Role of the Psi Packaging Signal and Dimerization Initiation Sequence in the Organization of Rous Sarcoma Virus Gag-gRNA Co-Condensates
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmids
2.2. Protein Expression, Purification, and Labeling
2.3. RNA Production by In Vitro Transcription and Fluorescent Labeling
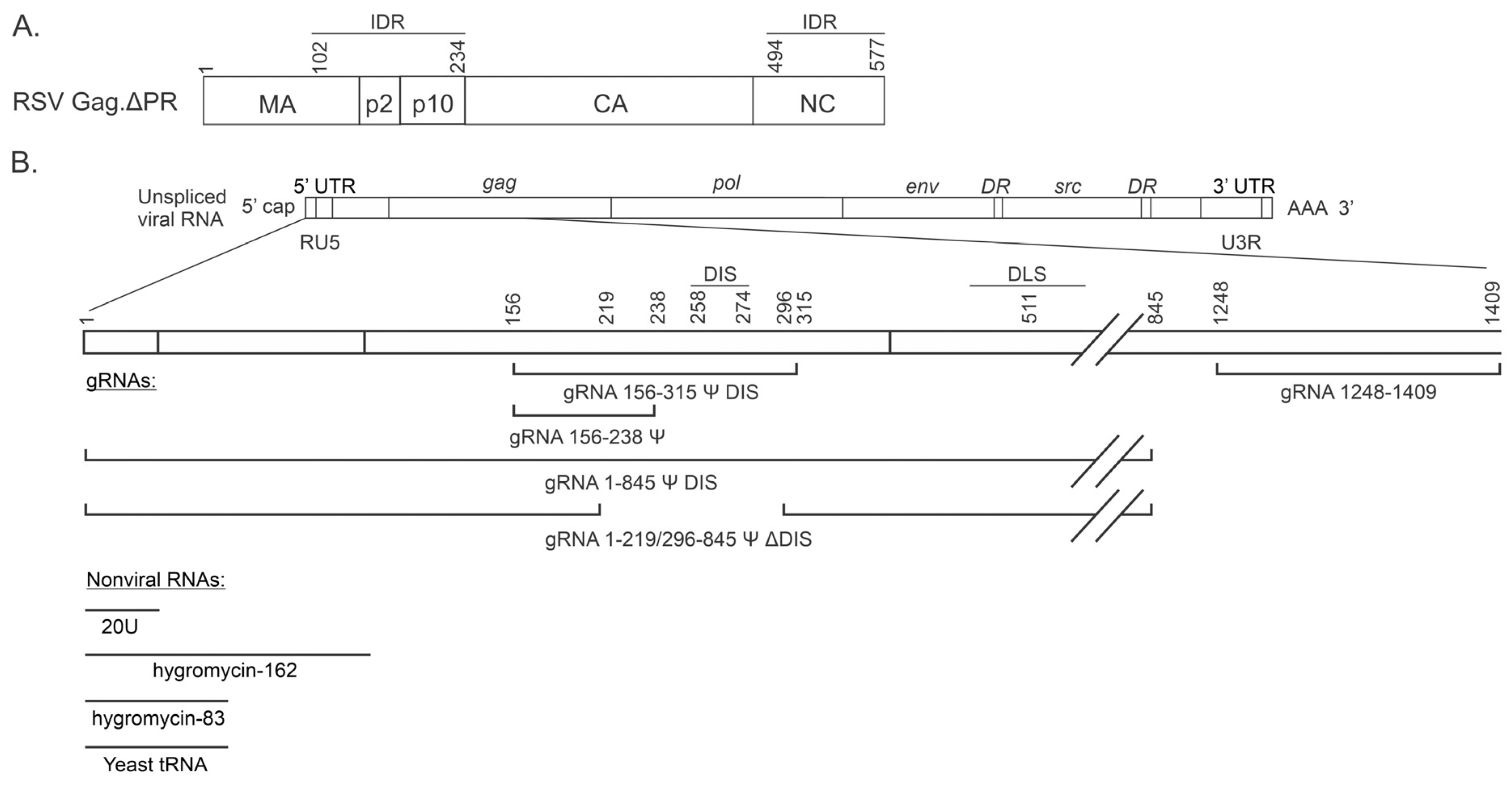
2.4. RNA Sequences
2.5. In Vitro Condensate Formation and Imaging
2.6. Quantitative Image Analysis of In Vitro Condensates
2.7. Förster Resonance Energy Transfer (FRET)
2.8. Fluorescence Recovery After Photobleaching (FRAP)
2.9. Quantitative Image and Statistical Analyses
2.10. RNA Secondary Structure Prediction
3. Results
3.1. Elucidating the Effect of Viral and Nonviral RNAs on the Biophysical Properties of RSV Gag Biomolecular Condensates
3.2. Viral and Nonviral RNAs Greatly Influence RSV Gag Condensates at a Low Gag Concentration (2.5 μM) and at a Ratio of 2 RNAs per 3000 Gag Molecules
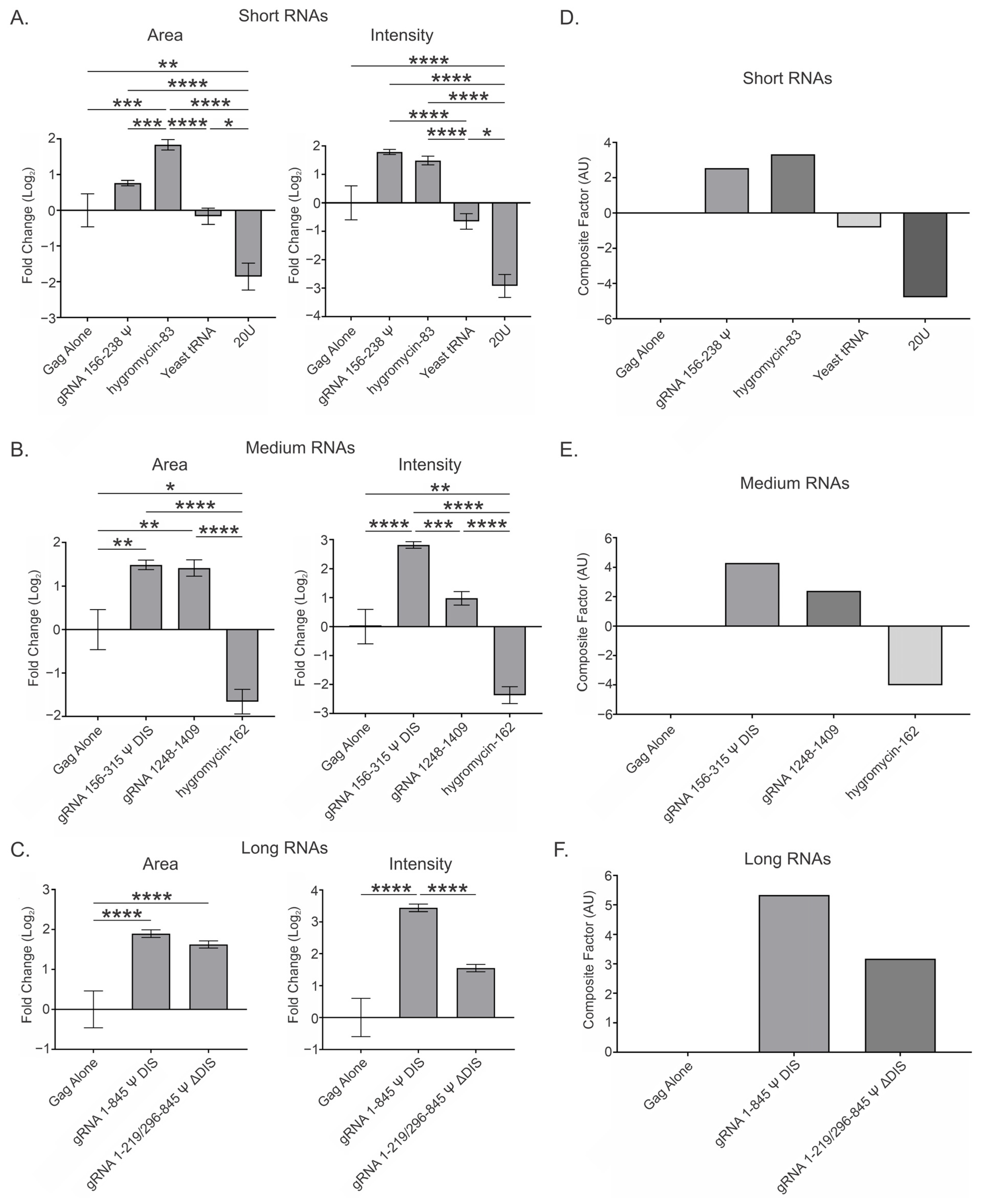
3.3. Overall Effect of Viral and Nonviral RNAs at a Low Gag Concentration (2.5 μM) and Range of RNA Ratios (0.5, 1, 2, and 4) per 3000 Gag Molecules
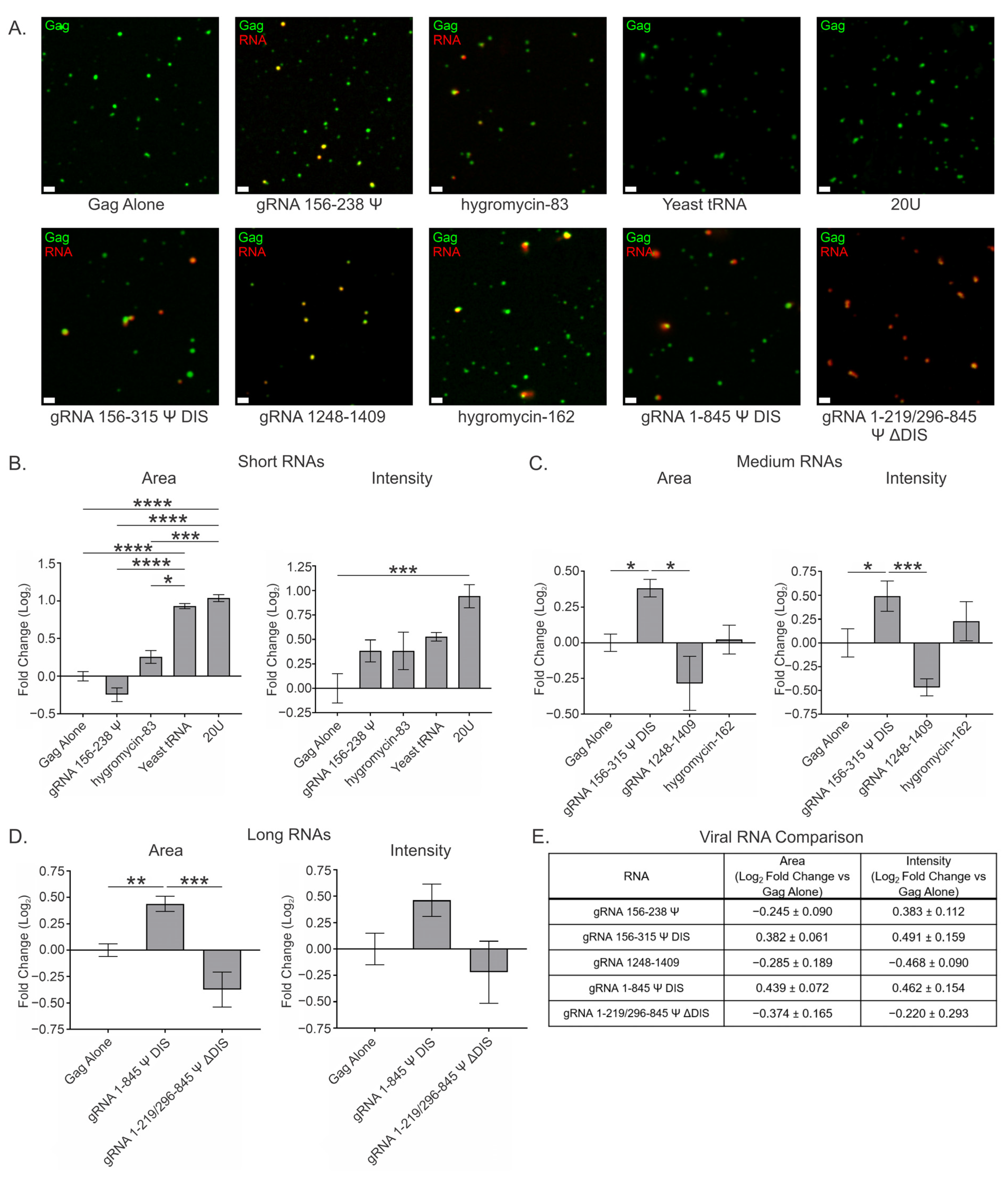
3.4. Viral and Nonviral RNAs Modestly Affect Gag Condensates at a High Gag Concentration (10 μM) at a Ratio of 2 RNAs per 3000 Gag Molecules
3.5. Overall Effect of Viral and Nonviral RNAs at High Gag Concentration (10 μM) and Range of RNA Ratios (0.5, 1, 2, and 4) per 3000 Gag Molecules
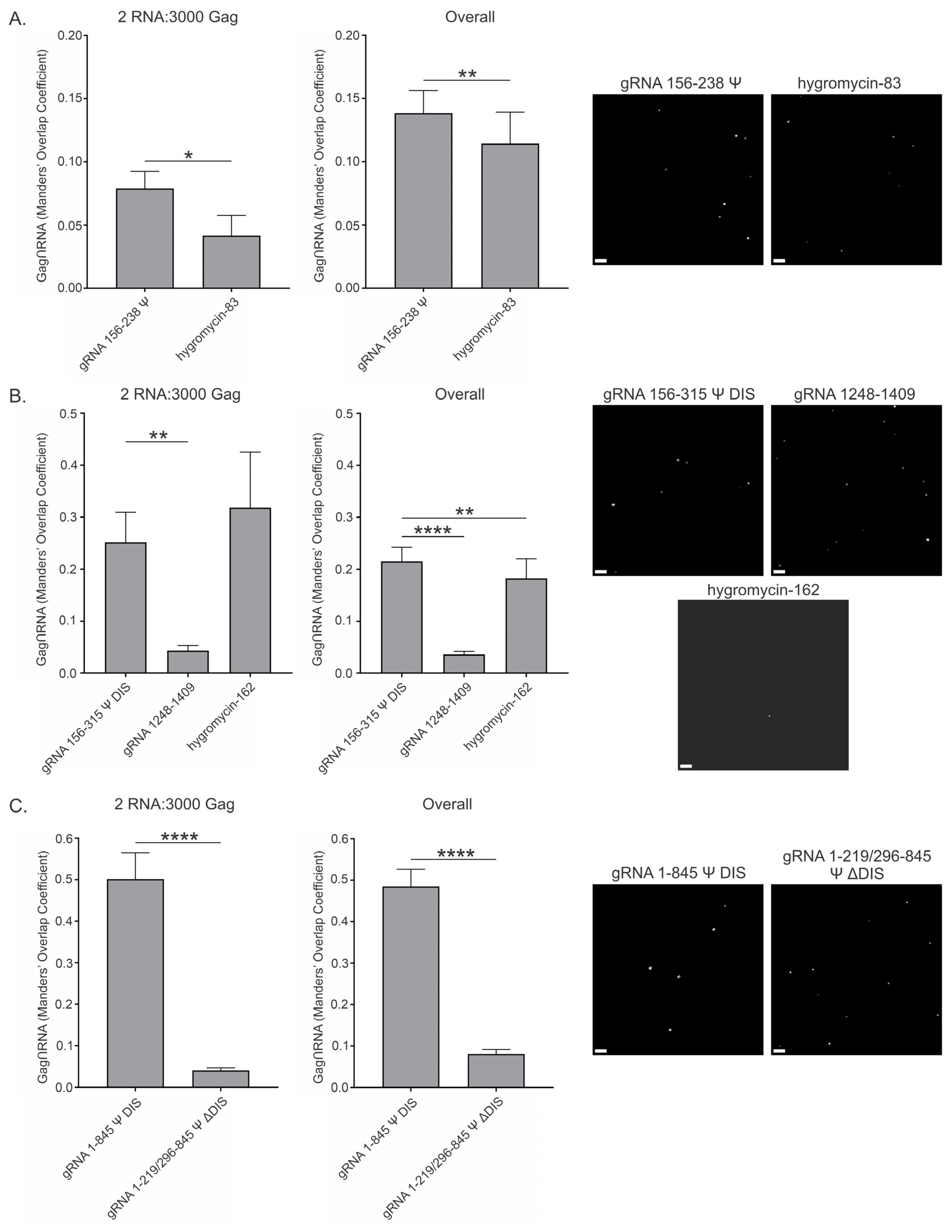
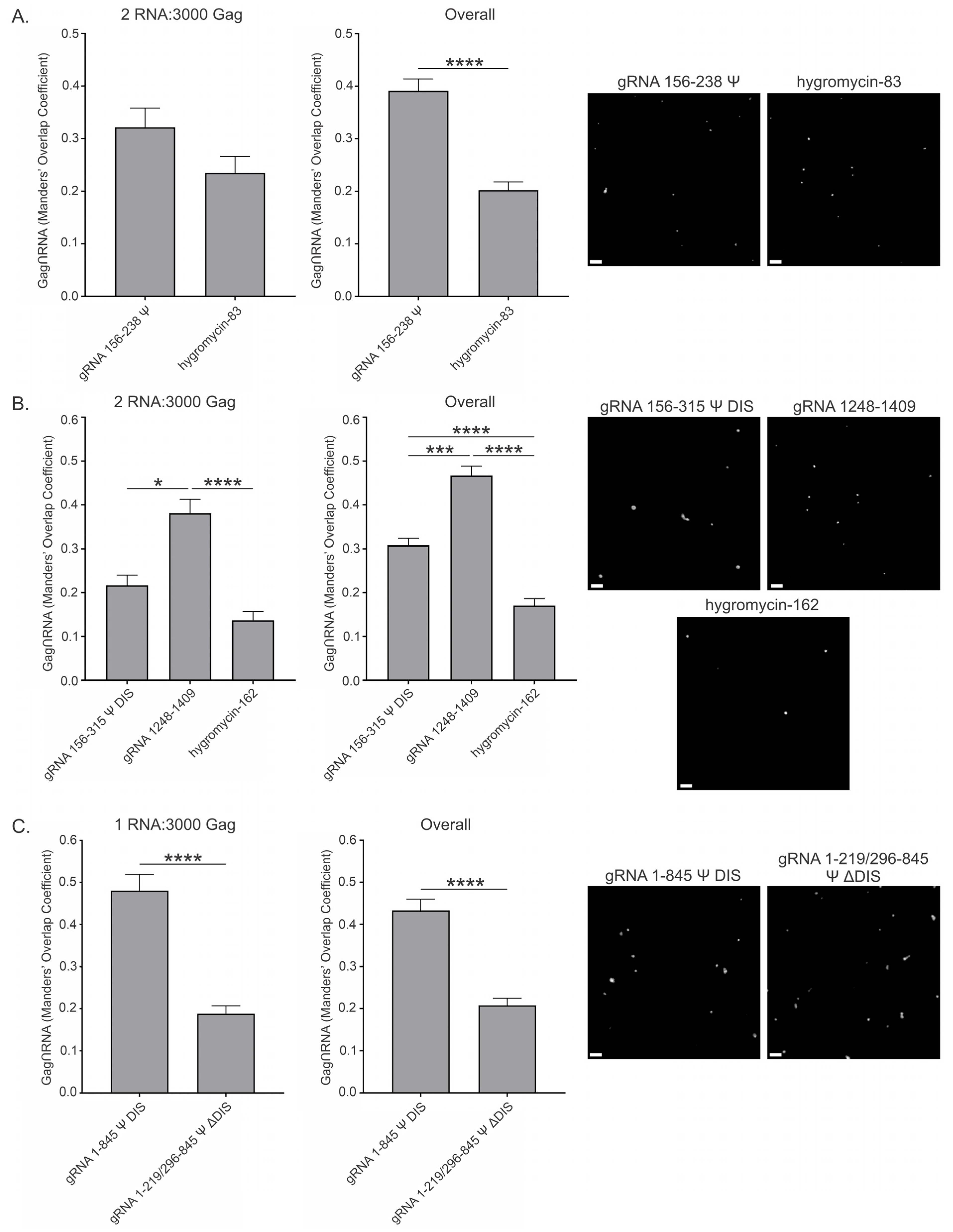
3.6. RSV Gag Colocalization at 2.5 μM and 10 μM with Viral and Nonviral RNAs
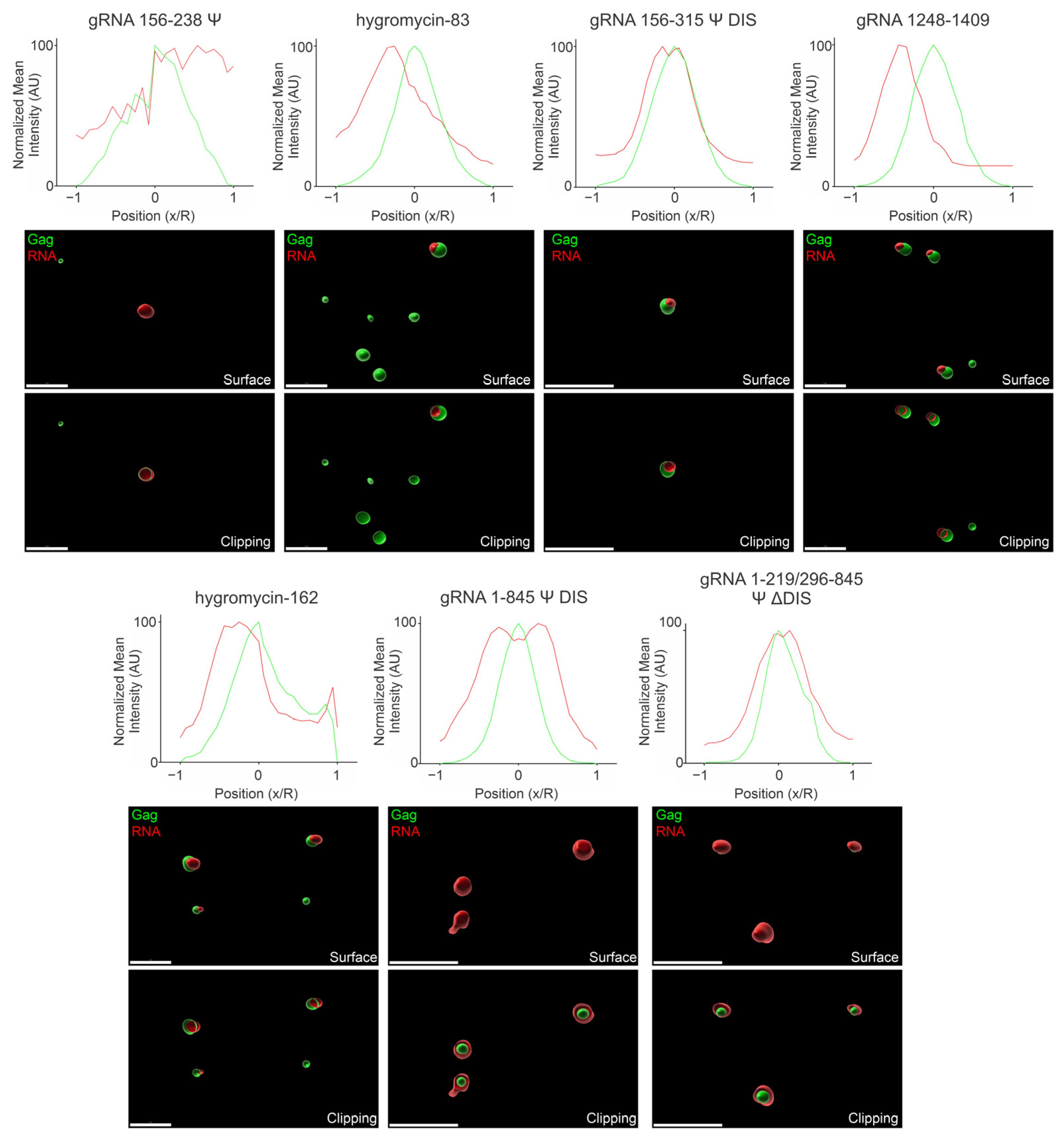
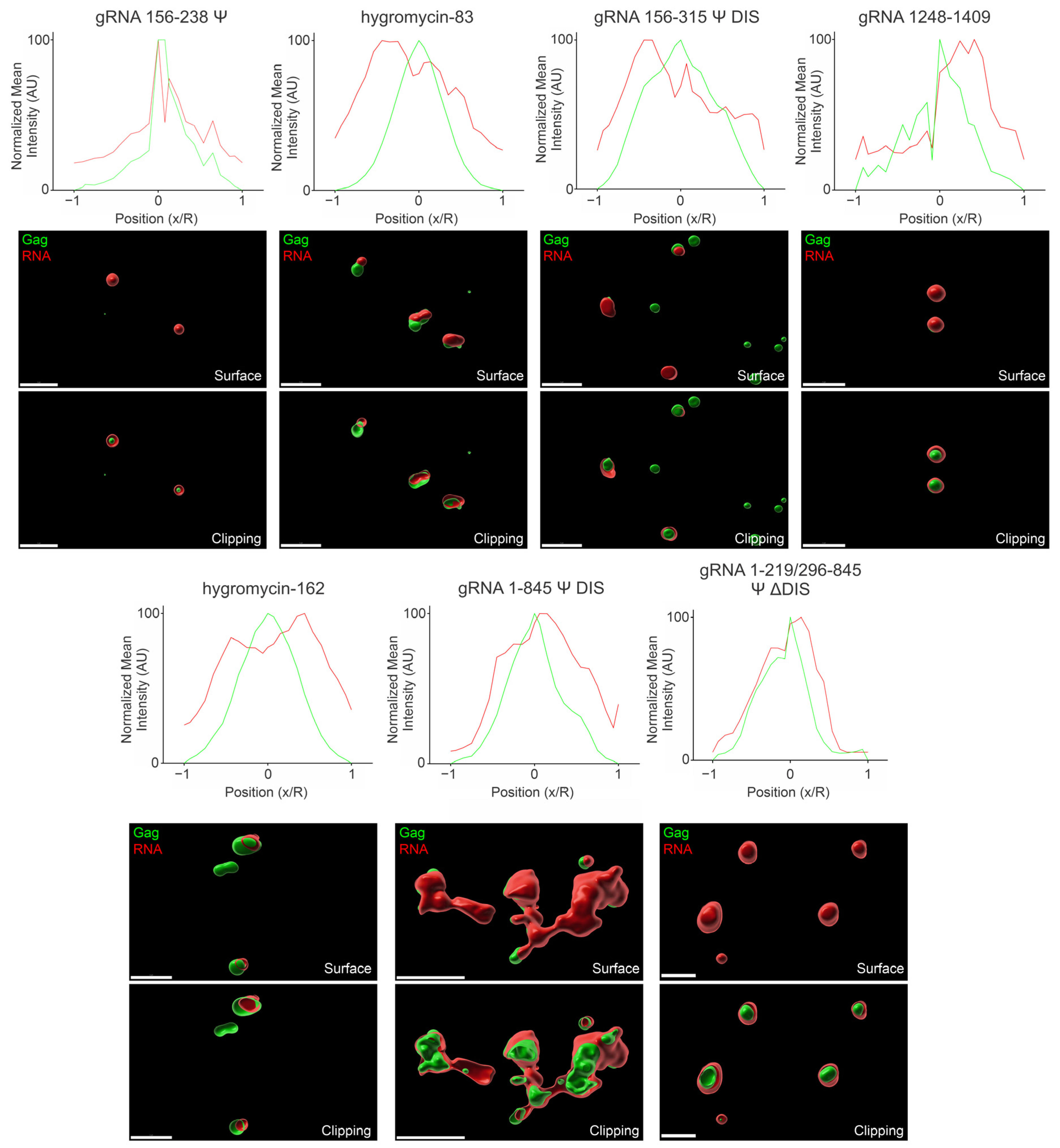
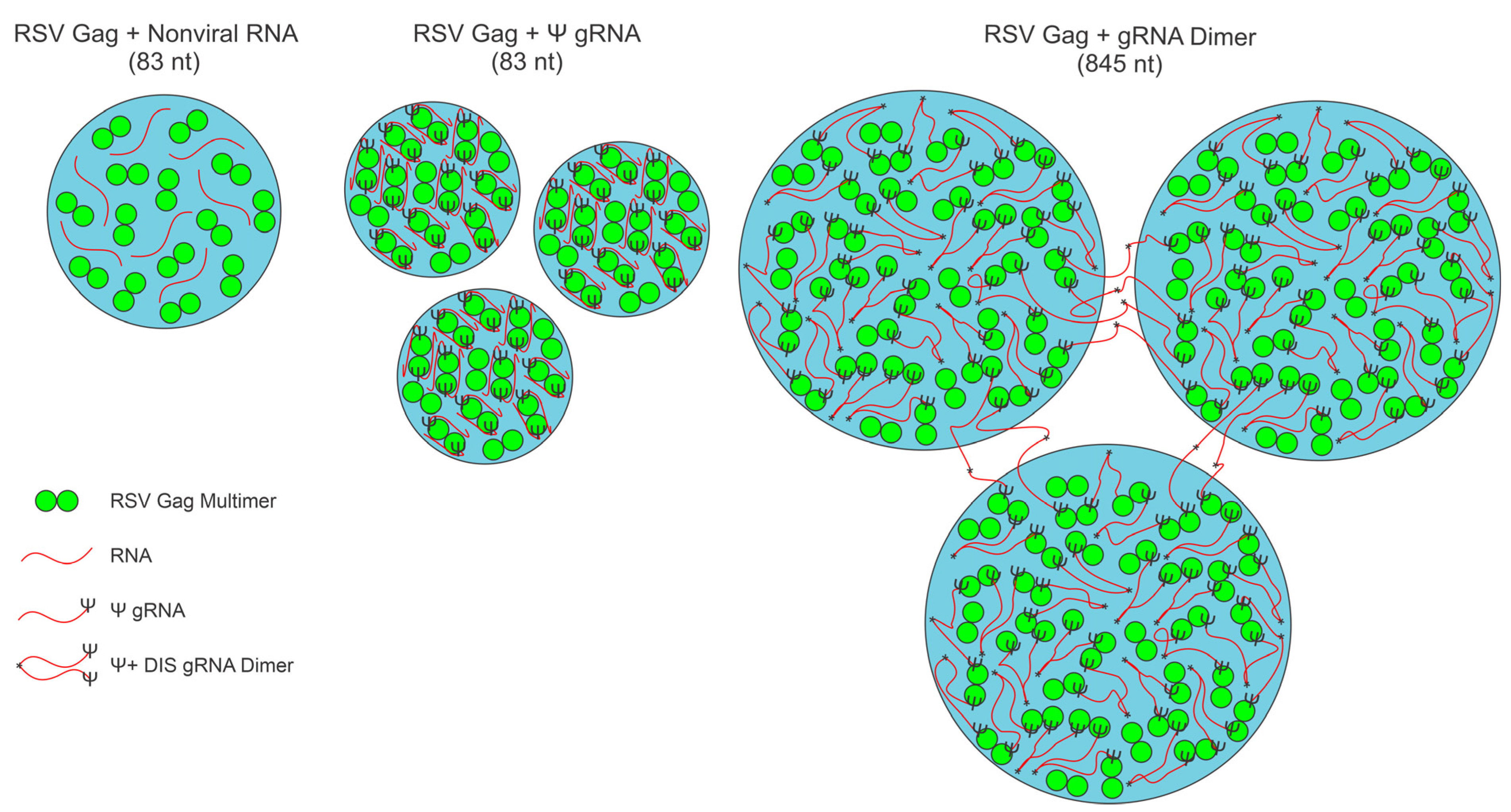
3.7. Three-Dimensional Spatial Organization of Gag-RNA Co-Condensates
3.8. Förster Resonance Energy Transfer (FRET) Analysis of Gag-RNA Co-Condensates
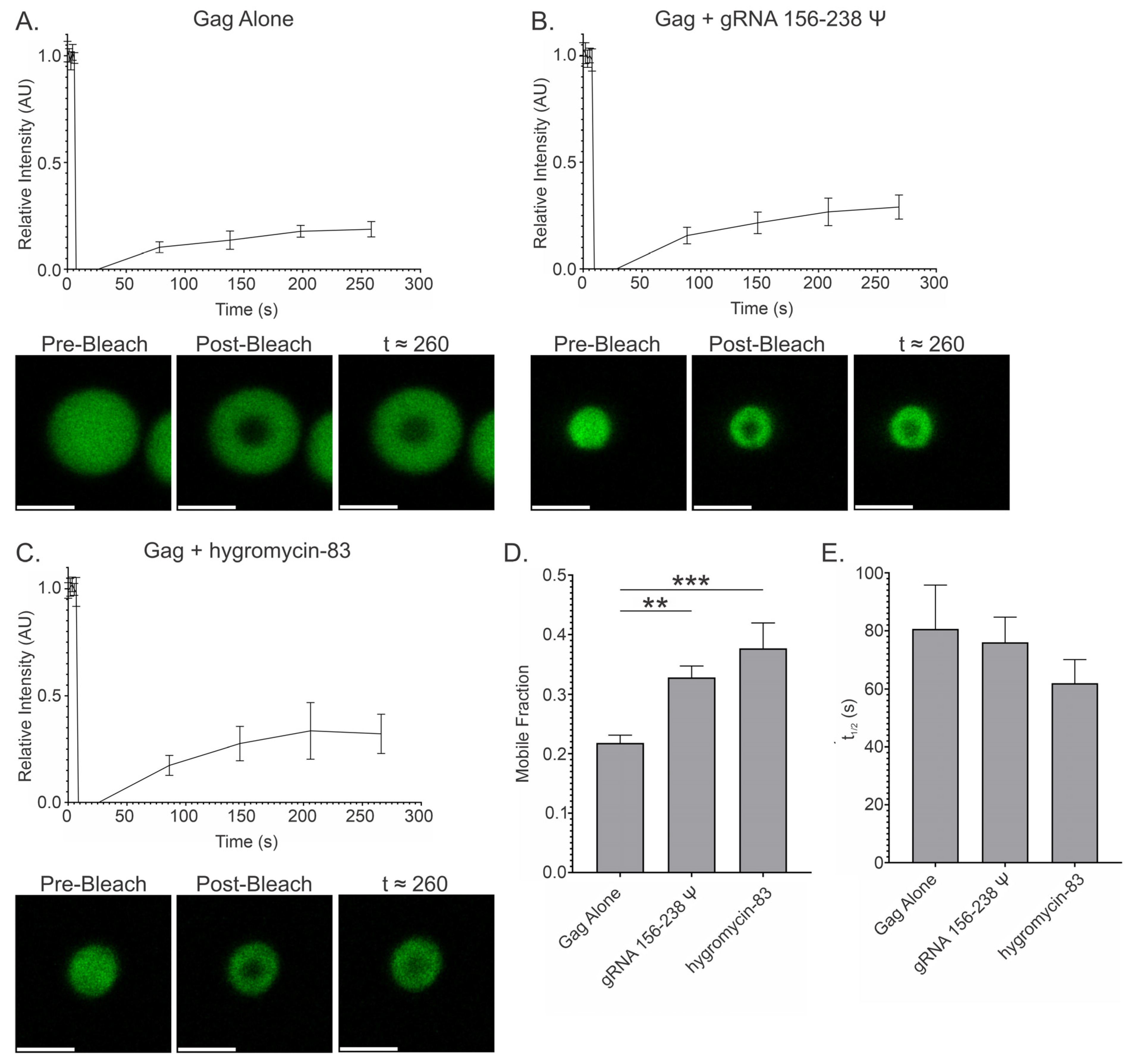
3.9. Fluorescence Recovery After Photobleaching (FRAP) of Gag-RNA Co-Condensates
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Alberti, S.; Hyman, A.A. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell. Biol. 2021, 22, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Kaddis Maldonado, R.; Lambert, G.S.; Rice, B.L.; Sudol, M.; Flanagan, J.M.; Parent, L.J. The Rous sarcoma virus Gag Polyprotein Forms Biomolecular Condensates Driven by Intrinsically-disordered Regions. J. Mol. Biol. 2023, 435, 168182. [Google Scholar] [CrossRef] [PubMed]
- Monette, A.; Niu, M.; Maldonado, R.K.; Chang, J.; Lambert, G.S.; Flanagan, J.M.; Cochrane, A.; Parent, L.J.; Mouland, A.J. Influence of HIV-1 Genomic RNA on the Formation of Gag Biomolecular Condensates. J. Mol. Biol. 2023, 435, 168190. [Google Scholar] [CrossRef]
- Sabari, B.R.; Dall’Agnese, A.; Boija, A.; Klein, I.A.; Coffey, E.L.; Shrinivas, K.; Abraham, B.J.; Hannett, N.M.; Zamudio, A.V.; Manteiga, J.C.; et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science 2018, 361, eaar3958. [Google Scholar] [CrossRef]
- Lyons, H.; Veettil, R.T.; Pradhan, P.; Fornero, C.; De La Cruz, N.; Ito, K.; Eppert, M.; Roeder, R.G.; Sabari, B.R. Functional partitioning of transcriptional regulators by patterned charge blocks. Cell 2023, 186, 327–345.e328. [Google Scholar] [CrossRef]
- Jain, S.; Wheeler, J.R.; Walters, R.W.; Agrawal, A.; Barsic, A.; Parker, R. ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell 2016, 164, 487–498. [Google Scholar] [CrossRef]
