Exploring the Contribution of TLR7 to Sex-Based Disparities in Respiratory Syncytial Virus (RSV)-Induced Inflammation and Immunity
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Infections
2.2. Lung Gene Expression Analysis by qPCR
2.3. Assessment of Airway Inflammation and Differential Cell Counting
2.4. Ex Vivo Infection of Alveolar Macrophages
2.5. Measurement of Antibody Levels in BALF
2.6. Statistical Analysis
3. Results
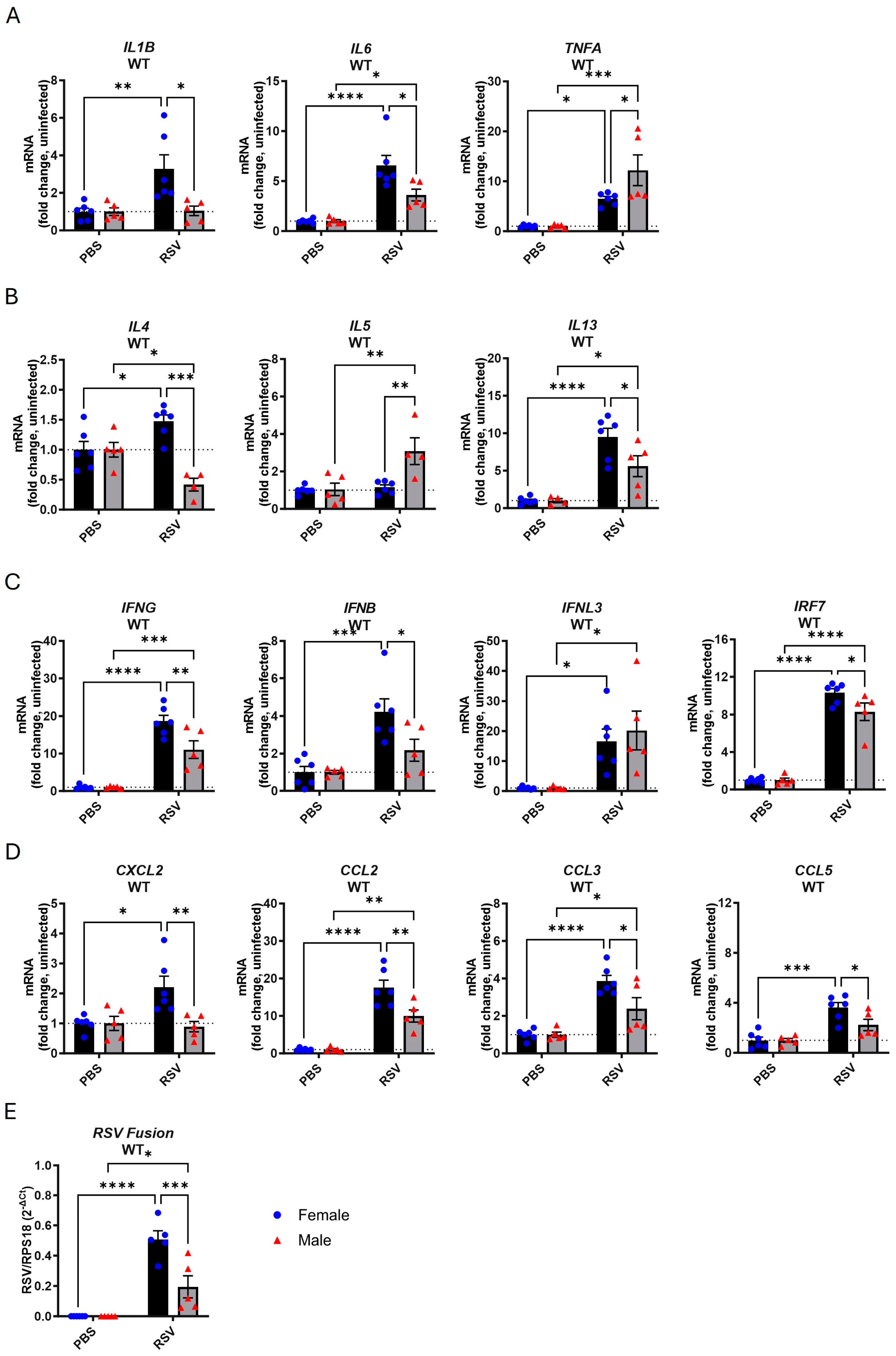
3.1. Female Mice Exhibit Higher Inflammatory Gene Expression in the Lungs Following Acute RSV Infection
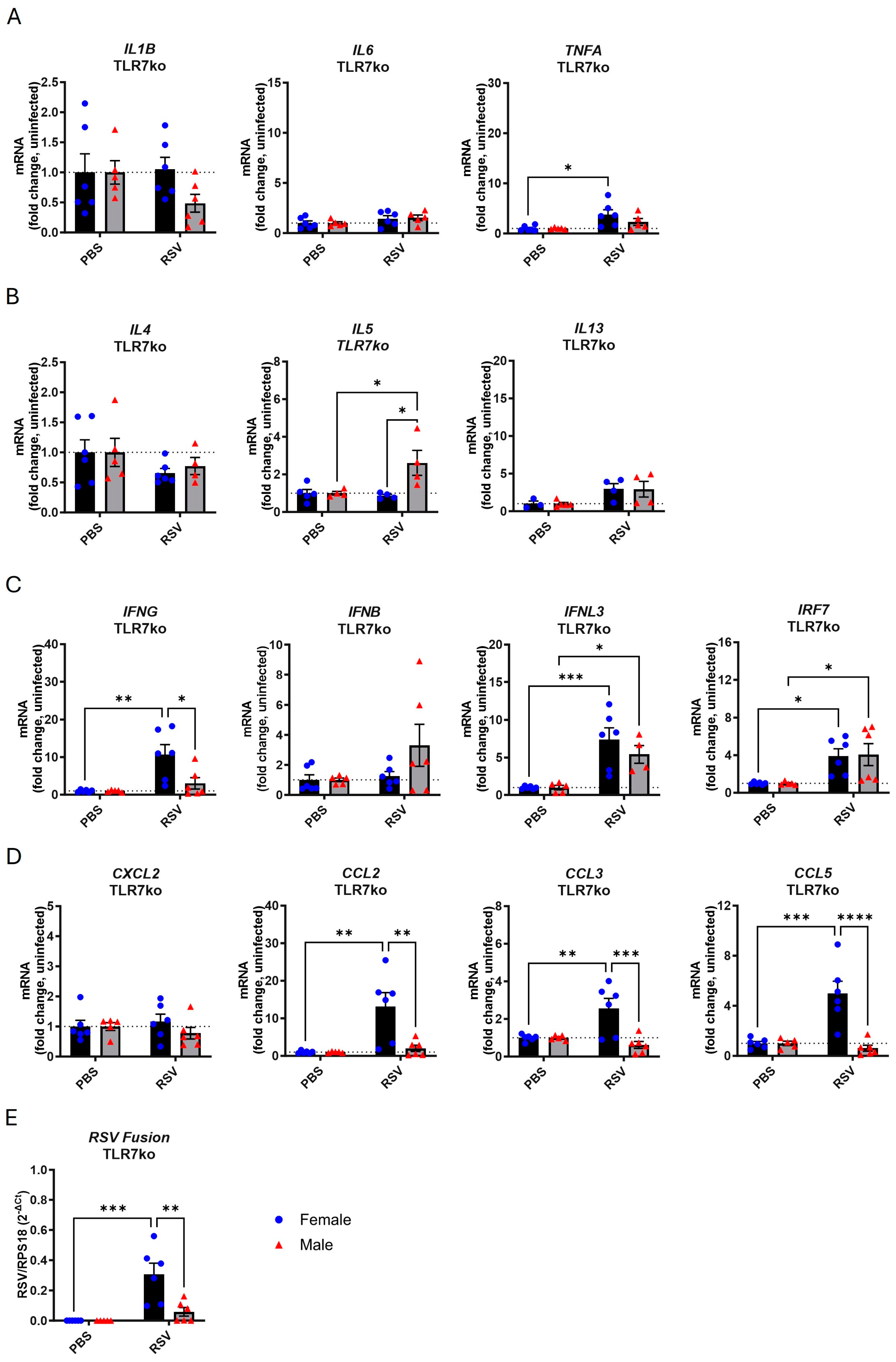
3.2. TLR7 Deficiency Reduces Cytokine Gene Expression in Response to Acute RSV Infection in Both Sexes
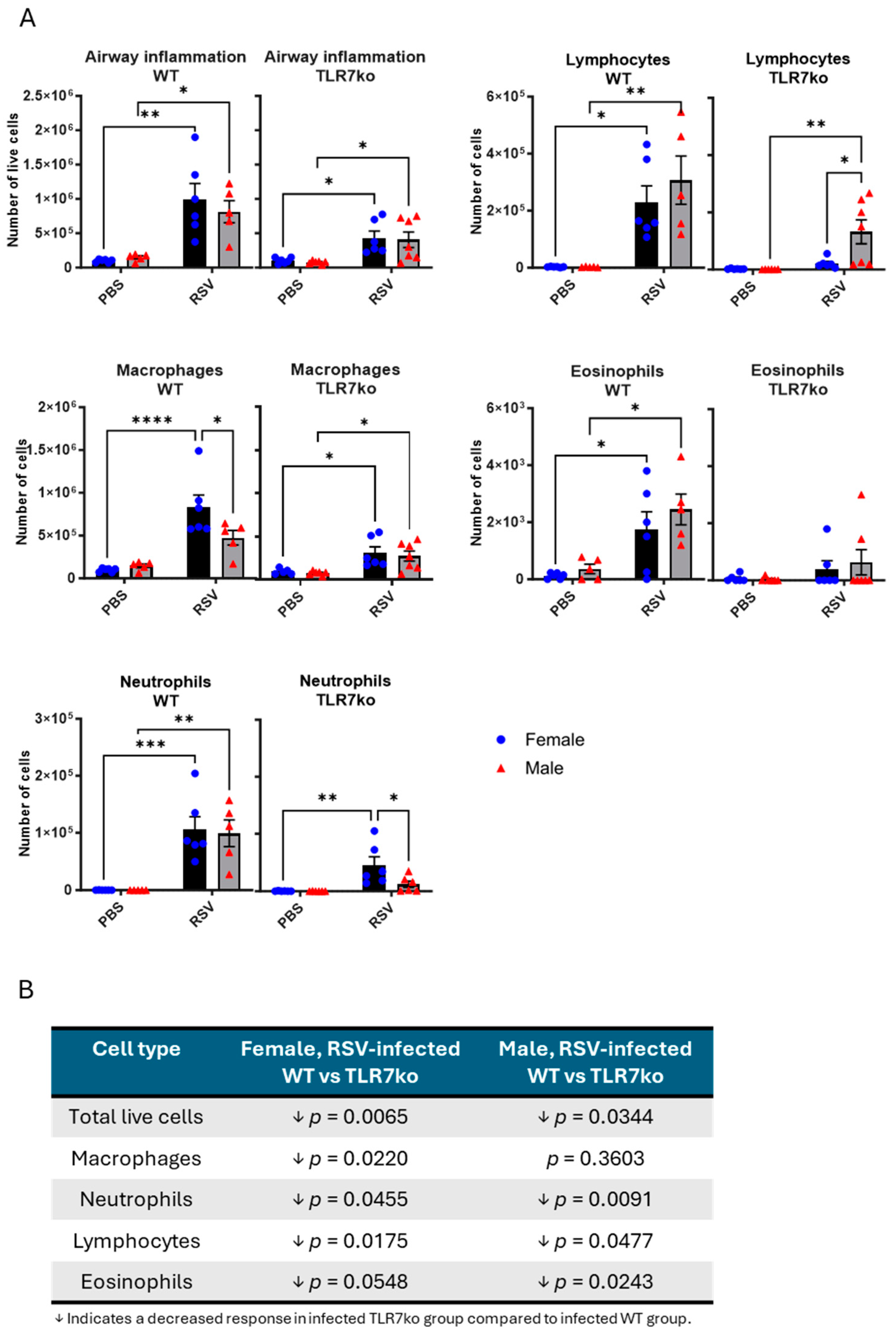
3.3. Female Mice Exhibit a Greater Influx of Macrophages into the Airways Following Acute RSV Infection
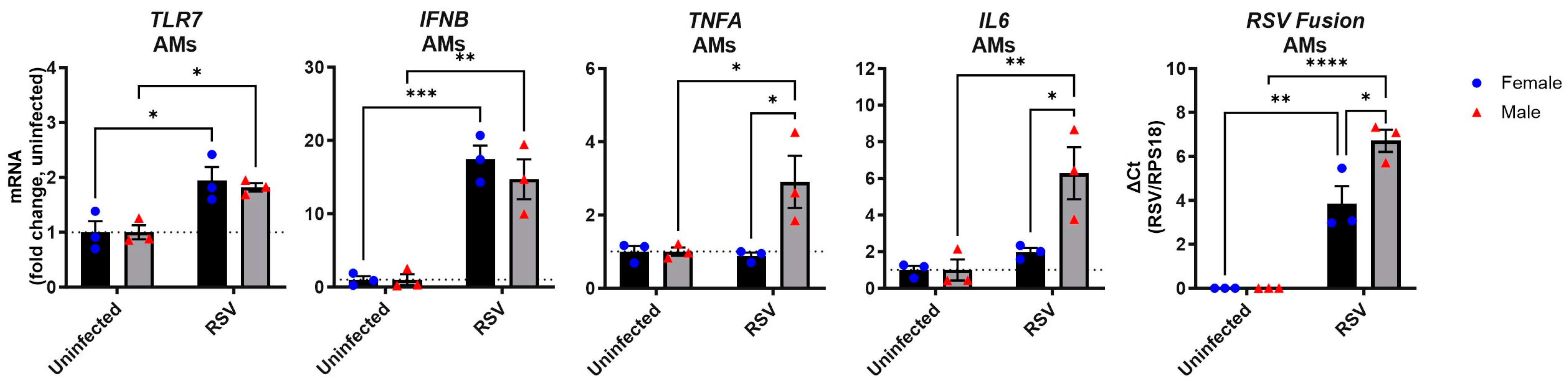
3.4. RSV Infection of Male Alveolar Macrophages Induces a Stronger Proinflammatory Cytokine Response than in Female Cells
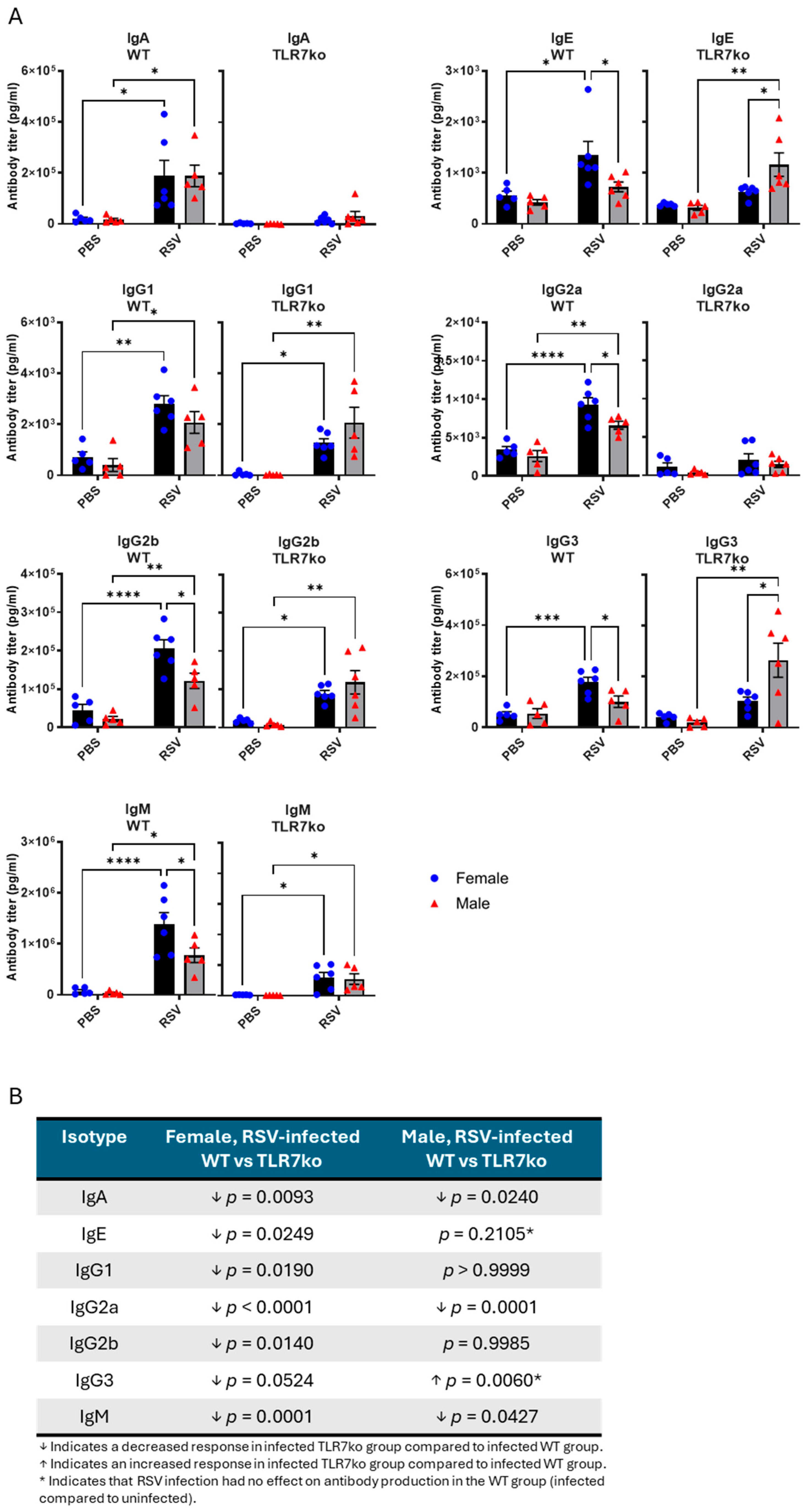
3.5. Female Mice Exhibit Higher Antibody Titers Following Acute RSV Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BALF | Bronchoalveolar lavage fluid |
| COPD | Chronic obstructive pulmonary disease |
| IAV | Influenza A virus |
| IFN | Interferon |
| LRT | Lower respiratory tract |
| TLR7 | Toll-like receptor 7 |
| TLR7ko | Toll-like receptor 7 knockout |
| PBS | Phosphate-buffered saline |
| PFU | Plaque-forming units |
| RSV | Respiratory syncytial virus |
| WT | Wild type |
References
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Barr, R.; Green, C.A.; Sande, C.J.; Drysdale, S.B. Respiratory syncytial virus: Diagnosis, prevention and management. Ther. Adv. Infect. Dis. 2019, 6, 2049936119865798. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Van-Tam, J.S.; O’Leary, M.; Martin, E.T.; Heijnen, E.; Callendret, B.; Fleischhackl, R.; Comeaux, C.; Tran, T.M.P.; Weber, K. Burden of respiratory syncytial virus infection in older and high-risk adults: A systematic review and meta-analysis of the evidence from developed countries. Eur. Respir. Rev. 2022, 31, 220105. [Google Scholar] [CrossRef]
- Topalidou, X.; Kalergis, A.M.; Papazisis, G. Respiratory Syncytial Virus Vaccines: A Review of the Candidates and the Approved Vaccines. Pathogens 2023, 12, 1259. [Google Scholar] [CrossRef]
- Walsh, E.E.; Pérez Marc, G.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.; Goswami, J.; Baqui, A.H.; Doreski, P.A.; Perez-Marc, G.; Zaman, K.; Monroy, J.; Duncan, C.J.A.; Ujiie, M.; Rämet, M.; et al. Efficacy and Safety of an mRNA-Based RSV PreF Vaccine in Older Adults. N. Engl. J. Med. 2023, 389, 2233–2244. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E.; Jones, J.M.; Roper, L.E.; Prill, M.M.; Ortega-Sanchez, I.R.; Moulia, D.L.; Wallace, M.; Godfrey, M.; Broder, K.R.; Tepper, N.K.; et al. Use of the Pfizer Respiratory Syncytial Virus Vaccine During Pregnancy for the Prevention of Respiratory Syncytial Virus-Associated Lower Respiratory Tract Disease in Infants: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1115–1122. [Google Scholar] [CrossRef]
