Virucidal Potential of 3,3′,4,4′,5,5′-Hexahydroxy-trans-Stilbene Against Respiratory Syncytial Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Virus Stock
2.3. RSV Infection Assay
2.4. MTT Assay
2.5. Statistics
3. Results
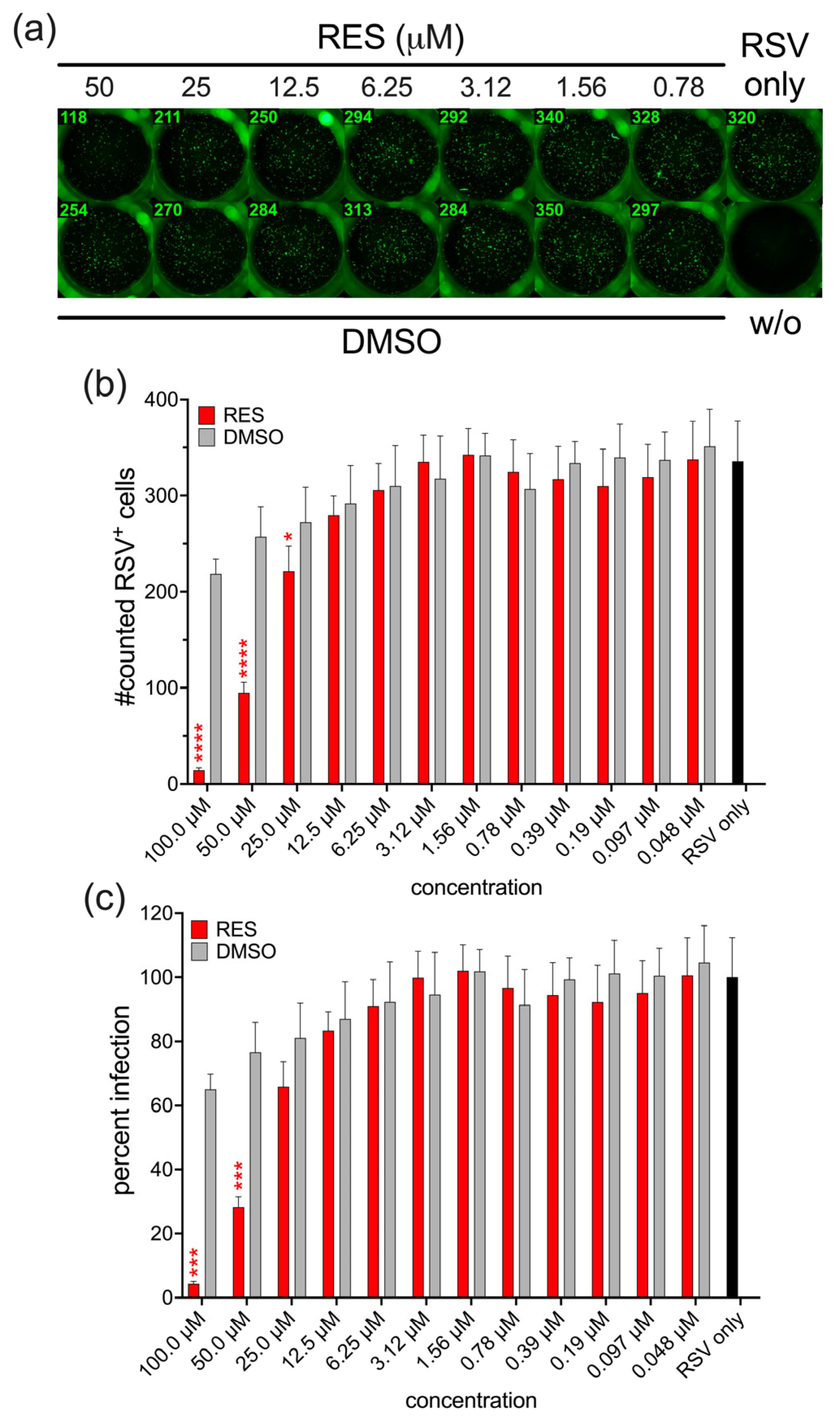
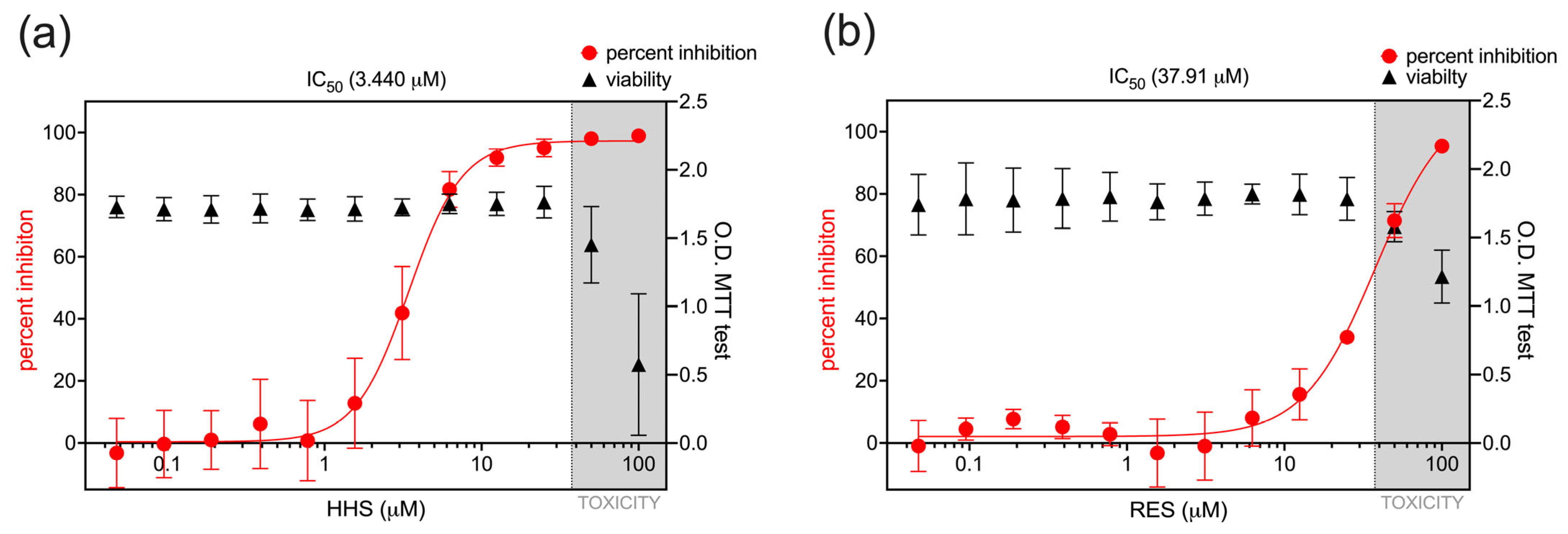
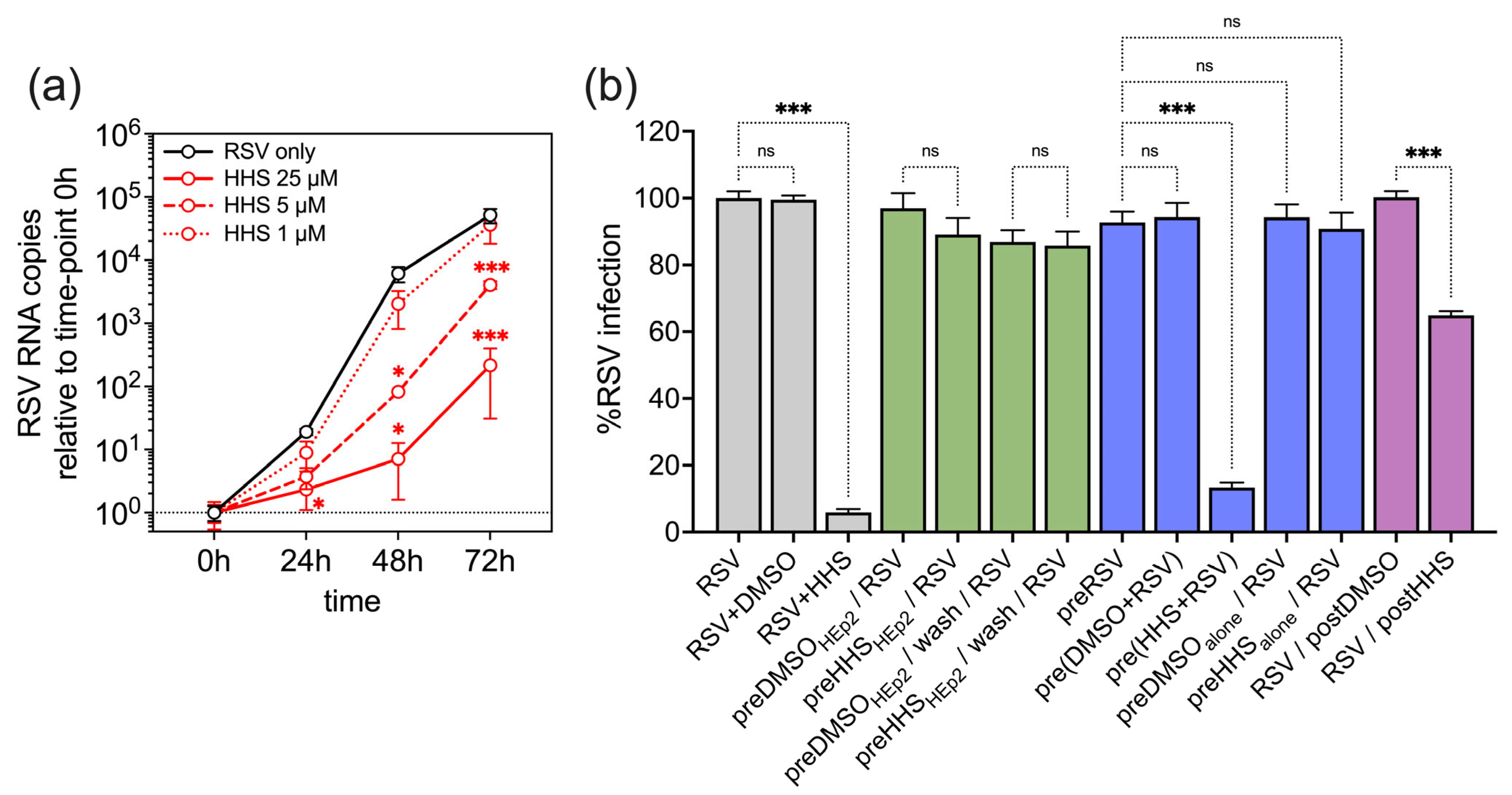
3.1. Antiviral Activity of HHS on RSV Infection of HEp-2 Cells
3.2. Antiviral Activity of HHS Related to a Direct Interaction of HHS with RSV
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RSV | Respiratory syncytial virus |
| HHS | 3,3′,4,4′,5,5′-hexahydroxy-trans-stilbene |
| RES | resveratrol |
| DMSO | dimethyl sulfoxide |
References
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simoes, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P. First RSV vaccine approvals. Lancet Microbe 2023, 4, e577. [Google Scholar] [CrossRef] [PubMed]
- Blanken, M.O.; Rovers, M.M.; Molenaar, J.M.; Winkler-Seinstra, P.L.; Meijer, A.; Kimpen, J.L.; Bont, L.; Dutch, R.S.V.N.N. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N. Engl. J. Med. 2013, 368, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Drysdale, S.B.; Cathie, K.; Flamein, F.; Knuf, M.; Collins, A.M.; Hill, H.C.; Kaiser, F.; Cohen, R.; Pinquier, D.; Felter, C.T.; et al. Nirsevimab for Prevention of Hospitalizations Due to RSV in Infants. N. Engl. J. Med. 2023, 389, 2425–2435. [Google Scholar] [CrossRef]
- Gatt, D.; Martin, I.; AlFouzan, R.; Moraes, T.J. Prevention and Treatment Strategies for Respiratory Syncytial Virus (RSV). Pathogens 2023, 12, 154. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef]
