Myocarditis and Inflammatory Cardiomyopathy in Dilated Heart Failure
Abstract
1. Introduction
2. Viruses’ Position in Pathogenesis
2.1. Viral Agents
- Coronaviridae
- Adenoviruses and enteroviruses
- Parvovirus B19
- Herpesviridae
- HIV, hepatitis C virus and influenza A and B viruses.
2.2. Immune Cells’ Function
2.3. Myocarditis Autoimmunity Profile
- Autoantibodies specific to the heart
- How genes interact with the environment
3. General Concept of Diagnosing and Prognosis
3.1. Clinical Settings
3.2. Immaging Diagnostics
3.3. Endomyocardial Biopsy to Diagnose Myocarditis
3.4. The Process of Genetic Testing and the Use of Biomarkers
3.4.1. Genetic Testing
3.4.2. The Role of Microrna Profiles in EMB Specimens and Blood
3.4.3. Novel Diagnostic Procedure: EMB Transcriptome—Based Biomarker
3.4.4. Blood Related Biomarkers
4. Treating Patients
4.1. Treating Heart Failure and Abnormal Heart Rhythms
4.2. Pharmaceutical and Biological Products
4.2.1. The Heart Muscle Has Been Inflamed, Without Being Infected with a Virus
4.2.2. Patient Diagnosed with Viral Positive Inflammatory Cardiomyopathy
5. New Types of Administered Medications
- Aldosterone antagonists
- Cannabidiol and antagomirs
- Modulating the gut microbiome
- Immunotherapies
- ▪
- Soluble anti-CAR antibody. A pair of investigations centred on a treatment involving an engineered soluble CAR that has been fused to the carboxy terminus of human IgG, a process that has been demonstrated to curtail virus uptake into host cells, with the objective of curtailing the progression of both acute [281] and chronic [282] CVB3-induced myocarditis in murine subjects. Nonetheless, the feasibility of this approach in human subjects remains to be ascertained.
- ▪
- Anti-IL-1β and anti-IL-1 receptor antibodies. Studies of viral and autoimmune myocarditis in animal models have yielded evidence that lends support to a central role for NRLP3 inflammasome signalling and subsequent IL-1β production in the etiopathogenesis of myocardial inflammation [283] (68). The administration of an anti-mouse IL-1 receptor antibody at various phases of enteroviral infection has been demonstrated to impede the progression of chronic viral myocarditis in murine models by attenuating inflammation, interstitial fibrosis, and deleterious cardiac remodelling [283]. There is supporting evidence from one clinical study [284] and a number of case reports [285,286] for the use of an anti-IL-1β monoclonal antibody in the management of patients with a recurrence of pericarditis. The underway ARAMIS [287] and RHAPSODY [288] trials have been developed to evaluate the effectiveness of IL-1β-blocking medications in individuals diagnosed with myocarditis and concomitant pericarditis.
- ▪
- Anti-IL-17 antibody. The present body of research indicates that heightened IL-17-related responses and the onset of profibrotic mechanisms have been correlated with an elevated risk of mortality in murine models of CVB3-induced myocarditis [289] and with a diminished rate of restorative functional recuperation in human myocarditis cases [124]. It has been demonstrated that TH17 cells can induce the development of dilated cardiomyopathy in murine models [118]. Conversely, Treg cells have been observed to offer a protective effect against myocarditis by moderating inflammatory responses [96,119]. A clinical trial involving the administration of secukinumab, a monoclonal antibody targeting IL-17, has been put forward as a potential therapeutic approach.
- ▪
- Cell-based therapies. The clinical implementation of Treg cells [290] and the utilisation of IL-2 agonists [291] whose function is to stimulate Treg cell proliferation and to enhance the survival and suppressor function of mature Treg cells [292] represent complementary strategies to elevate the Treg cell to TH17 cell ratio. It has been posited that an alternative cell-based strategy may entail the utilisation of mesenchymal stromal cells, which have been demonstrated to enhance the population of Treg cells [101] and have been observed to exert immunomodulatory and cardioprotective functions in murine models of myocardial inflammation [85,94,293], potentially through the moderation of the cardiosplenic interface. The safety and efficacy of therapy with allogeneic mesenchymal stromal cells has been demonstrated in patients with non-ischaemic DCM in the POSEIDON-DCM trial [294]. The POSEIDON-DCM trial revealed that the LVEF exhibited a notable enhancement following autologous mesenchymal stromal cell therapy, yet this observation was exclusively confined to patients devoid of a pathogenic gene variant associated with DCM. This finding underscores the significance of a patient’s genetic profile in determining their responsiveness to mesenchymal stromal cell therapy [295]. A notable finding in this regard was the significant correlation between this response and a considerable decrease in circulating TNF levels [294], thereby pointing to an immunomodulatory therapeutic effect. In conclusion, this set of observations signifies the potential for cell-based therapy in the management of subjects diagnosed with inflammation-induced cardiomyopathy, thereby underscoring the necessity for further investigation in this area through well-designed clinical trials.