- Rice, B.L.; Kaddis, R.J.; Stake, M.S.; Lochmann, T.L.; Parent, L.J. Interplay between the alpharetroviral Gag protein and SR proteins SF2 and SC35 in the nucleus. Front. Microbiol. 2015, 6, 925. [Google Scholar] [CrossRef]
- Lochmann, T.L.; Bann, D.V.; Ryan, E.P.; Beyer, A.R.; Mao, A.; Cochrane, A.; Parent, L.J. NC-mediated nucleolar localization of retroviral gag proteins. Virus Res. 2013, 171, 304–318. [Google Scholar] [CrossRef]
- Boeynaems, S.; Alberti, S.; Fawzi, N.L.; Mittag, T.; Polymenidou, M.; Rousseau, F.; Schymkowitz, J.; Shorter, J.; Wolozin, B.; Van Den Bosch, L.; et al. Protein Phase Separation: A New Phase in Cell Biology. Trends Cell Biol. 2018, 28, 420–435. [Google Scholar] [CrossRef]
- Brangwynne, C.P.; Eckmann, C.R.; Courson, D.S.; Rybarska, A.; Hoege, C.; Gharakhani, J.; Julicher, F.; Hyman, A.A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 2009, 324, 1729–1732. [Google Scholar] [CrossRef]
- Alberti, S. Phase separation in biology. Curr. Biol. 2017, 27, R1097–R1102. [Google Scholar] [CrossRef] [PubMed]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, R.J.; Hyman, A.A. Controlling compartmentalization by non-membrane-bound organelles. Philos. Trans. R Soc. Lond. B Biol. Sci. 2018, 373, 20170193. [Google Scholar] [CrossRef] [PubMed]
- Mouland, A.J.; Parent, L.; Weber, S.C.; Holehouse, A.S. Virus Induced Membraneless Organelles and Biomolecular Condensates. J. Mol. Biol. 2023, 435, 168213. [Google Scholar] [CrossRef]
- Sagan, S.M.; Weber, S.C. Let’s phase it: Viruses are master architects of biomolecular condensates. Trends. Biochem. Sci. 2023, 48, 229–243. [Google Scholar] [CrossRef]
- Asimi, V.; Sampath Kumar, A.; Niskanen, H.; Riemenschneider, C.; Hetzel, S.; Naderi, J.; Fasching, N.; Popitsch, N.; Du, M.; Kretzmer, H.; et al. Hijacking of transcriptional condensates by endogenous retroviruses. Nat. Genet. 2022, 54, 1238–1247. [Google Scholar] [CrossRef]
- Rovnak, J.; Quackenbush, S.L. Exploitation of the Mediator complex by viruses. PLoS Pathog. 2022, 18, e1010422. [Google Scholar] [CrossRef]
- Pei, J.; Beri, N.R.; Zou, A.J.; Hubel, P.; Dorando, H.K.; Bergant, V.; Andrews, R.D.; Pan, J.; Andrews, J.M.; Sheehan, K.C.F.; et al. Nuclear-localized human respiratory syncytial virus NS1 protein modulates host gene transcription. Cell Rep. 2021, 37, 109803. [Google Scholar] [CrossRef]
- Vijayalingam, S.; Chinnadurai, G. Adenovirus L-E1A activates transcription through mediator complex-dependent recruitment of the super elongation complex. J. Virol. 2013, 87, 3425–3434. [Google Scholar] [CrossRef]
- Charman, M.; Grams, N.; Kumar, N.; Halko, E.; Dybas, J.M.; Abbott, A.; Lum, K.K.; Blumenthal, D.; Tsopurashvili, E.; Weitzman, M.D. A viral biomolecular condensate coordinates assembly of progeny particles. Nature 2023, 616, 332–338. [Google Scholar] [CrossRef]
- Martin, E.W.; Iserman, C.; Olety, B.; Mitrea, D.M.; Klein, I.A. Biomolecular Condensates as Novel Antiviral Targets. J. Mol. Biol. 2024, 436, 168380. [Google Scholar] [CrossRef] [PubMed]
- Alston, J.J.; Soranno, A. Condensation Goes Viral: A Polymer Physics Perspective. J. Mol. Biol. 2023, 435, 167988. [Google Scholar] [CrossRef] [PubMed]
- Borodavka, A.; Acker, J. Seeing Biomolecular Condensates Through the Lens of Viruses. Annu. Rev. Virol. 2023, 10, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.S.; Rice, B.L.; Maldonado, R.J.K.; Chang, J.; Parent, L.J. Comparative analysis of retroviral Gag-host cell interactions: Focus on the nuclear interactome. Retrovirology 2024, 21, 13. [Google Scholar] [CrossRef]