- Garegnani, L.; Styrmisdóttir, L.; Roson Rodriguez, P.; Escobar Liquitay, C.M.; Esteban, I.; Franco, J.V. Palivizumab for preventing severe respiratory syncytial virus (RSV) infection in children. Cochrane Database Syst. Rev. 2021, 11, Cd013757. [Google Scholar] [CrossRef]
- Simões, E.A.F.; Madhi, S.A.; Muller, W.J.; Atanasova, V.; Bosheva, M.; Cabañas, F.; Baca Cots, M.; Domachowske, J.B.; Garcia-Garcia, M.L.; Grantina, I.; et al. Efficacy of nirsevimab against respiratory syncytial virus lower respiratory tract infections in preterm and term infants, and pharmacokinetic extrapolation to infants with congenital heart disease and chronic lung disease: A pooled analysis of randomised controlled trials. Lancet Child Adolesc. Health 2023, 7, 180–189. [Google Scholar] [CrossRef]
- Ursin, R.L.; Klein, S.L. Sex Differences in Respiratory Viral Pathogenesis and Treatments. Annu. Rev. Virol. 2021, 8, 393–414. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Balsells, E.; Wastnedge, E.; Singleton, R.; Rasmussen, Z.A.; Zar, H.J.; Rath, B.A.; Madhi, S.A.; Campbell, S.; Vaccari, L.C.; et al. Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: Systematic review and meta-analysis. J. Glob. Health 2015, 5, 020416. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, Y.; Tsubaki, T.; Nakayama, S.; Sawada, K.; Taguchi, K.; Tateno, N.; Toba, T. Gender analysis in acute bronchiolitis due to respiratory syncytial virus. Pediatr. Allergy Immunol. 2006, 17, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Simoes, E.A. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J. Pediatr. 2003, 143, S118–S126. [Google Scholar] [CrossRef]
- Papadopoulos, N.G.; Gourgiotis, D.; Javadyan, A.; Bossios, A.; Kallergi, K.; Psarras, S.; Tsolia, M.N.; Kafetzis, D. Does respiratory syncytial virus subtype influences the severity of acute bronchiolitis in hospitalized infants? Respir. Med. 2004, 98, 879–882. [Google Scholar] [CrossRef]
- Rosas-Salazar, C.; Chirkova, T.; Gebretsadik, T.; Chappell, J.D.; Peebles, R.S., Jr.; Dupont, W.D.; Jadhao, S.J.; Gergen, P.J.; Anderson, L.J.; Hartert, T.V. Respiratory syncytial virus infection during infancy and asthma during childhood in the USA (INSPIRE): A population-based, prospective birth cohort study. Lancet 2023, 401, 1669–1680. [Google Scholar] [CrossRef]
- Chowdhury, N.U.; Guntur, V.P.; Newcomb, D.C.; Wechsler, M.E. Sex and gender in asthma. Eur. Respir. Rev. 2021, 30, 210067. [Google Scholar] [CrossRef]
- Nagayama, Y.; Tsubaki, T.; Sawada, K.; Taguchi, K.; Nakayama, S.; Toba, T. Age and sex as factors of response to RSV infections among those with previous history of wheezing. Pediatr. Allergy Immunol. 2006, 17, 376–381. [Google Scholar] [CrossRef]