- Bostanghadiri, N.; Pormohammad, A.; Chirani, A.S.; Pouriran, R.; Erfanimanesh, S.; Hashemi, A. Comprehensive review on the antimicrobial potency of the plant polyphenol Resveratrol. Biomed. Pharmacother. 2017, 95, 1588–1595. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- Docherty, J.J.; Fu, M.M.; Stiffler, B.S.; Limperos, R.J.; Pokabla, C.M.; DeLucia, A.L. Resveratrol inhibition of herpes simplex virus replication. Antivir. Res. 1999, 43, 145–155. [Google Scholar] [CrossRef]
- Docherty, J.J.; Sweet, T.J.; Bailey, E.; Faith, S.A.; Booth, T. Resveratrol inhibition of varicella-zoster virus replication in vitro. Antivir. Res. 2006, 72, 171–177. [Google Scholar] [CrossRef]
- Evers, D.L.; Wang, X.; Huong, S.M.; Huang, D.Y.; Huang, E.S. 3,4′,5-Trihydroxy-trans-stilbene (resveratrol) inhibits human cytomegalovirus replication and virus-induced cellular signaling. Antivir. Res. 2004, 63, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Yiu, C.Y.; Chen, S.Y.; Chang, L.K.; Chiu, Y.F.; Lin, T.P. Inhibitory effects of resveratrol on the Epstein-Barr virus lytic cycle. Molecules 2010, 15, 7115–7124. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sinha, N.; Kodidela, S.; Godse, S.; Singla, B.; Singh, U.P.; Bhat, H.K. Resveratrol and its analogs suppress HIV replication, oxidative stress, and inflammation in macrophages. NeuroImmune Pharm. Ther. 2023, 2, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Palamara, A.T.; Nencioni, L.; Aquilano, K.; De Chiara, G.; Hernandez, L.; Cozzolino, F.; Ciriolo, M.R.; Garaci, E. Inhibition of influenza A virus replication by resveratrol. J. Infect. Dis. 2005, 191, 1719–1729. [Google Scholar] [CrossRef]
- Ter Ellen, B.M.; Dinesh Kumar, N.; Bouma, E.M.; Troost, B.; van de Pol, D.P.I.; van der Ende-Metselaar, H.H.; Apperloo, L.; van Gosliga, D.; van den Berge, M.; Nawijn, M.C.; et al. Resveratrol and Pterostilbene Inhibit SARS-CoV-2 Replication in Air-Liquid Interface Cultured Human Primary Bronchial Epithelial Cells. Viruses 2021, 13, 1335. [Google Scholar] [CrossRef]