- Fonin, A.V.; Darling, A.L.; Kuznetsova, I.M.; Turoverov, K.K.; Uversky, V.N. Intrinsically disordered proteins in crowded milieu: When chaos prevails within the cellular gumbo. Cell Mol. Life Sci. 2018, 75, 3907–3929. [Google Scholar] [CrossRef]
- Uversky, V.N. Intrinsically disordered proteins in overcrowded milieu: Membrane-less organelles, phase separation, and intrinsic disorder. Curr. Opin. Struct. Biol. 2017, 44, 18–30. [Google Scholar] [CrossRef]
- Uversky, V.N. Proteins without unique 3D structures: Biotechnological applications of intrinsically unstable/disordered proteins. Biotechnol. J. 2015, 10, 356–366. [Google Scholar] [CrossRef]
- Lin, Y.; Protter, D.S.; Rosen, M.K.; Parker, R. Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins. Mol. Cell 2015, 60, 208–219. [Google Scholar] [CrossRef]
- Tauber, D.; Tauber, G.; Parker, R. Mechanisms and Regulation of RNA Condensation in RNP Granule Formation. Trends. Biochem. Sci. 2020, 45, 764–778. [Google Scholar] [CrossRef]
- Meric, C.; Spahr, P.F. Rous sarcoma virus nucleic acid-binding protein p12 is necessary for viral 70S RNA dimer formation and packaging. J. Virol. 1986, 60, 450–459. [Google Scholar] [CrossRef]
- Katz, R.A.; Terry, R.W.; Skalka, A.M. A conserved cis-acting sequence in the 5’ leader of avian sarcoma virus RNA is required for packaging. J. Virol. 1986, 59, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Kaddis Maldonado, R.J.; Parent, L.J. Orchestrating the Selection and Packaging of Genomic RNA by Retroviruses: An Ensemble of Viral and Host Factors. Viruses 2016, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Lear, A.L.; Haddrick, M.; Heaphy, S. A study of the dimerization of Rous sarcoma virus RNA in vitro and in vivo. Virology 1995, 212, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Mangel, W.F.; Delius, H.; Duesberg, P.H. Structure and molecular weight of the 60-70S RNA and the 30-40S RNA of the Rous sarcoma virus. Proc. Natl. Acad. Sci. USA 1974, 71, 4541–4545. [Google Scholar] [CrossRef]
- Murti, K.G.; Bondurant, M.; Tereba, A. Secondary structural features in the 70S RNAs of Moloney murine leukemia and Rous sarcoma viruses as observed by electron microscopy. J. Virol. 1981, 37, 411–419. [Google Scholar] [CrossRef]
- Garbitt, R.A.; Albert, J.A.; Kessler, M.D.; Parent, L.J. trans-acting inhibition of genomic RNA dimerization by Rous sarcoma virus matrix mutants. J. Virol. 2001, 75, 260–268. [Google Scholar] [CrossRef]
- Banks, J.D.; Linial, M.L. Secondary structure analysis of a minimal avian leukosis-sarcoma virus packaging signal. J. Virol. 2000, 74, 456–464. [Google Scholar] [CrossRef]
- Zhou, J.; McAllen, J.K.; Tailor, Y.; Summers, M.F. High affinity nucleocapsid protein binding to the muPsi RNA packaging signal of Rous sarcoma virus. J. Mol. Biol. 2005, 349, 976–988. [Google Scholar] [CrossRef]
- Zhou, J.; Bean, R.L.; Vogt, V.M.; Summers, M. Solution structure of the Rous sarcoma virus nucleocapsid protein: muPsi RNA packaging signal complex. J. Mol. Biol. 2007, 365, 453–467. [Google Scholar] [CrossRef]
- Webb, J.A.; Jones, C.P.; Parent, L.J.; Rouzina, I.; Musier-Forsyth, K. Distinct binding interactions of HIV-1 Gag to Psi and non-Psi RNAs: Implications for viral genomic RNA packaging. RNA 2013, 19, 1078–1088. [Google Scholar] [CrossRef]
- Rye-McCurdy, T.; Olson, E.D.; Liu, S.; Binkley, C.; Reyes, J.P.; Thompson, B.R.; Flanagan, J.M.; Parent, L.J.; Musier-Forsyth, K. Functional Equivalence of Retroviral MA Domains in Facilitating Psi RNA Binding Specificity by Gag. Viruses 2016, 8, 256. [Google Scholar] [CrossRef] [PubMed]
