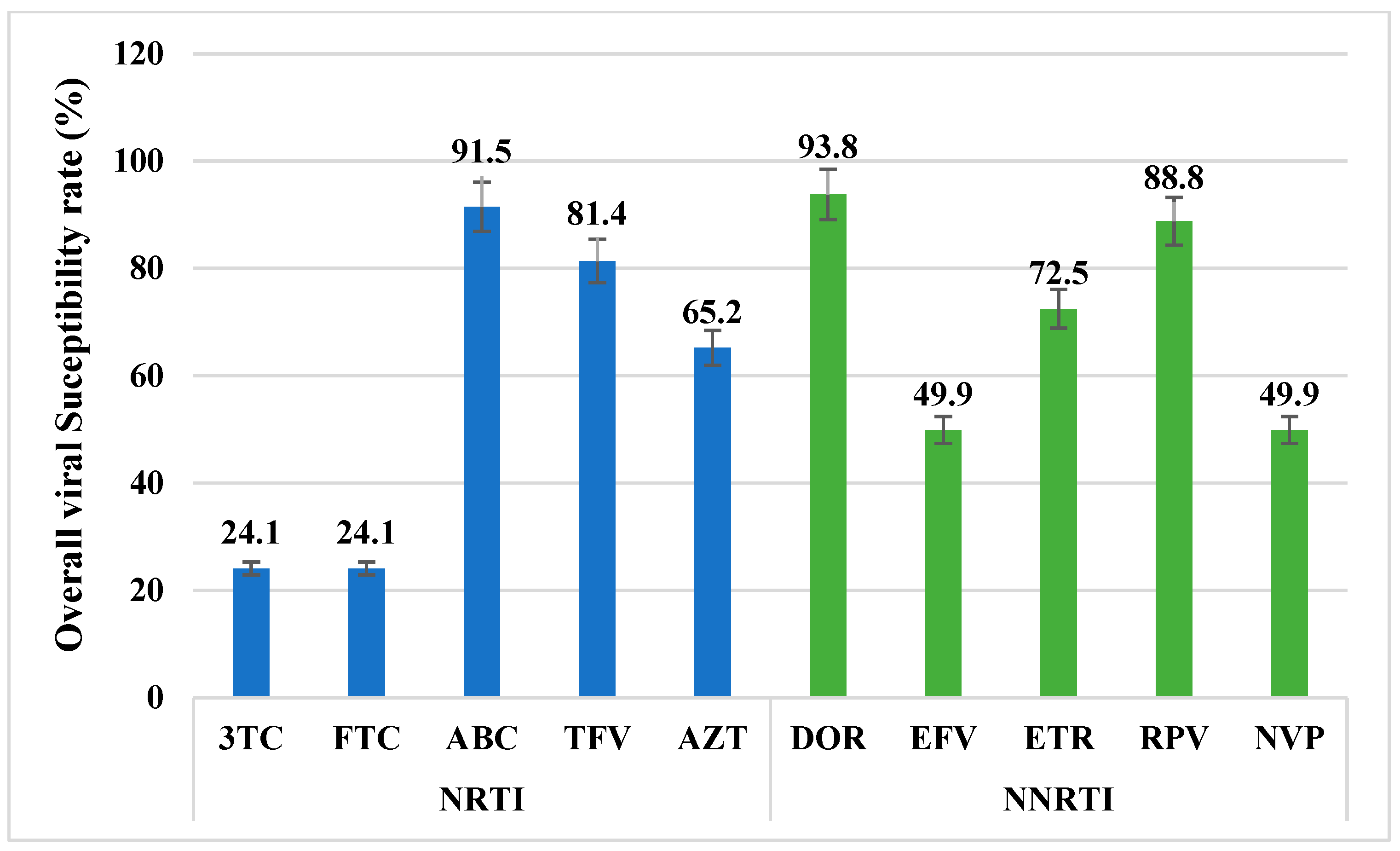
3.3. Antiretroviral Drugs Overall Susceptibility Rate
3.4. Covariation of Major Reverse Transcriptase DRMs with M184V Mutation
3.5. Major Reverse Transcriptase DRMs Involved in Positive Correlations with M184V