- Malinczak, C.A.; Fonseca, W.; Rasky, A.J.; Ptaschinski, C.; Morris, S.; Ziegler, S.F.; Lukacs, N.W. Sex-associated TSLP-induced immune alterations following early-life RSV infection leads to enhanced allergic disease. Mucosal Immunol. 2019, 12, 969–979. [Google Scholar] [CrossRef]
- Sundaram, M.E.; Meece, J.K.; Sifakis, F.; Gasser, R.A., Jr.; Belongia, E.A. Medically attended respiratory syncytial virus infections in adults aged ≥ 50 years: Clinical characteristics and outcomes. Clin. Infect. Dis. 2014, 58, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Long, C.E.; Schnabel, K.C. Respiratory syncytial virus infections in previously healthy working adults. Clin. Infect. Dis. 2001, 33, 792–796. [Google Scholar] [CrossRef]
- Yu, J.; Liu, N.; Zhu, Y.; Wang, W.; Fan, X.; Yuan, X.; Xu, J.; Zheng, B.; Luan, L. Comparative study on the epidemiological characteristics and hazards of respiratory syncytial virus and influenza virus infections among elderly people. BMC Infect. Dis. 2024, 24, 1129. [Google Scholar] [CrossRef]
- McCaw, J.M.; Howard, P.F.; Richmond, P.C.; Nissen, M.; Sloots, T.; Lambert, S.B.; Lai, M.; Greenberg, M.; Nolan, T.; McVernon, J. Household transmission of respiratory viruses-assessment of viral, individual and household characteristics in a population study of healthy Australian adults. BMC Infect. Dis. 2012, 12, 345. [Google Scholar] [CrossRef]
- vom Steeg, L.G.; Klein, S.L. SeXX Matters in Infectious Disease Pathogenesis. PLoS Pathog. 2016, 12, e1005374. [Google Scholar] [CrossRef]
- Márquez, E.J.; Chung, C.H.; Marches, R.; Rossi, R.J.; Nehar-Belaid, D.; Eroglu, A.; Mellert, D.J.; Kuchel, G.A.; Banchereau, J.; Ucar, D. Sexual-dimorphism in human immune system aging. Nat. Commun. 2020, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, R.; Trovato, M.; Manco, R.; D’Apice, L.; De Berardinis, P. Exploiting viral sensing mediated by Toll-like receptors to design innovative vaccines. Npj Vaccines 2021, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Souyris, M.; Cenac, C.; Azar, P.; Daviaud, D.; Canivet, A.; Grunenwald, S.; Pienkowski, C.; Chaumeil, J.; Mejía, J.E.; Guéry, J.C. TLR7 escapes X chromosome inactivation in immune cells. Sci. Immunol. 2018, 3, eaap8855. [Google Scholar] [CrossRef]
- Berghöfer, B.; Frommer, T.; Haley, G.; Fink, L.; Bein, G.; Hackstein, H. TLR7 ligands induce higher IFN-alpha production in females. J. Immunol. 2006, 177, 2088–2096. [Google Scholar] [CrossRef]
- Griesbeck, M.; Ziegler, S.; Laffont, S.; Smith, N.; Chauveau, L.; Tomezsko, P.; Sharei, A.; Kourjian, G.; Porichis, F.; Hart, M.; et al. Sex Differences in Plasmacytoid Dendritic Cell Levels of IRF5 Drive Higher IFN-α Production in Women. J. Immunol. 2015, 195, 5327–5336. [Google Scholar] [CrossRef]