- Eckwahl, M.J.; Telesnitsky, A.; Wolin, S.L. Host RNA Packaging by Retroviruses: A Newly Synthesized Story. MBio 2016, 7, e02025-15. [Google Scholar] [CrossRef] [PubMed]
- Giles, K.E.; Caputi, M.; Beemon, K.L. Packaging and reverse transcription of snRNAs by retroviruses may generate pseudogenes. RNA 2004, 10, 299–307. [Google Scholar] [CrossRef]
- Cen, S.; Javanbakht, H.; Kim, S.; Shiba, K.; Craven, R.; Rein, A.; Ewalt, K.; Schimmel, P.; Musier-Forsyth, K.; Kleiman, L. Retrovirus-specific packaging of aminoacyl-tRNA synthetases with cognate primer tRNAs. J. Virol. 2002, 76, 13111–13115. [Google Scholar] [CrossRef]
- Campbell, S.; Vogt, V.M. Self-assembly in vitro of purified CA-NC proteins from Rous sarcoma virus and human immunodeficiency virus type 1. J. Virol. 1995, 69, 6487–6497. [Google Scholar] [CrossRef]
- Ma, Y.M.; Vogt, V.M. Nucleic acid binding-induced Gag dimerization in the assembly of Rous sarcoma virus particles in vitro. J. Virol. 2004, 78, 52–60. [Google Scholar] [CrossRef]
- Ma, Y.M.; Vogt, V.M. Rous sarcoma virus Gag protein-oligonucleotide interaction suggests a critical role for protein dimer formation in assembly. J. Virol. 2002, 76, 5452–5462. [Google Scholar] [CrossRef]
- Liu, S.; Kaddis Maldonado, R.; Rye-McCurdy, T.; Binkley, C.; Bah, A.; Chen, E.C.; Rice, B.L.; Parent, L.J.; Musier-Forsyth, K. Rous Sarcoma Virus Genomic RNA Dimerization Capability In Vitro Is Not a Prerequisite for Viral Infectivity. Viruses 2020, 12, 568. [Google Scholar] [CrossRef]
- Aronoff, R.; Linial, M. Specificity of retroviral RNA packaging. J. Virol. 1991, 65, 71–80. [Google Scholar] [CrossRef]
- Chen, E.C.; Maldonado, R.J.K.; Parent, L.J. Visualizing Rous Sarcoma Virus Genomic RNA Dimerization in the Nucleus, Cytoplasm, and at the Plasma Membrane. Viruses 2021, 13, 903. [Google Scholar] [CrossRef]
- Rice, B.L.; Stake, M.S.; Parent, L.J. TNPO3-Mediated Nuclear Entry of the Rous Sarcoma Virus Gag Protein Is Independent of the Cargo-Binding Domain. J. Virol. 2020, 94, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Banks, J.D.; Yeo, A.; Green, K.; Cepeda, F.; Linial, M.L. A minimal avian retroviral packaging sequence has a complex structure. J. Virol. 1998, 72, 6190–6194. [Google Scholar] [CrossRef] [PubMed]
- Banks, J.D.; Kealoha, B.O.; Linial, M.L. An Mpsi-containing heterologous RNA, but not env mRNA, is efficiently packaged into avian retroviral particles. J. Virol. 1999, 73, 8926–8933. [Google Scholar] [CrossRef] [PubMed]
- Fossé, P.; Motté, N.; Roumier, A.; Gabus, C.; Muriaux, D.; Darlix, J.L.; Paoletti, J. A short autocomplementary sequence plays an essential role in avian sarcoma-leukosis virus RNA dimerization. Biochemistry 1996, 35, 16601–16609. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.N.; Allen, N.E.; Hobbs, J.N.; Alborn, W.E.; Kirst, H.A.; Paschal, J.W. Genetic and enzymatic basis of hygromycin B resistance in Escherichia coli. Antimicrob. Agents Chemother. 1983, 24, 689–695. [Google Scholar] [CrossRef]
- Gudleski, N.; Flanagan, J.M.; Ryan, E.P.; Bewley, M.C.; Parent, L.J. Directionality of nucleocytoplasmic transport of the retroviral gag protein depends on sequential binding of karyopherins and viral RNA. Proc. Natl. Acad. Sci. USA 2010, 107, 9358–9363. [Google Scholar] [CrossRef]