3.6. Major Reverse Transcriptase DRMs Involved in Negative Correlations with M184V
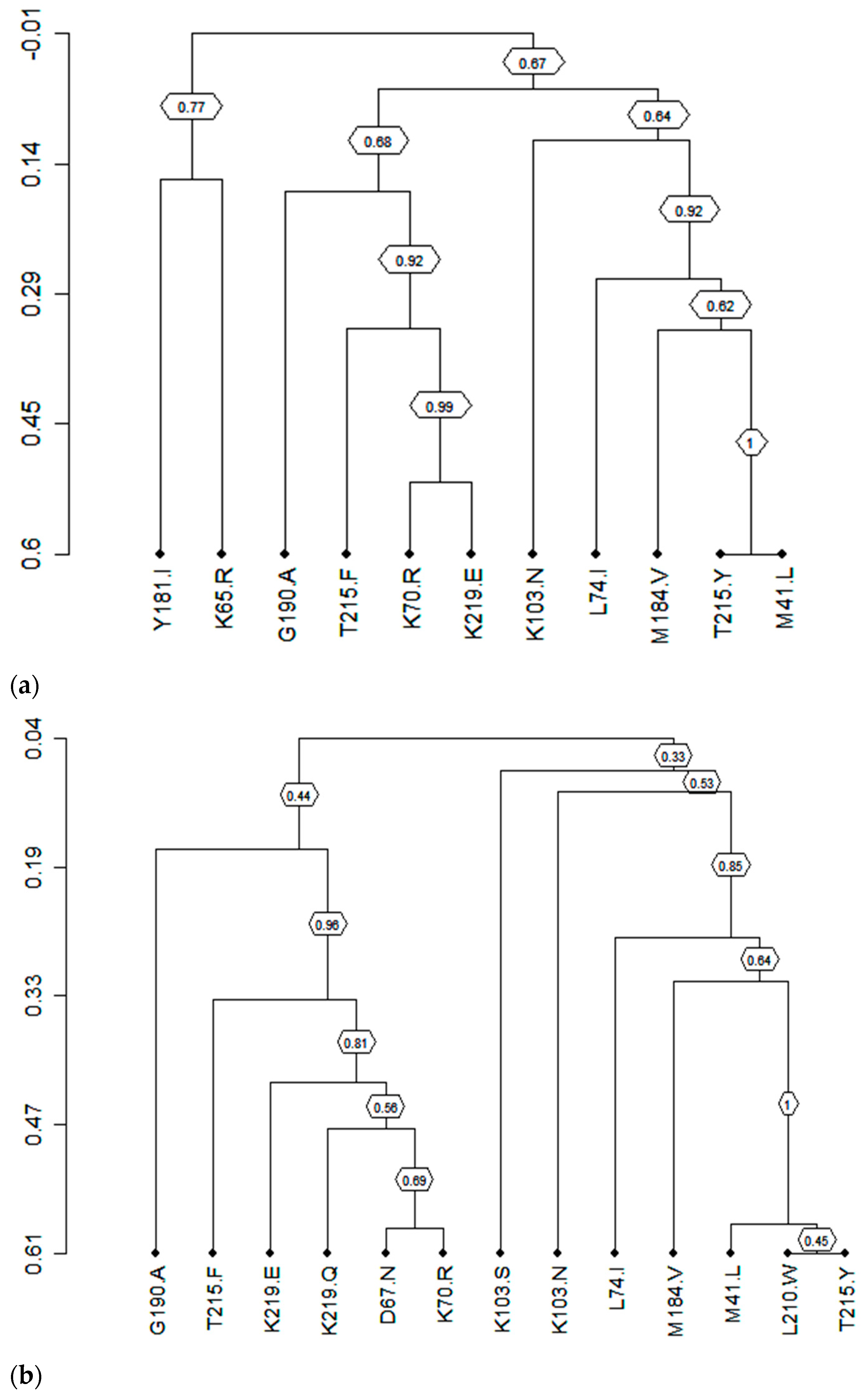
3.7. Clusters of Correlated Mutations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNAIDS Global HIV & AIDS Statistics—Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 18 May 2024).