- Spiering, A.E.; de Vries, T.J. Why Females Do Better: The X Chromosomal TLR7 Gene-Dose Effect in COVID-19. Front. Immunol. 2021, 12, 756262. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Carballa, A.; Pardo-Seco, J.; Pischedda, S.; Rivero-Calle, I.; Butler-Laporte, G.; Richards, J.B.; Viz-Lasheras, S.; Martinón-Torres, F.; Salas, A. Sex-biased expression of the TLR7 gene in severe COVID-19 patients: Insights from transcriptomics and epigenomics. Environ. Res. 2022, 215, 114288. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Boisson, B.; Onodi, F.; Matuozzo, D.; Moncada-Velez, M.; Maglorius Renkilaraj, M.R.L.; Zhang, P.; Meertens, L.; Bolze, A.; Materna, M.; et al. X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci. Immunol. 2021, 6, eabl4348. [Google Scholar] [CrossRef]
- Fallerini, C.; Daga, S.; Mantovani, S.; Benetti, E.; Picchiotti, N.; Francisci, D.; Paciosi, F.; Schiaroli, E.; Baldassarri, M.; Fava, F.; et al. Association of Toll-like receptor 7 variants with life-threatening COVID-19 disease in males: Findings from a nested case-control study. Elife 2021, 10, e67569. [Google Scholar] [CrossRef]
- Fink, A.L.; Engle, K.; Ursin, R.L.; Tang, W.Y.; Klein, S.L. Biological sex affects vaccine efficacy and protection against influenza in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 12477–12482. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.A.; Liong, S.; Liong, F.; Coward-Smith, M.; Trollope, G.S.; Oseghale, O.; Erlich, J.R.; Brooks, R.D.; Logan, J.M.; Hickey, S.; et al. TLR7 promotes chronic airway disease in RSV-infected mice. Front. Immunol. 2023, 14, 1240552. [Google Scholar] [CrossRef]
- Lukacs, N.W.; Smit, J.J.; Mukherjee, S.; Morris, S.B.; Nunez, G.; Lindell, D.M. Respiratory virus-induced TLR7 activation controls IL-17-associated increased mucus via IL-23 regulation. J. Immunol. 2010, 185, 2231–2239. [Google Scholar] [CrossRef]
- Malinczak, C.A.; Fonseca, W.; Mire, M.M.; Parolia, A.; Chinnaiyan, A.; Rasky, A.J.; Morris, S.; Yagi, K.; Bermick, J.R.; Lukacs, N.W. Sex-associated early-life viral innate immune response is transcriptionally associated with chromatin remodeling of type-I IFN-inducible genes. Mucosal Immunol. 2023, 16, 578–592. [Google Scholar] [CrossRef]
- Liong, S.; Liong, F.; Mohsenipour, M.; Hill-Yardin, E.L.; Miles, M.A.; Selemidis, S. Early-Life Respiratory Syncytial Virus (RSV) Infection Triggers Immunological Changes in Gut-Associated Lymphoid Tissues in a Sex-Dependent Manner in Adulthood. Cells 2024, 13, 1728. [Google Scholar] [CrossRef]
- Graham, B.S.; Perkins, M.D.; Wright, P.F.; Karzon, D.T. Primary respiratory syncytial virus infection in mice. J. Med. Virol. 1988, 26, 153–162. [Google Scholar] [CrossRef]
- Habibi, M.S.; Thwaites, R.S.; Chang, M.; Jozwik, A.; Paras, A.; Kirsebom, F.; Varese, A.; Owen, A.; Cuthbertson, L.; James, P.; et al. Neutrophilic inflammation in the respiratory mucosa predisposes to RSV infection. Science 2020, 370, eaba9301. [Google Scholar] [CrossRef] [PubMed]
- Montaño Mendoza, V.M.; Mendez Cortina, Y.A.; Rodríguez-Perea, A.L.; Fernandez, G.J.; Rugeles, M.T.; Velilla Hernandez, P.A.; Cardona Maya, W.D. Biological sex and age-related differences shape the antiviral response to SARS-CoV-2 infection. Heliyon 2023, 9, e13045. [Google Scholar] [CrossRef]