- Wang, J.; Choi, J.M.; Holehouse, A.S.; Lee, H.O.; Zhang, X.; Jahnel, M.; Maharana, S.; Lemaitre, R.; Pozniakovsky, A.; Drechsel, D.; et al. A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 2018, 174, 688–699.e616. [Google Scholar] [CrossRef]
- Iserman, C.; Roden, C.A.; Boerneke, M.A.; Sealfon, R.S.G.; McLaughlin, G.A.; Jungreis, I.; Fritch, E.J.; Hou, Y.J.; Ekena, J.; Weidmann, C.A.; et al. Genomic RNA Elements Drive Phase Separation of the SARS-CoV-2 Nucleocapsid. Mol. Cell 2020, 80, 1078–1091.e1076. [Google Scholar] [CrossRef]
- Guillén-Boixet, J.; Kopach, A.; Holehouse, A.S.; Wittmann, S.; Jahnel, M.; Schlüßler, R.; Kim, K.; Trussina, I.R.E.A.; Wang, J.; Mateju, D.; et al. RNA-Induced Conformational Switching and Clustering of G3BP Drive Stress Granule Assembly by Condensation. Cell 2020, 181, 346–361.e317. [Google Scholar] [CrossRef]
- Kelley, F.M.; Favetta, B.; Regy, R.M.; Mittal, J.; Schuster, B.S. Amphiphilic proteins coassemble into multiphasic condensates and act as biomolecular surfactants. Proc. Natl. Acad. Sci. USA 2021, 118, e2109967118. [Google Scholar] [CrossRef]
- Koulouras, G.; Panagopoulos, A.; Rapsomaniki, M.A.; Giakoumakis, N.N.; Taraviras, S.; Lygerou, Z. EasyFRAP-web: A web-based tool for the analysis of fluorescence recovery after photobleaching data. Nucleic Acids Res. 2018, 46, W467–W472. [Google Scholar] [CrossRef] [PubMed]
- Reuter, J.S.; Mathews, D.H. RNAstructure: Software for RNA secondary structure prediction and analysis. BMC Bioinform. 2010, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.J.; Gloor, J.W.; Mathews, D.H. Improved RNA secondary structure prediction by maximizing expected pair accuracy. RNA 2009, 15, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.Z.; Simon, A.E. RNAcanvas: Interactive drawing and exploration of nucleic acid structures. Nucleic Acids Res. 2023, 51, W501–W508. [Google Scholar] [CrossRef]
- Nadaraia-Hoke, S.; Bann, D.V.; Lochmann, T.L.; Gudleski-O’Regan, N.; Parent, L.J. Alterations in the MA and NC domains modulate phosphoinositide-dependent plasma membrane localization of the Rous sarcoma virus Gag protein. J. Virol. 2013, 87, 3609–3615. [Google Scholar] [CrossRef]
- Bishop, J.M.; Levinson, W.E.; Sullivan, D.; Fanshier, L.; Quintrell, N.; Jackson, J. The low molecular weight RNAs of Rous sarcoma virus. II. The 7 S RNA. Virology 1970, 42, 927–937. [Google Scholar] [CrossRef]
- Erikson, E.; Erikson, R.L.; Henry, B.; Pace, N.R. Comparison of oligonucleotides produced by RNase T1 digestion of 7 S RNA from avian and murine oncornaviruses and from uninfected cells. Virology 1973, 53, 40–46. [Google Scholar] [CrossRef]
- Sawyer, R.C.; Dahlberg, J.E. Small RNAs of Rous sarcoma virus: Characterization by two-dimensional polyacrylamide gel electrophoresis and fingerprint analysis. J. Virol. 1973, 12, 1226–1237. [Google Scholar] [CrossRef]
- Maurel, S.; Mougel, M. Murine leukemia virus RNA dimerization is coupled to transcription and splicing processes. Retrovirology 2010, 7, 64. [Google Scholar] [CrossRef]
- Maldonado, R.J.K.; Rice, B.; Chen, E.C.; Tuffy, K.M.; Chiari, E.F.; Fahrbach, K.M.; Hope, T.J.; Parent, L.J. Visualizing Association of the Retroviral Gag Protein with Unspliced Viral RNA in the Nucleus. mBio 2020, 11, e00524-20. [Google Scholar] [CrossRef]
- Schwartz, D.E.; Tizard, R.; Gilbert, W. Nucleotide sequence of Rous sarcoma virus. Cell 1983, 32, 853–869. [Google Scholar] [CrossRef] [PubMed]
- Grese, Z.R.; Bastos, A.C.; Mamede, L.D.; French, R.L.; Miller, T.M.; Ayala, Y.M. Specific RNA interactions promote TDP-43 multivalent phase separation and maintain liquid properties. EMBO Rep. 2021, 22, e53632. [Google Scholar] [CrossRef] [PubMed]