- Fokam, J.; Sosso, S.M.; Yagai, B.; Billong, S.C.; Mbadie, R.E.D.; Simo, R.K.; Edimo, S.V.; Nka, A.D.; Ayissi, A.T.; Yimga, J.F.; et al. Viral suppression in adults, adolescents and children receiving antiretroviral therapy in Cameroon: Adolescents at high risk of virological failure in the era of “test and treat”. AIDS Res. Ther. 2019, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Røge, B.; Barfod, T.; Kirk, O.; Katzenstein, T.; Obel, N.; Nielsen, H.; Pedersen, C.; Mathiesen, L.; Lundgren, J.; Gerstoft, J. Resistance profiles and adherence at primary virological failure in three different highly active antiretroviral therapy regimens: Analysis of failure rates in a randomized study. HIV Med. 2004, 5, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Meresse, M.; March, L.; Kouanfack, C.; Bonono, R.-C.; Boyer, S.; Laborde-Balen, G.; Aghokeng, A.; Suzan-Monti, M.; Delaporte, E.; Spire, B.; et al. Patterns of adherence to antiretroviral therapy and HIV drug resistance over time in the S tratall ANRS 12110/ ESTHER trial in C ameroon. HIV Med. 2014, 15, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Sigaloff, K.C.; Calis, J.C.; Geelen, S.P.; van Vugt, M.; de Wit, T.F.R. HIV-1-resistance-associated mutations after failure of first-line antiretroviral treatment among children in resource-poor regions: A systematic review. Lancet Infect. Dis. 2011, 11, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Cortez, K.J.; Maldarelli, F. Clinical Management of HIV Drug Resistance. Viruses 2011, 3, 347–378. [Google Scholar] [CrossRef]
- Conway, B. The Role of Adherence to Antiretroviral Therapy in the Management of HIV Infection. Am. J. Ther. 2007, 45, S14–S18. [Google Scholar] [CrossRef]
- Fokam, J.; Chenwi, C.A.; Tala, V.; Takou, D.; Santoro, M.M.; Teto, G.; Dambaya, B.; Anubodem, F.; Semengue, E.N.J.; Beloumou, G.; et al. Pre-Treatment HIV Drug Resistance and Genetic Diversity in Cameroon: Implications for First-Line Regimens. Viruses 2023, 15, 1458. [Google Scholar] [CrossRef] [PubMed]
- Spreen, W.R.; Margolis, D.A.; Pottage, J.C. Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr. Opin. HIV AIDS 2013, 8, 565–571. [Google Scholar] [CrossRef]
- Michailidis, E.; Marchand, B.; Kodama, E.N.; Singh, K.; Matsuoka, M.; Kirby, K.A.; Ryan, E.M.; Sawani, A.M.; Nagy, E.; Ashida, N.; et al. Mechanism of Inhibition of HIV-1 Reverse Transcriptase by 4′-Ethynyl-2-fluoro-2′-deoxyadenosine Triphosphate, a Translocation-defective Reverse Transcriptase Inhibitor. J. Biol. Chem. 2009, 284, 35681–35691. [Google Scholar]
- Michailidis, E.; Huber, A.D.; Ryan, E.M.; Ong, Y.T.; Leslie, M.D.; Matzek, K.B.; Singh, K.; Marchand, B.; Hagedorn, A.N.; Kirby, K.A.; et al. 4′-Ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) Inhibits HIV-1 Reverse Transcriptase with Multiple Mechanisms. J. Biol. Chem. 2014, 289, 24533–24548. [Google Scholar] [CrossRef]
- Schürmann, D.; Rudd, D.J.; Zhang, S.; De Lepeleire, I.; Robberechts, M.; Friedman, E.; Keicher, C.; Hüser, A.; Hofmann, J.; A Grobler, J.; et al. Safety, pharmacokinetics, and antiretroviral activity of islatravir (ISL, MK-8591), a novel nucleoside reverse transcriptase translocation inhibitor, following single-dose administration to treatment-naive adults infected with HIV-1: An open-label, phase 1b, consecutive-panel trial. Lancet HIV 2020, 7, e164–e172. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.P.; Zang, X.; Barrett, S.; Goodey, A.; Heimbach, T.; Weissler, V.L.; Leyssens, C.; Reynders, T.; Vargo, R.; Liu, Y.; et al. Next-Generation Islatravir Implants Projected To Provide Yearly HIV Prophylaxis—CROI Conference. 2021. Available online: https://www.croiconference.org/abstract/next-generation-islatravir-implants-projected-to-provide-yearly-hiv-prophylaxis/ (accessed on 14 May 2024).