- van der Made, C.I.; Simons, A.; Schuurs-Hoeijmakers, J.; van den Heuvel, G.; Mantere, T.; Kersten, S.; van Deuren, R.C.; Steehouwer, M.; van Reijmersdal, S.V.; Jaeger, M.; et al. Presence of Genetic Variants Among Young Men with Severe COVID-19. JAMA 2020, 324, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Getts, D.R.; Chastain, E.M.; Terry, R.L.; Miller, S.D. Virus infection, antiviral immunity, and autoimmunity. Immunol. Rev. 2013, 255, 197–209. [Google Scholar] [CrossRef]
- Souyris, M.; Mejía, J.E.; Chaumeil, J.; Guéry, J.C. Female predisposition to TLR7-driven autoimmunity: Gene dosage and the escape from X chromosome inactivation. Semin. Immunopathol. 2019, 41, 153–164. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, J.; Liu, Q.; Xu, S. Neurological risks of COVID-19 in women: The complex immunology underpinning sex differences. Front. Immunol. 2023, 14, 1281310. [Google Scholar] [CrossRef]
- Zuo, Y.; Navaz, S.; Liang, W.; Li, C.; Ayers, C.R.; Rysenga, C.E.; Harbaugh, A.; Norman, G.L.; Solow, E.B.; Bermas, B.; et al. Prevalence of Antiphospholipid Antibodies and Association with Incident Cardiovascular Events. JAMA Netw. Open 2023, 6, e236530. [Google Scholar] [CrossRef]
- Liu, G.; Haw, T.J.; Starkey, M.R.; Philp, A.M.; Pavlidis, S.; Nalkurthi, C.; Nair, P.M.; Gomez, H.M.; Hanish, I.; Hsu, A.C.; et al. TLR7 promotes smoke-induced experimental lung damage through the activity of mast cell tryptase. Nat. Commun. 2023, 14, 7349. [Google Scholar] [CrossRef] [PubMed]
- Birring, S.S.; Brightling, C.E.; Bradding, P.; Entwisle, J.J.; Vara, D.D.; Grigg, J.; Wardlaw, A.J.; Pavord, I.D. Clinical, radiologic, and induced sputum features of chronic obstructive pulmonary disease in nonsmokers: A descriptive study. Am. J. Respir. Crit. Care Med. 2002, 166, 1078–1083. [Google Scholar] [CrossRef]
- Laurent, P.; Yang, C.; Rendeiro, A.F.; Nilsson-Payant, B.E.; Carrau, L.; Chandar, V.; Bram, Y.; tenOever, B.R.; Elemento, O.; Ivashkiv, L.B.; et al. Sensing of SARS-CoV-2 by pDCs and their subsequent production of IFN-I contribute to macrophage-induced cytokine storm during COVID-19. Sci. Immunol. 2022, 7, eadd4906. [Google Scholar] [CrossRef]
- Miles, M.A.; Liong, S.; Liong, F.; Trollope, G.S.; Wang, H.; Brooks, R.D.; Bozinovski, S.; O’Leary, J.J.; Brooks, D.A.; Selemidis, S. TLR7 Promotes Acute Inflammatory-Driven Lung Dysfunction in Influenza-Infected Mice but Prevents Late Airway Hyperresponsiveness. Int. J. Mol. Sci. 2024, 25, 13699. [Google Scholar] [CrossRef]
- Scotland, R.S.; Stables, M.J.; Madalli, S.; Watson, P.; Gilroy, D.W. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood 2011, 118, 5918–5927. [Google Scholar] [CrossRef] [PubMed]
- Huret, C.; Ferrayé, L.; David, A.; Mohamed, M.; Valentin, N.; Charlotte, F.; Savignac, M.; Goodhardt, M.; Guéry, J.C.; Rougeulle, C.; et al. Altered X-chromosome inactivation predisposes to autoimmunity. Sci. Adv. 2024, 10, eadn6537. [Google Scholar] [CrossRef] [PubMed]