- Mountain, G.A.; Keating, C.D. Formation of Multiphase Complex Coacervates and Partitioning of Biomolecules within them. Biomacromolecules 2020, 21, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Meyer, M.O.; Bevilacqua, P.C.; Keating, C.D. Phase-specific RNA accumulation and duplex thermodynamics in multiphase coacervate models for membraneless organelles. Nat. Chem. 2022, 14, 1110–1117. [Google Scholar] [CrossRef]
- Roden, C.; Gladfelter, A.S. RNA contributions to the form and function of biomolecular condensates. Nat. Rev. Mol. Cell. Biol. 2021, 22, 183–195. [Google Scholar] [CrossRef]
- Vogt, V.M.; Simon, M.N. Mass determination of rous sarcoma virus virions by scanning transmission electron microscopy. J. Virol. 1999, 73, 7050–7055. [Google Scholar] [CrossRef]
- Briggs, J.A.; Simon, M.N.; Gross, I.; Kräusslich, H.G.; Fuller, S.D.; Vogt, V.M.; Johnson, M.C. The stoichiometry of Gag protein in HIV-1. Nat. Struct. Mol. Biol. 2004, 11, 672–675. [Google Scholar] [CrossRef]
- Morelli, C.; Faltova, L.; Capasso Palmiero, U.; Makasewicz, K.; Papp, M.; Jacquat, R.P.B.; Pinotsi, D.; Arosio, P. RNA modulates hnRNPA1A amyloid formation mediated by biomolecular condensates. Nat. Chem. 2024, 16, 1052–1061. [Google Scholar] [CrossRef]
- Molliex, A.; Temirov, J.; Lee, J.; Coughlin, M.; Kanagaraj, A.P.; Kim, H.J.; Mittag, T.; Taylor, J.P. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 2015, 163, 123–133. [Google Scholar] [CrossRef]
- Lee, K.H.; Zhang, P.; Kim, H.J.; Mitrea, D.M.; Sarkar, M.; Freibaum, B.D.; Cika, J.; Coughlin, M.; Messing, J.; Molliex, A.; et al. C9orf72 Dipeptide Repeats Impair the Assembly, Dynamics, and Function of Membrane-Less Organelles. Cell 2016, 167, 774–788.e717. [Google Scholar] [CrossRef]
- de Vries, T.; Novakovic, M.; Ni, Y.; Smok, I.; Inghelram, C.; Bikaki, M.; Sarnowski, C.P.; Han, Y.; Emmanouilidis, L.; Padroni, G.; et al. Specific protein-RNA interactions are mostly preserved in biomolecular condensates. Sci. Adv. 2024, 10, eadm7435. [Google Scholar] [CrossRef] [PubMed]
- Bose, M.; Rankovic, B.; Mahamid, J.; Ephrussi, A. An architectural role of specific RNA-RNA interactions in oskar granules. Nat. Cell. Biol. 2024, 26, 1934–1942. [Google Scholar] [CrossRef] [PubMed]
- Tuffy, K.M.; Maldonado, R.J.K.; Chang, J.; Rosenfeld, P.; Cochrane, A.; Parent, L.J. HIV-1 Gag Forms Ribonucleoprotein Complexes with Unspliced Viral RNA at Transcription Sites. Viruses 2020, 12, 1281. [Google Scholar] [CrossRef]
- Le Sage, V.; Cinti, A.; Valiente-Echeverría, F.; Mouland, A.J. Proteomic analysis of HIV-1 Gag interacting partners using proximity-dependent biotinylation. Virol. J. 2015, 12, 138. [Google Scholar] [CrossRef]
- Jäger, S.; Cimermancic, P.; Gulbahce, N.; Johnson, J.R.; McGovern, K.E.; Clarke, S.C.; Shales, M.; Mercenne, G.; Pache, L.; Li, K.; et al. Global landscape of HIV–human protein complexes. Nature 2011, 481, 365. [Google Scholar] [CrossRef]
- Engeland, C.E.; Oberwinkler, H.; Schümann, M.; Krause, E.; Müller, G.A.; Kräusslich, H.-G. The Cellular Protein Lyric Interacts with HIV-1 Gag. J. Virol. 2011, 85, 13322–13332. [Google Scholar] [CrossRef]
- Engeland, C.E.; Brown, N.P.; Börner, K.; Schümann, M.; Krause, E.; Kaderali, L.; Müller, G.A.; Kräusslich, H.-G. Proteome analysis of the HIV-1 Gag interactome. Virology 2014, 460–461, 194–206. [Google Scholar] [CrossRef]