- Diamond, T.L.; Goh, S.L.; Ngo, W.; Rodriguez, S.; Xu, M.; Klein, D.J.; Grobler, J.A.; Asante-Appiah, E. No antagonism or cross-resistance and a high barrier to the emergence of resistance in vitro for the combination of islatravir and lenacapavir. Antimicrob. Agents Chemother. 2024, 68, e0033424. [Google Scholar] [CrossRef] [PubMed]
- Cilento, M.E.; Reeve, A.B.; Michailidis, E.; Ilina, T.V.; Nagy, E.; Mitsuya, H.; Parniak, M.A.; Tedbury, P.R.; Sarafianos, S.G. Development of Human Immunodeficiency Virus Type 1 Resistance to 4′-Ethynyl-2-Fluoro-2′-Deoxyadenosine Starting with Wild-Type or Nucleoside Reverse Transcriptase Inhibitor-Resistant Strains. Antimicrob. Agents Chemother. 2021, 65, e01167-21. [Google Scholar] [CrossRef]
- Diamond, T.L.; Ngo, W.; Xu, M.; Goh, S.L.; Rodriguez, S.; Lai, M.-T.; Asante-Appiah, E.; Grobler, J.A. Islatravir Has a High Barrier to Resistance and Exhibits a Differentiated Resistance Profile from Approved Nucleoside Reverse Transcriptase Inhibitors (NRTIs). Antimicrob. Agents Chemother. 2022, 66, e00133-22. [Google Scholar] [CrossRef] [PubMed]
- Takou, D.; Fokam, J.; Teto, G.; Santoro, M.-M.; Ceccherini-Silberstein, F.; Nanfack, A.J.; Sosso, S.M.; Dambaya, B.; Salpini, R.; Billong, S.C.; et al. HIV-1 drug resistance testing is essential for heavily-treated patients switching from first- to second-line regimens in resource-limited settings: Evidence from routine clinical practice in Cameroon. BMC Infect. Dis. 2019, 19, 246. [Google Scholar] [CrossRef]
- Diallo, K.; Götte, M.; Wainberg, M.A. Molecular Impact of the M184V Mutation in Human Immunodeficiency Virus Type 1 Reverse Transcriptase. Antimicrob. Agents Chemother. 2003, 47, 3377–3383. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.Y.; Cara, A.; Gallo, R.C.; Lori, F. Low levels of deoxynucleotides in peripheral blood lymphocytes: A strategy to inhibit human immunodeficiency virus type 1 replication. Proc. Natl. Acad. Sci. USA 1993, 90, 8925–8928. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Gao, Q.; Li, X.; Parniak, M.A.; Wainberg, M.A. Novel mutation in the human immunodeficiency virus type 1 reverse transcriptase gene that encodes cross-resistance to 2′,3′-dideoxyinosine and 2′,3′-dideoxycytidine. J. Virol. 1992, 66, 7128–7135. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.D.; Anton, K.E.; Mulato, A.S.; Lamy, P.D.; Cherrington, J.M. Human Immunodeficiency Virus Type 1 Expressing the Lamivudine-Associated M184V Mutation in Reverse Transcriptase Shows Increased Susceptibility to Adefovir and Decreased Replication Capability In Vitro. J. Infect. Dis. 1999, 179, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Clair, M.H.S.; Martin, J.L.; Tudor-Williams, G.; Bach, M.C.; Vavro, C.L.; King, D.M.; Kellam, P.; Kemp, S.D.; Larder, B.A. Resistance to ddI and Sensitivity to AZT Induced by a Mutation in HIV-1 Reverse Transcriptase. Science 1991, 253, 1557–1559. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, M.; Alnadaf, T.; Cousens, D. Combination of mutations in human immunodeficiency virus type 1 reverse transcriptase required for resistance to the carbocyclic nucleoside 1592U89. Antimicrob. Agents Chemother. 1997, 41, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Masquelier, B.; Descamps, D.; Carrière, I.; Ferchal, F.; Collin, G.; Denayrolles, M.; Ruffault, A.; Chanzy, B.; Izopet, J.; Buffet-Janvresse, C.; et al. Zidovudine resensitization and dual HIV-1 resistance to zidovudine and lamivudine in the delta lamivudine roll-over study. Antivir. Ther. 1999, 4, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, M.; Schuurman, R.; de Jong, D.; van Leeuwen, R.; Lange, J.; Danner, S.; Keulen, W.; de Groot, T.; Boucher, C.A.B. Lamivudine-Resistant Human Immunodeficiency Virus Type 1 Variants (184V) Require Multiple Amino Acid Changes to Become Co-Resistant to Zidovudine In Vivo. J. Infect. Dis. 1997, 176, 398–405. [Google Scholar] [CrossRef]
- Aksamentov, I.; Roemer, C.; Hodcroft, E.B.; Neher, R.A. Nextclade: Clade assignment, mutation calling and quality control for viral genomes. J. Open Source Softw. 2021, 6, 3773. [Google Scholar] [CrossRef]
- Teto, G.; Nka, A.D.; Fokam, J.; Bouba, Y.; Takou, D.; Fabeni, L.; Carioti, L.; Armenia, D.; Semengue, E.N.J.; Dambaya, B.; et al. Detection of Gag C-terminal mutations among HIV-1 non-B subtypes in a subset of Cameroonian patients. Sci. Rep. 2022, 12, 1374. [Google Scholar] [CrossRef]
- Sing, T.; Svicher, V.; Beerenwinkel, N.; Ceccherini-Silberstein, F.; Däumer, M.; Kaiser, R.; Walter, H.; Korn, K.; Hoffmann, D.; Oette, M.; et al. Characterization of Novel HIV Drug Resistance Mutations Using Clustering, Multidimensional Scaling and SVM-Based Feature Ranking. In Knowledge Discovery in Databases: PKDD 2005; Jorge, A.M., Torgo, L., Brazdil, P., Camacho, R., Gama, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 3721, pp. 285–296. [Google Scholar]
- HIV Drug Resistance Database. Available online: https://hivdb.stanford.edu/ (accessed on 22 July 2024).
- Ceccherini-Silberstein, F.; Svicher, V.; Sing, T.; Artese, A.; Santoro, M.M.; Forbici, F.; Bertoli, A.; Alcaro, S.; Palamara, G.; Monforte, A.D.; et al. Characterization and Structural Analysis of Novel Mutations in Human Immunodeficiency Virus Type 1 Reverse Transcriptase Involved in the Regulation of Resistance to Nonnucleoside Inhibitors. J. Virol. 2007, 81, 11507–11519. [Google Scholar] [CrossRef] [PubMed]
- Smyth, R.P.; Davenport, M.P.; Mak, J. The origin of genetic diversity in HIV-1. Virus Res. 2012, 169, 415–429. [Google Scholar] [CrossRef] [PubMed]
- NIH. What to Start: Initial Combination Antiretroviral Regimens|NIH. 2022. Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/what-start-initial-combination (accessed on 22 July 2024).