- Murias, M.; Handler, N.; Erker, T.; Pleban, K.; Ecker, G.; Saiko, P.; Szekeres, T.; Jager, W. Resveratrol analogues as selective cyclooxygenase-2 inhibitors: Synthesis and structure-activity relationship. Bioorg. Med. Chem. 2004, 12, 5571–5578. [Google Scholar] [CrossRef]
- Saiko, P.; Pemberger, M.; Horvath, Z.; Savinc, I.; Grusch, M.; Handler, N.; Erker, T.; Jaeger, W.; Fritzer-Szekeres, M.; Szekeres, T. Novel resveratrol analogs induce apoptosis and cause cell cycle arrest in HT29 human colon cancer cells: Inhibition of ribonucleotide reductase activity. Oncol. Rep. 2008, 19, 1621–1626. [Google Scholar] [CrossRef]
- Paulitschke, V.; Schicher, N.; Szekeres, T.; Jager, W.; Elbling, L.; Riemer, A.B.; Scheiner, O.; Trimurtulu, G.; Venkateswarlu, S.; Mikula, M.; et al. 3,3′,4,4′,5,5′-hexahydroxystilbene impairs melanoma progression in a metastatic mouse model. J. Investig. Dermatol. 2010, 130, 1668–1679. [Google Scholar] [CrossRef]
- Fischhuber, K.; Banki, Z.; Kimpel, J.; Kragl, N.; Rossler, A.; Bolze, A.; Muellauer, B.; Angerer, J.; Nagy, G.; Nagy, E.; et al. Antiviral Potential of Azelastine against Major Respiratory Viruses. Viruses 2023, 15, 2300. [Google Scholar] [CrossRef]
- Srinivasan, V.; Brognaro, H.; Prabhu, P.R.; de Souza, E.E.; Gunther, S.; Reinke, P.Y.A.; Lane, T.J.; Ginn, H.; Han, H.; Ewert, W.; et al. Antiviral activity of natural phenolic compounds in complex at an allosteric site of SARS-CoV-2 papain-like protease. Commun. Biol. 2022, 5, 805. [Google Scholar] [CrossRef]