- Ritchie, C.; Cylinder, I.; Platt, E.J.; Barklis, E. Analysis of HIV-1 Gag Protein Interactions via Biotin Ligase Tagging. J. Virol. 2015, 89, 3988–4001. [Google Scholar] [CrossRef]
- Li, Y.; Frederick, K.M.; Haverland, N.A.; Ciborowski, P.; Belshan, M. Investigation of the HIV-1 matrix interactome during virus replication. PROTEOMICS Clin. Appl. 2016, 10, 156–163. [Google Scholar] [CrossRef]
- Decker, C.J.; Burke, J.M.; Mulvaney, P.K.; Parker, R. RNA is required for the integrity of multiple nuclear and cytoplasmic membrane-less RNP granules. EMBO J. 2022, 41, e110137. [Google Scholar] [CrossRef]
- Guo, L.; Shorter, J. It’s Raining Liquids: RNA Tunes Viscoelasticity and Dynamics of Membraneless Organelles. Mol. Cell 2015, 60, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Fay, M.M.; Anderson, P.J. The Role of RNA in Biological Phase Separations. J. Mol. Biol. 2018, 430, 4685–4701. [Google Scholar] [CrossRef] [PubMed]
- Laghmach, R.; Alshareedah, I.; Pham, M.; Raju, M.; Banerjee, P.R.; Potoyan, D.A. RNA chain length and stoichiometry govern surface tension and stability of protein-RNA condensates. iScience 2022, 25, 104105. [Google Scholar] [CrossRef] [PubMed]
- Cochard, A.; Garcia-Jove Navarro, M.; Piroska, L.; Kashida, S.; Kress, M.; Weil, D.; Gueroui, Z. RNA at the surface of phase-separated condensates impacts their size and number. Biophys. J. 2022, 121, 1675–1690. [Google Scholar] [CrossRef]
- Poudyal, R.R.; Sieg, J.P.; Portz, B.; Keating, C.D.; Bevilacqua, P.C. RNA sequence and structure control assembly and function of RNA condensates. RNA 2021, 27, 1589–1601. [Google Scholar] [CrossRef]
- Bevilacqua, P.C.; Williams, A.M.; Chou, H.L.; Assmann, S.M. RNA multimerization as an organizing force for liquid-liquid phase separation. RNA 2022, 28, 16–26. [Google Scholar] [CrossRef]
- Van Treeck, B.; Parker, R. Emerging Roles for Intermolecular RNA-RNA Interactions in RNP Assemblies. Cell 2018, 174, 791–802. [Google Scholar] [CrossRef]
- Henninger, J.E.; Oksuz, O.; Shrinivas, K.; Sagi, I.; LeRoy, G.; Zheng, M.M.; Andrews, J.O.; Zamudio, A.V.; Lazaris, C.; Hannett, N.M.; et al. RNA-Mediated Feedback Control of Transcriptional Condensates. Cell 2021, 184, 207–225.e224. [Google Scholar] [CrossRef]
- Little, S.C.; Sinsimer, K.S.; Lee, J.J.; Wieschaus, E.F.; Gavis, E.R. Independent and coordinate trafficking of single Drosophila germ plasm mRNAs. Nat. Cell. Biol. 2015, 17, 558–568. [Google Scholar] [CrossRef]
- Langdon, E.M.; Qiu, Y.; Ghanbari Niaki, A.; McLaughlin, G.A.; Weidmann, C.A.; Gerbich, T.M.; Smith, J.A.; Crutchley, J.M.; Termini, C.M.; Weeks, K.M.; et al. mRNA structure determines specificity of a polyQ-driven phase separation. Science 2018, 360, 922–927. [Google Scholar] [CrossRef]
- Ferrandon, D.; Koch, I.; Westhof, E.; Nüsslein-Volhard, C. RNA-RNA interaction is required for the formation of specific bicoid mRNA 3’ UTR-STAUFEN ribonucleoprotein particles. EMBO J. 1997, 16, 1751–1758. [Google Scholar] [CrossRef]
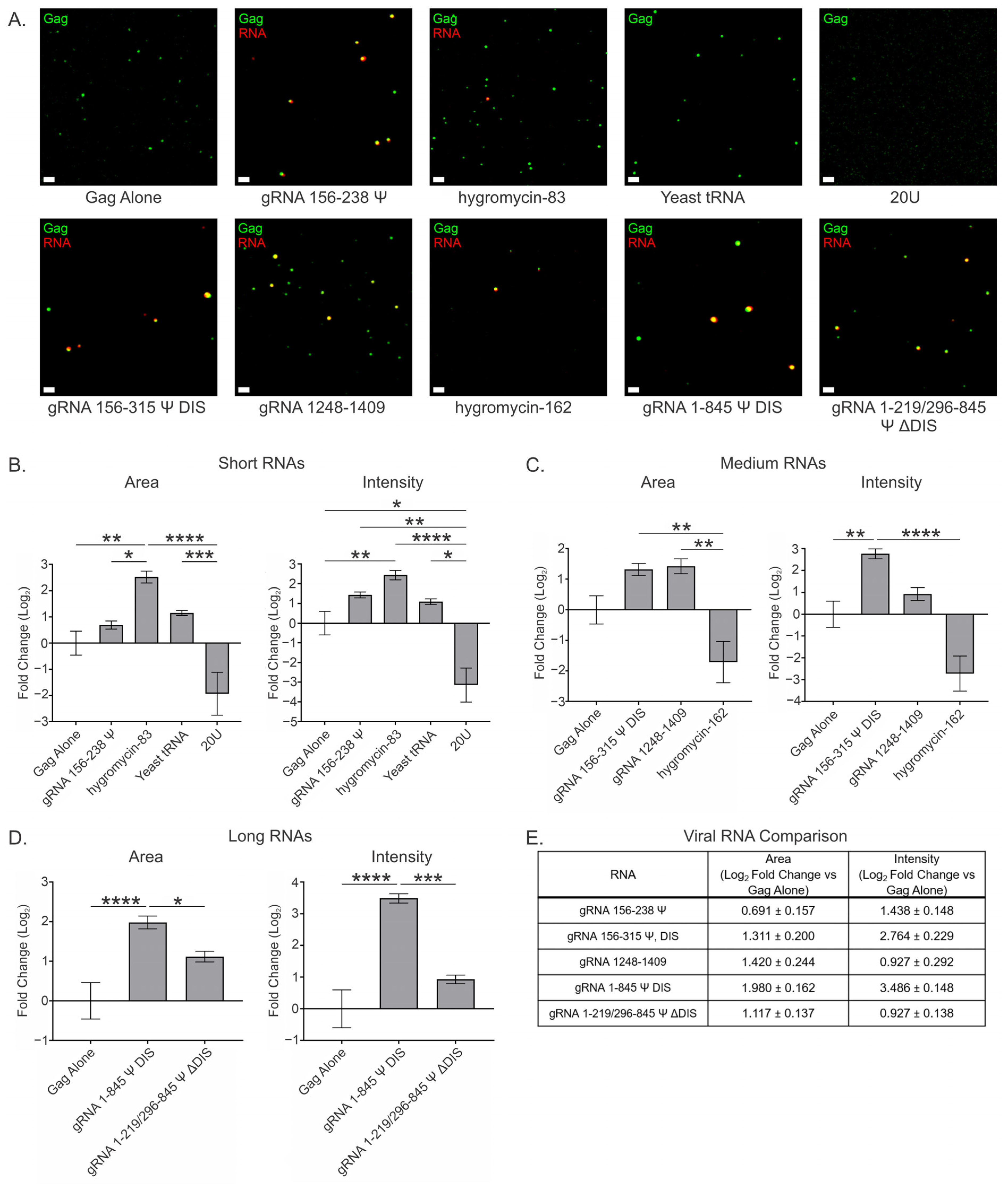


| RNA | Area (Log2 Fold Change) | Intensity (Log2 Fold Change) |
|---|---|---|
| gRNA 156–238 Ψ | 0.691 ± 0.157 | 1.438 ± 0.148 |
| hygromycin-83 | 2.515 ± 0.224 | 2.432 ± 0.239 |
| Yeast tRNA | 1.150 ± 0.095 | 1.092 ± 0.145 |
| 20U | −1.937 ± 0.821 | −3.146 ± 0.865 |
| gRNA 156–315 Ψ DIS | 1.311 ± 0.200 | 2.764 ± 0.229 |
| gRNA 1248–1409 | 1.420 ± 0.244 | 0.927 ± 0.292 |
| hygromycin-162 | −1.712 ± 0.678 | −2.724 ± 0.805 |
| gRNA 1–845 Ψ DIS | 1.980 ± 0.162 | 3.486 ± 0.148 |
| gRNA 1–219/296–845 Ψ ΔDIS | 1.117 ± 0.137 | 0.927 ± 0.138 |