- Lu, D.-Y.; Wu, H.-Y.; Yarla, N.S.; Xu, B.; Ding, J.; Lu, T.-R. HAART in HIV/AIDS Treatments: Future Trends. Infect. Disord.-Drug Targets 2018, 18, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Tzou, P.L.; Rhee, S.-Y.; Descamps, D.; Clutter, D.S.; Hare, B.; Mor, O.; Grude, M.; Parkin, N.; Jordan, M.R.; Bertagnolio, S.; et al. Integrase strand transfer inhibitor (INSTI)-resistance mutations for the surveillance of transmitted HIV-1 drug resistance. J. Antimicrob. Chemother. 2020, 75, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Ndashimye, E.; Li, Y.; Reyes, P.S.; Avino, M.; Olabode, A.S.; Kityo, C.M.; Kyeyune, F.; Nankya, I.; E Quiñones-Mateu, M.; Barr, S.D.; et al. High-level resistance to bictegravir and cabotegravir in subtype A- and D-infected HIV-1 patients failing raltegravir with multiple resistance mutations. J. Antimicrob. Chemother. 2021, 76, 2965–2974. [Google Scholar] [CrossRef]
- Ismail, S.D.; Pankrac, J.; Ndashimye, E.; Prodger, J.L.; Abrahams, M.-R.; Mann, J.F.S.; Redd, A.D.; Arts, E.J. Addressing an HIV cure in LMIC. Retrovirology 2021, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Cilento, M.E.; Wen, X.; Reeve, A.B.; Ukah, O.B.; Snyder, A.A.; Carrillo, C.M.; Smith, C.P.; Edwards, K.; Wahoski, C.C.; Kitzler, D.R.; et al. HIV-1 Resistance to Islatravir/Tenofovir Combination Therapy in Wild-Type or NRTI-Resistant Strains of Diverse HIV-1 Subtypes. Viruses 2023, 15, 1990. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.-M.; Yazdanpanah, Y.; Saud, A.A.; Bettacchi, C.; Anania, C.C.; DeJesus, E.; Klopfer, S.O.; Grandhi, A.; Eves, K.; Robertson, M.N.; et al. Islatravir in combination with doravirine for treatment-naive adults with HIV-1 infection receiving initial treatment with islatravir, doravirine, and lamivudine: A phase 2b, randomised, double-blind, dose-ranging trial. Lancet HIV 2021, 8, e324–e333. [Google Scholar] [CrossRef]
- Brenner, B.G.; Oliveira, M.; Ibanescu, R.-I.; Routy, J.-P.; Thomas, R. Doravirine responses to HIV-1 viruses bearing mutations to NRTIs and NNRTIs under in vitro selective drug pressure. J. Antimicrob. Chemother. 2023, 78, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.-T.; Feng, M.; Xu, M.; Ngo, W.; Diamond, T.L.; Hwang, C.; Grobler, J.A.; Hazuda, D.J.; Asante-Appiah, E. Doravirine and Islatravir Have Complementary Resistance Profiles and Create a Combination with a High Barrier to Resistance. Antimicrob. Agents Chemother. 2022, 66, e0222321. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Ibanescu, R.I.; Pham, H.T.; Brenner, B.; Mesplède, T.; Wainberg, M.A. The M184I/V and K65R nucleoside resistance mutations in HIV-1 prevent the emergence of resistance mutations against dolutegravir. AIDS 2016, 30, 2267–2273. [Google Scholar] [CrossRef]