- Reshamwala, D.; Shroff, S.; Sheik Amamuddy, O.; Laquintana, V.; Denora, N.; Zacheo, A.; Lampinen, V.; Hytonen, V.P.; Tastan Bishop, O.; Krol, S.; et al. Polyphenols Epigallocatechin Gallate and Resveratrol, and Polyphenol-Functionalized Nanoparticles Prevent Enterovirus Infection through Clustering and Stabilization of the Viruses. Pharmaceutics 2021, 13, 1182. [Google Scholar] [CrossRef]
- Lin, C.J.; Lin, H.J.; Chen, T.H.; Hsu, Y.A.; Liu, C.S.; Hwang, G.Y.; Wan, L. Polygonum cuspidatum and its active components inhibit replication of the influenza virus through toll-like receptor 9-induced interferon beta expression. PLoS ONE 2015, 10, e0117602. [Google Scholar] [CrossRef]
- Xiong, Y.; Tao, K.; Li, T.; Ou, W.; Zhou, Y.; Zhang, W.; Wang, S.; Qi, R.; Ji, J. Resveratrol inhibits respiratory syncytial virus replication by targeting heparan sulfate proteoglycans. Food Funct. 2024, 15, 1948–1962. [Google Scholar] [CrossRef] [PubMed]
- Zlodeeva, P.D.; Shekunov, E.V.; Ostroumova, O.S.; Efimova, S.S. The Degree of Hydroxylation of Phenolic Rings Determines the Ability of Flavonoids and Stilbenes to Inhibit Calcium-Mediated Membrane Fusion. Nutrients 2023, 15, 1121. [Google Scholar] [CrossRef] [PubMed]
- Jager, W.; Kicker, E.; Hardt, M.; Gawish, R.; Gattinger, P.; Bohmdorfer, M.; Knapp, S.; Valenta, R.; Zatloukal, K.; Szekeres, T. Identification of a Synthetic Polyhydroxyphenolic Resveratrol Analogue, 3,3′,4,4′,5,5′-Hexahydroxy-trans-Stilbene with Anti-SARS-CoV-2 Activity. Molecules 2023, 28, 2612. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Quashie, P.K.; Mesplede, T.; Xu, H.; Quan, Y.; Jaeger, W.; Szekeres, T.; Wainberg, M.A. A resveratrol analog termed 3,3′,4,4′,5,5′-hexahydroxy-trans-stilbene is a potent HIV-1 inhibitor. J. Med. Virol. 2015, 87, 2054–2060. [Google Scholar] [CrossRef]