- Lakshmikanth, T.; Consiglio, C.; Sardh, F.; Forlin, R.; Wang, J.; Tan, Z.; Barcenilla, H.; Rodriguez, L.; Sugrue, J.; Noori, P.; et al. Immune system adaptation during gender-affirming testosterone treatment. Nature 2024, 633, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Bouman, A.; Schipper, M.; Heineman, M.J.; Faas, M.M. Gender difference in the non-specific and specific immune response in humans. Am. J. Reprod. Immunol. 2004, 52, 19–26. [Google Scholar] [CrossRef]
- Marriott, I.; Bost, K.L.; Huet-Hudson, Y.M. Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: A possible mechanism for gender-based differences in endotoxic shock susceptibility. J. Reprod. Immunol. 2006, 71, 12–27. [Google Scholar] [CrossRef]
- Gupta, V.; Singh, S.M. Gender dimorphism in the myeloid differentiation of bone marrow precursor cells in a murine host bearing a T cell lymphoma. J. Reprod. Immunol. 2007, 74, 90–102. [Google Scholar] [CrossRef]
- Tipton, A.J.; Sullivan, J.C. Sex differences in T cells in hypertension. Clin. Ther. 2014, 36, 1882–1900. [Google Scholar] [CrossRef]
- Kadel, S.; Kovats, S. Sex Hormones Regulate Innate Immune Cells and Promote Sex Differences in Respiratory Virus Infection. Front. Immunol. 2018, 9, 1653. [Google Scholar] [CrossRef]
- Bianchi, I.; Lleo, A.; Gershwin, M.E.; Invernizzi, P. The X chromosome and immune associated genes. J. Autoimmun. 2012, 38, J187–J192. [Google Scholar] [CrossRef]
- Yee Mon, K.J.; Goldsmith, E.; Watson, N.B.; Wang, J.; Smith, N.L.; Rudd, B.D. Differential Sensitivity to IL-12 Drives Sex-Specific Differences in the CD8+ T Cell Response to Infection. Immunohorizons 2019, 3, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Syrett, C.M.; Kramer, M.C.; Basu, A.; Atchison, M.L.; Anguera, M.C. Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proc. Natl. Acad. Sci. USA 2016, 113, E2029–E2038. [Google Scholar] [CrossRef]
- Heer, A.K.; Shamshiev, A.; Donda, A.; Uematsu, S.; Akira, S.; Kopf, M.; Marsland, B.J. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J. Immunol. 2007, 178, 2182–2191. [Google Scholar] [CrossRef] [PubMed]
- Hewagama, A.; Patel, D.; Yarlagadda, S.; Strickland, F.M.; Richardson, B.C. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes Immun. 2009, 10, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Fuseini, H.; Yung, J.A.; Cephus, J.Y.; Zhang, J.; Goleniewska, K.; Polosukhin, V.V.; Peebles, R.S., Jr.; Newcomb, D.C. Testosterone Decreases House Dust Mite-Induced Type 2 and IL-17A-Mediated Airway Inflammation. J. Immunol. 2018, 201, 1843–1854. [Google Scholar] [CrossRef]
- Cephus, J.Y.; Stier, M.T.; Fuseini, H.; Yung, J.A.; Toki, S.; Bloodworth, M.H.; Zhou, W.; Goleniewska, K.; Zhang, J.; Garon, S.L.; et al. Testosterone Attenuates Group 2 Innate Lymphoid Cell-Mediated Airway Inflammation. Cell Rep. 2017, 21, 2487–2499. [Google Scholar] [CrossRef]