| First Line (n = 671) | Second Line (n = 470) | Third Line (n = 29) | Overall (n= 1170) | p-Value a | ||
|---|---|---|---|---|---|---|
| Gender | Female (%) | 437 (65.1) | 264 (56.2) | 13 (44.8) | 714 (61.0) | 0.032 |
| Male (%) | 234 (34.9) | 206 (43.8) | 16 (55.2) | 456 (39.0) | ||
| Age (years), median (IQR) | 41 [33–47] | 39 [20–48] | 17 [9–38] | 40 [28–47] | <0.001 | |
| CD4 (cells/µL), median (IQR) | 186 [63–361] | 195 [58–374] | 157 [63–665] | 190 [63–665] | 0.941 | |
| Viral load (Log10 copies/mL), median (IQR) | 5 [4.2–5.5] | 5 [3.8–5.3] | 5 [3.4–5.3] | 5 [3.5–5.3] | <0.001 | |
| ART regimen n (%) | 2NRTIs + EFV | 424 (63.2) | 1 (0.2) | - | 425 (36.3) | |
| 2NRTIs + NVP | 210 (31.3) | - | - | 210 (17.9) | ||
| 2NRTIs + ATV/r | 2 (0.3) | 314 (66.8) | - | 316 (27.0) | ||
| 2NRTIs + LPV/r | - | 140 (29.8) | - | 140 (12.0) | ||
| 2NRTIs + DRV/r | - | 3 (0.6) | - | 3 (0.3) | ||
| 2NRTIs + NFV/r | - | 1 (0.2) | - | 1 (0.9 × 10−1) | ||
| 2NRTIs + DTG | 34 (2.9) | - | 22 (75.9) | 56 (4.8) | ||
| Others | 1 (0.1) | 11 (2.3) | 7 (24.1) | 19 (1.6) | ||
| ART Line | RT Mutation | Frequency (%) a | Covariated Mutations | Frequency (%) b | Covariated Frequency (%) | phi | p-Value c |
|---|---|---|---|---|---|---|---|
| I | M184V | 561 (83.61) | K70R | 122 (18.18) | 84 (12.52) | 0.19 | 6.69 × 10−6 |
| L74I | 111 (16.54) | 74 (11.03) | 0.18 | 6.69 × 10−6 | |||
| T215F | 109 (16.24) | 88 (13.11) | 0.18 | 1.89 × 10−5 | |||
| M41L | 149 (22.21) | 120 (17.88) | 0.19 | 2.60 × 10−5 | |||
| T215Y | 111 (16.54 | 92 (13.71) | 0.18 | 4.79 × 10−5 | |||
| K219E | 97 (14.46) | 68 10.13) | 0.14 | 7.38 × 10−3 | |||
| K65R | 188 (28.02) | 108 (16.10) | −0.26 | 2.15 × 10−7 | |||
| K103N | 378 (56.33) | 304 (45.31) | 0.16 | 3.37 × 10−3 | |||
| G190A | 135 (20.12) | 106 (15.80) | 0.12 | 4.91 × 10−2 | |||
| Y181I | 9 (1.34) | 1 (0.15) | −0.16 | 3.09 × 10−2 | |||
| II | 327 (69.57) | T215Y | 110 (23.40) | 94 (20.00) | 0.34 | 9.73 × 10−14 | |
| M41L | 136 (28.93) | 114 (24.26) | 0.33 | 1.08 × 10−11 | |||
| D67N | 83 (17.66) | 75 (15.96) | 0.28 | 1.73 × 10−9 | |||
| T215F | 94 (20.00) | 87 (18.51) | 0.28 | 2.06 × 10−9 | |||
| L210W | 73 (15.53) | 63 (13.40) | 0.26 | 4.93 × 10−8 | |||
| K70R | 93 (19.79) | 81 (17.23) | 0.26 | 2.01 × 10−7 | |||
| L74I | 61 (12.98) | 50 (10.64) | 0.20 | 2.35 × 10−4 | |||
| K219E | 53 (11.28) | 45 (9.57) | 0.19 | 3.31 × 10−4 | |||
| K219Q | 52 (11.06) | 43 (9.15) | 0.19 | 5.25 × 10−4 | |||
| G190A | 97 (20.64) | 84 (17.87) | 0.22 | 3.49 × 10−5 | |||
| K103N | 185 (39.36) | 136 (28.94) | 0.16 | 1.59 × 10−2 | |||
| K103S | 27 (5.74) | 21 (4.47) | 0.14 | 2.77 × 10−2 | |||
| III | 18 (62.1) | T215Y | 5 (17.24) | 4 (13.79) | 0.36 | 0.13 | |