| RNA | Area (Log2 Fold Change) | Intensity (Log2 Fold Change) |
|---|---|---|
| gRNA 156–238 Ψ | 0.762 ± 0.075 | 1.785 ± 0.090 |
| hygromycin-83 | 1.833 ± 0.147 | 1.488 ± 0.150 |
| Yeast tRNA | −0.167 ± 0.228 | −0.653 ± 0.273 |
| 20U | −1.857 ± 0.377 | −2.921 ± 0.403 |
| gRNA 156–315 Ψ DIS | 1.489 ± 0.107 | 2.813 ± 0.115 |
| gRNA 1248–1409 | 1.416 ± 0.187 | 0.979 ± 0.230 |
| hygromycin-162 | −1.661 ± 0.281 | −2.369 ± 0.290 |
| gRNA 1–845 Ψ DIS | 1.896 ± 0.095 | 3.439 ± 0.117 |
| gRNA 1–219/296–845 Ψ ΔDIS | 1.625 ± 0.090 | 1.552 ± 0.117 |
| RNA | Area (Log2 Fold Change) | Intensity (Log2 Fold Change) |
|---|---|---|
| gRNA 156–238 Ψ | −0.245 ± 0.090 | 0.383 ± 0.112 |
| hygromycin-83 | 0.256 ± 0.084 | 0.383 ± 0.191 |
| Yeast tRNA | 0.931 ± 0.035 | 0.528 ± 0.043 |
| 20U | 1.036 ± 0.045 | 0.943 ± 0.118 |
| gRNA 156–315 Ψ DIS | 0.382 ± 0.061 | 0.491 ± 0.159 |
| gRNA 1248–1409 | −0.285 ± 0.189 | −0.468 ± 0.090 |
| hygromycin-162 | 0.022 ± 0.101 | 0.229 ± 0.206 |
| gRNA 1–845 Ψ DIS * | 0.439 ± 0.072 | 0.462 ± 0.154 |
| gRNA 1–219/296–845 Ψ ΔDIS * | −0.374 ± 0.165 | −0.220 ± 0.293 |
| RNA | Area (Log2 Fold Change) | Intensity (Log2 Fold Change) |
|---|---|---|
| gRNA 156–238 Ψ | 0.004 ± 0.053 | 0.816 ± 0.069 |
| hygromycin-83 | 0.334 ± 0.048 | 0.455 ± 0.079 |
| Yeast tRNA | 0.732 ± 0.060 | 0.254 ± 0.060 |
| 20U | 0.939 ± 0.031 | 1.029 ± 0.052 |
| gRNA 156–315 Ψ DIS | 0.281 ± 0.045 | 0.656 ± 0.096 |
| gRNA 1248–1409 | −0.085 ± 0.072 | 0.341 ± 0.069 |
| hygromycin-162 | −0.038 ± 0.046 | 0.233 ± 0.119 |
| gRNA 1–845 Ψ DIS | 0.610 ± 0.057 | 0.638 ± 0.094 |
| gRNA 1–219/296–845 Ψ ΔDIS | −0.442 ± 0.108 | −0.224 ± 0.187 |
| RNA | 2.5 μM Gag | 10 μM Gag | ||
|---|---|---|---|---|
| 2 RNA:3000 Gag | Overall | 2 RNA:3000 Gag | Overall | |
| gRNA 156–238 Ψ | 0.079 ± 0.014 | 0.138 ± 0.018 | 0.321 ± 0.037 | 0.391 ± 0.023 |
| hygromycin-83 | 0.042 ± 0.016 | 0.114 ± 0.025 | 0.235 ± 0.031 | 0.202 ± 0.015 |
| Yeast tRNA | N.D. | N.D. | N.D. | N.D. |
| 20U | N.D. | N.D. | N.D. | N.D. |
| gRNA 156–315 Ψ DIS | 0.252 ± 0.058 | 0.216 ± 0.027 | 0.217 ± 0.023 | 0.308 ± 0.016 |
| gRNA 1248–1409 | 0.043 ± 0.010 | 0.036 ± 0.006 | 0.380 ± 0.032 | 0.467 ± 0.021 |
| hygromycin-162 | 0.318 ± 0.107 | 0.183 ± 0.038 | 0.137 ± 0.020 | 0.171 ± 0.016 |
| gRNA 1–845 Ψ DIS | 0.501 ± 0.064 | 0.485 ± 0.041 | 0.480 ± 0.039 | 0.433 ± 0.027 |
| gRNA 1–219/296–845 Ψ ΔDIS | 0.041 ± 0.006 | 0.081 ± 0.011 | 0.188 ± 0.018 | 0.207 ± 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambert, G.S.; Maldonado, R.J.K.; Parent, L.J. Role of the Psi Packaging Signal and Dimerization Initiation Sequence in the Organization of Rous Sarcoma Virus Gag-gRNA Co-Condensates. Viruses 2025, 17, 97. https://doi.org/10.3390/v17010097
Lambert GS, Maldonado RJK, Parent LJ. Role of the Psi Packaging Signal and Dimerization Initiation Sequence in the Organization of Rous Sarcoma Virus Gag-gRNA Co-Condensates. Viruses. 2025; 17(1):97. https://doi.org/10.3390/v17010097
Chicago/Turabian StyleLambert, Gregory S., Rebecca J. Kaddis Maldonado, and Leslie J. Parent. 2025. "Role of the Psi Packaging Signal and Dimerization Initiation Sequence in the Organization of Rous Sarcoma Virus Gag-gRNA Co-Condensates" Viruses 17, no. 1: 97. https://doi.org/10.3390/v17010097
APA StyleLambert, G. S., Maldonado, R. J. K., & Parent, L. J. (2025). Role of the Psi Packaging Signal and Dimerization Initiation Sequence in the Organization of Rous Sarcoma Virus Gag-gRNA Co-Condensates. Viruses, 17(1), 97. https://doi.org/10.3390/v17010097