- Jeisy-Scott, V.; Davis, W.G.; Patel, J.R.; Bowzard, J.B.; Shieh, W.J.; Zaki, S.R.; Katz, J.M.; Sambhara, S. Increased MDSC accumulation and Th2 biased response to influenza A virus infection in the absence of TLR7 in mice. PLoS ONE 2011, 6, e25242. [Google Scholar] [CrossRef]
- Luo, H.; Jia, T.; Chen, J.; Zeng, S.; Qiu, Z.; Wu, S.; Li, X.; Lei, Y.; Wang, X.; Wu, W.; et al. The Characterization of Disease Severity Associated IgG Subclasses Response in COVID-19 Patients. Front. Immunol. 2021, 12, 632814. [Google Scholar] [CrossRef]
- Dakhama, A.; Lee, Y.M.; Ohnishi, H.; Jing, X.; Balhorn, A.; Takeda, K.; Gelfand, E.W. Virus-specific IgE enhances airway responsiveness on reinfection with respiratory syncytial virus in newborn mice. J. Allergy Clin. Immunol. 2009, 123, 138–145.e135. [Google Scholar] [CrossRef]
- Moisan, J.; Camateros, P.; Thuraisingam, T.; Marion, D.; Koohsari, H.; Martin, P.; Boghdady, M.L.; Ding, A.; Gaestel, M.; Guiot, M.C.; et al. TLR7 ligand prevents allergen-induced airway hyperresponsiveness and eosinophilia in allergic asthma by a MYD88-dependent and MK2-independent pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L987–L995. [Google Scholar] [CrossRef]
- Leaker, B.R.; Singh, D.; Lindgren, S.; Almqvist, G.; Eriksson, L.; Young, B.; O’Connor, B. Effects of the Toll-like receptor 7 (TLR7) agonist, AZD8848, on allergen-induced responses in patients with mild asthma: A double-blind, randomised, parallel-group study. Respir. Res. 2019, 20, 288. [Google Scholar] [CrossRef]
- Matsui, H.; Tomizawa, H.; Eiho, K.; Kashiwazaki, Y.; Edwards, S.; Biffen, M.; Bell, J.P.; Bahl, A.; Leishman, A.J.; Murray, C.M.; et al. Mechanism of action of inhibition of allergic immune responses by a novel antedrug TLR7 agonist. J. Immunol. 2012, 189, 5194–5205. [Google Scholar] [CrossRef] [PubMed]
- Møller-Larsen, S.; Nyegaard, M.; Haagerup, A.; Vestbo, J.; Kruse, T.A.; Børglum, A.D. Association analysis identifies TLR7 and TLR8 as novel risk genes in asthma and related disorders. Thorax 2008, 63, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Shikhagaie, M.M.; Andersson, C.K.; Mori, M.; Kortekaas Krohn, I.; Bergqvist, A.; Dahl, R.; Ekblad, E.; Hoffmann, H.J.; Bjermer, L.; Erjefält, J.S. Mapping of TLR5 and TLR7 in central and distal human airways and identification of reduced TLR expression in severe asthma. Clin. Exp. Allergy 2014, 44, 184–196. [Google Scholar] [CrossRef]
- Roponen, M.; Yerkovich, S.T.; Hollams, E.; Sly, P.D.; Holt, P.G.; Upham, J.W. Toll-like receptor 7 function is reduced in adolescents with asthma. Eur. Respir. J. 2010, 35, 64–71. [Google Scholar] [CrossRef]
- Rupani, H.; Martinez-Nunez, R.T.; Dennison, P.; Lau, L.C.; Jayasekera, N.; Havelock, T.; Francisco-Garcia, A.S.; Grainge, C.; Howarth, P.H.; Sanchez-Elsner, T. Toll-like Receptor 7 Is Reduced in Severe Asthma and Linked to an Altered MicroRNA Profile. Am. J. Respir. Crit. Care Med. 2016, 194, 26–37. [Google Scholar] [CrossRef]