| D67N | 5 (17.24) | 2 (6.90) | 0.36 | 0.13 | |||
| M41L | 7 (24.14) | 3 (10.34) | 0.27 | 0.20 | |||
| K70R | 4 (13.79) | 3 (10.34) | 0.31 | 0.27 | |||
| Y115F | 3 (10.34) | 3 (10.34) | 0.27 | 0.27 | |||
| L210W | 3 (10.34) | 2 (6.90) | 0.27 | 0.27 | |||
| T215F | 2 (6.90) | 1 (3.45) | 0.21 | 0.27 | |||
| K103N | 12 (41.38) | 10 (34.48) | 0.37 | 0.06 | |||
| Y188L | 3 (10.34) | 2 (6.90) | 0.27 | 0.27 | |||
| G190A | 3 (10.34) | 4 (13.79) | 0.27 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nka, A.D.; Bouba, Y.; Tsapi Lontsi, W.R.; Gouissi Anguechia, D.-H.; Teto, G.; Ka’e, A.c.; Semengue, E.N.J.; Ambe Chenwi, C.; Takou, D.; Forgwei, L.; et al. Tenofovir and Doravirine Are Potential Reverse-Transcriptase Analogs in Combination with the New Reverse-Transcriptase Translocation Inhibitor (Islatravir) Among Treatment-Experienced Patients in Cameroon: Designing Future Treatment Strategies for Low- and Middle-Income Countries. Viruses 2025, 17, 69. https://doi.org/10.3390/v17010069
Nka AD, Bouba Y, Tsapi Lontsi WR, Gouissi Anguechia D-H, Teto G, Ka’e Ac, Semengue ENJ, Ambe Chenwi C, Takou D, Forgwei L, et al. Tenofovir and Doravirine Are Potential Reverse-Transcriptase Analogs in Combination with the New Reverse-Transcriptase Translocation Inhibitor (Islatravir) Among Treatment-Experienced Patients in Cameroon: Designing Future Treatment Strategies for Low- and Middle-Income Countries. Viruses. 2025; 17(1):69. https://doi.org/10.3390/v17010069
Chicago/Turabian StyleNka, Alex Durand, Yagai Bouba, Wilfried Rooker Tsapi Lontsi, Davy-Hyacinte Gouissi Anguechia, Georges Teto, Aude christelle Ka’e, Ezechiel Ngoufack Jagni Semengue, Collins Ambe Chenwi, Désiré Takou, Lum Forgwei, and et al. 2025. "Tenofovir and Doravirine Are Potential Reverse-Transcriptase Analogs in Combination with the New Reverse-Transcriptase Translocation Inhibitor (Islatravir) Among Treatment-Experienced Patients in Cameroon: Designing Future Treatment Strategies for Low- and Middle-Income Countries" Viruses 17, no. 1: 69. https://doi.org/10.3390/v17010069
APA StyleNka, A. D., Bouba, Y., Tsapi Lontsi, W. R., Gouissi Anguechia, D.-H., Teto, G., Ka’e, A. c., Semengue, E. N. J., Ambe Chenwi, C., Takou, D., Forgwei, L., Tekoh, T. A.-K., Ngueko, A. M. K., Fokou, B. B., Efakika Gabisa, J., Tchouaket, M. C. T., TognaPabo, W. L., Ayuk Ngwese, D. T., Njiki Bikoi, J., Armenia, D., ... Fokam, J. (2025). Tenofovir and Doravirine Are Potential Reverse-Transcriptase Analogs in Combination with the New Reverse-Transcriptase Translocation Inhibitor (Islatravir) Among Treatment-Experienced Patients in Cameroon: Designing Future Treatment Strategies for Low- and Middle-Income Countries. Viruses, 17(1), 69. https://doi.org/10.3390/v17010069