- Liu, G.; Xiong, S.; Xiang, Y.F.; Guo, C.W.; Ge, F.; Yang, C.R.; Zhang, Y.J.; Wang, Y.F.; Kitazato, K. Antiviral activity and possible mechanisms of action of pentagalloylglucose (PGG) against influenza A virus. Arch. Virol. 2011, 156, 1359–1369. [Google Scholar] [CrossRef]
- Arakawa, T.; Yamasaki, H.; Ikeda, K.; Ejima, D.; Naito, T.; Koyama, A.H. Antiviral and virucidal activities of natural products. Curr. Med. Chem. 2009, 16, 2485–2497. [Google Scholar] [CrossRef]
- Sepulveda, C.S.; Garcia, C.C.; Damonte, E.B. Inhibition of arenavirus infection by thiuram and aromatic disulfides. Antivir. Res. 2010, 87, 329–337. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bánki, Z.; Wolf, L.; Müllauer, B.; Geisler-Moroder, D.; Borena, W.; Jäger, W.; Szekeres, T. Virucidal Potential of 3,3′,4,4′,5,5′-Hexahydroxy-trans-Stilbene Against Respiratory Syncytial Virus. Viruses 2025, 17, 1287. https://doi.org/10.3390/v17101287
Bánki Z, Wolf L, Müllauer B, Geisler-Moroder D, Borena W, Jäger W, Szekeres T. Virucidal Potential of 3,3′,4,4′,5,5′-Hexahydroxy-trans-Stilbene Against Respiratory Syncytial Virus. Viruses. 2025; 17(10):1287. https://doi.org/10.3390/v17101287
Chicago/Turabian StyleBánki, Zoltán, Leonie Wolf, Brigitte Müllauer, Daniel Geisler-Moroder, Wegene Borena, Walter Jäger, and Thomas Szekeres. 2025. "Virucidal Potential of 3,3′,4,4′,5,5′-Hexahydroxy-trans-Stilbene Against Respiratory Syncytial Virus" Viruses 17, no. 10: 1287. https://doi.org/10.3390/v17101287
APA StyleBánki, Z., Wolf, L., Müllauer, B., Geisler-Moroder, D., Borena, W., Jäger, W., & Szekeres, T. (2025). Virucidal Potential of 3,3′,4,4′,5,5′-Hexahydroxy-trans-Stilbene Against Respiratory Syncytial Virus. Viruses, 17(10), 1287. https://doi.org/10.3390/v17101287