- Binns, E.; Tuckerman, J.; Licciardi, P.V.; Wurzel, D. Respiratory syncytial virus, recurrent wheeze and asthma: A narrative review of pathophysiology, prevention and future directions. J. Paediatr. Child. Health 2022, 58, 1741–1746. [Google Scholar] [CrossRef] [PubMed]
- Torcia, M.G.; Nencioni, L.; Clemente, A.M.; Civitelli, L.; Celestino, I.; Limongi, D.; Fadigati, G.; Perissi, E.; Cozzolino, F.; Garaci, E.; et al. Sex differences in the response to viral infections: TLR8 and TLR9 ligand stimulation induce higher IL10 production in males. PLoS ONE 2012, 7, e39853. [Google Scholar] [CrossRef]
- Youness, A.; Cenac, C.; Faz-López, B.; Grunenwald, S.; Barrat, F.J.; Chaumeil, J.; Mejía, J.E.; Guéry, J.C. TLR8 escapes X chromosome inactivation in human monocytes and CD4(+) T cells. Biol. Sex. Differ. 2023, 14, 60. [Google Scholar] [CrossRef]
- Khan, N.; Summers, C.W.; Helbert, M.R.; Arkwright, P.D. Effects of age, gender, and immunosuppressive agents on in vivo toll-like receptor pathway responses. Hum. Immunol. 2010, 71, 372–376. [Google Scholar] [CrossRef]

| Gene | Female, RSV-Infected WT vs. TLR7ko | Male, RSV-Infected WT vs. TLR7ko |
|---|---|---|
| IL1B | ↓ p = 0.0025 | p = 0.6172 * |
| NLRP3 | ↓ p < 0.0001 | ↓ p = 0.0431 * |
| IL18 | ↓ p = 0.0938 | p = 0.8545 * |
| IL6 | ↓ p = 0.0004 | p = 0.1204 |
| TNFA | ↓ p = 0.0731 | ↓ p = 0.0020 |
| IL4 | ↓ p < 0.0001 | ↓ p = 0.0961 * |
| IL5 | p = 0.8696 * | p = 0.7319 |
| IL13 | ↓ p = 0.0028 | p = 0.2654 |
| IFNG | ↓ p = 0.0144 | ↓ p = 0.0217 |
| IFNB | ↓ p = 0.0466 | p = 0.6202 * |
| IFNL3 | p = 0.1950 | ↓ p = 0.0554 |
| IRF7 | ↓ p < 0.0001 | ↓ p = 0.0066 |
| CXCL2 | ↓ p = 0.0200 | p = 0.9447 * |
| CCL2 | p = 0.3583 | ↓ p = 0.0612 |
| CCL3 | ↓ p = 0.0154 | ↓ p = 0.0123 |
| CCL5 | p = 0.2243 | p = 0.1553 * |
| RSV F | ↓ p = 0.0866 | p = 0.1996 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miles, M.A.; Huttmann, T.D.; Liong, S.; Liong, F.; O’Leary, J.J.; Brooks, D.A.; Selemidis, S. Exploring the Contribution of TLR7 to Sex-Based Disparities in Respiratory Syncytial Virus (RSV)-Induced Inflammation and Immunity. Viruses 2025, 17, 428. https://doi.org/10.3390/v17030428
Miles MA, Huttmann TD, Liong S, Liong F, O’Leary JJ, Brooks DA, Selemidis S. Exploring the Contribution of TLR7 to Sex-Based Disparities in Respiratory Syncytial Virus (RSV)-Induced Inflammation and Immunity. Viruses. 2025; 17(3):428. https://doi.org/10.3390/v17030428
Chicago/Turabian StyleMiles, Mark A., Thomas D. Huttmann, Stella Liong, Felicia Liong, John J. O’Leary, Doug A. Brooks, and Stavros Selemidis. 2025. "Exploring the Contribution of TLR7 to Sex-Based Disparities in Respiratory Syncytial Virus (RSV)-Induced Inflammation and Immunity" Viruses 17, no. 3: 428. https://doi.org/10.3390/v17030428
APA StyleMiles, M. A., Huttmann, T. D., Liong, S., Liong, F., O’Leary, J. J., Brooks, D. A., & Selemidis, S. (2025). Exploring the Contribution of TLR7 to Sex-Based Disparities in Respiratory Syncytial Virus (RSV)-Induced Inflammation and Immunity. Viruses, 17(3), 428. https://doi.org/10.3390/v17030428