6. Mechanical Circulatory Support in Patients with Fulminant Myocarditis
7. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richardson, P.; McKenna, W.; Bristow, M.; Maisch, B.; Mautner, B.; O’Connell, J.; Olsen, E.; Thiene, G.; Goodwin, J.; Gyarfas, I.; et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology task force on the definition and classification of cardiomyopathies. Circulation 1996, 93, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Giles, T.D. New WHO/ISFC classification of cardiomyopathies: A task not completed. Circulation 1997, 96, 2081–2082. [Google Scholar]
- Sánchez Torres, R.J.; Calderón, R. The cardiomyopathies, a review for the primary physician. Puerto Rico Health Sci. J. 2004, 23, 285–292. [Google Scholar]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Şahan, E.; Şahan, S.; Karamanlıoğlu, M.; Gul, M.; Tufekcioğlu, O. The MOGE(S) classification: A TNM-like classification for cardiomyopathies. Herz 2016, 41, 503–506. [Google Scholar] [CrossRef]
- Ammirati, E.; Cipriani, M.; Moro, C.; Raineri, C.; Pini, D.; Sormani, P.; Mantovani, R.; Varrenti, M.; Pedrotti, P.; Conca, C.; et al. Clinical presentation and outcome in a contemporary cohort of patients with acute myocarditis. Circulation 2018, 138, 1088–1099. [Google Scholar] [CrossRef]
- Ammirati, E.; Varrenti, M.; Sormani, P.; Bernasconi, D.; Moro, C.; Grosu, A.; D’Elia, S.; Raineri, C.; Quattrocchi, G.; Milazzo, A.; et al. Long-term prognostic performance of cardiac magnetic resonance imaging markers versus complicated clinical presentation after an acute myocarditis. Int. J. Cardiol. 2024, 417, 132567. [Google Scholar] [CrossRef]
- Ginsberg, F.; Parrillo, J.E. Fulminant myocarditis. Crit. Care Clin. 2013, 29, 465–483. [Google Scholar] [CrossRef]
- Van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement from the American Heart Association. Circulation 2017, 136, e232–e268. [Google Scholar] [CrossRef]
- Kociol, R.D.; Cooper, L.T.; Fang, J.C.; Moslehi, J.J.; Pang, P.S.; Sabe, M.A.; Shah, R.V.; Sims, D.B.; Thiene, G.; Vardeny, O.; et al. Recognition and initial management of fulminant myocarditis: A scientific statement from the American Heart Association. Circulation 2020, 141, e69–e92. [Google Scholar] [CrossRef]
- Chou, H.W.; Wang, C.H.; Lin, L.Y.; Chi, N.H.; Chou, N.K.; Yu, H.Y.; Chen, Y.S. Prognostic factors for heart recovery in adult patients with acute fulminant myocarditis and cardiogenic shock supported with extracorporeal membrane oxygenation. J. Crit. Care 2020, 57, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Cipriani, M.; Lilliu, M.; Sormani, P.; Varrenti, M.; Raineri, C.; Petrella, D.; Garascia, A.; Pedrotti, P.; Roghi, A.; et al. Survival and Left Ventricular Function Changes in Fulminant Versus Nonfulminant Acute Myocarditis. Circulation 2017, 136, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Veronese, G.; Brambatti, M.; Merlo, M.; Cipriani, M.; Potena, L.; Sormani, P.; Aoki, T.; Sugimura, K.; Sawamura, A.; et al. Fulminant versus acute nonfulminant myocarditis in patients with left ventricular systolic dysfunction. J. Am. Coll. Cardiol. 2019, 74, 299–311. [Google Scholar] [CrossRef]
- Dominguez, F.; Kuhl, U.; Pieske, B.; Garcia-Pavia, P.; Tschöpe, C. Update on myocarditis and inflammatory cardiomyopathy: Reemergence of endomyocardial biopsy. Rev. Esp. Cardiol. (Engl. Ed). 2016, 69, 178–187. [Google Scholar] [CrossRef]
- Trachtenberg, B.H.; Hare, J.M. Inflammatory cardiomyopathic syndromes. Circ. Res. 2017, 121, 803–818. [Google Scholar] [CrossRef]
- Hu, J.R.; Florido, R.; Lipson, E.J.; Naidoo, J.; Ardehali, R.; Tocchetti, C.G.; Lyon, A.R.; Padera, R.F.; Johnson, D.B.; Moslehi, J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc. Res. 2019, 115, 854–868. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Adler, Y.; Agostini, C.; Allanore, Y.; Anastasakis, A.; Arad, M.; Böhm, M.; Charron, P.; Elliott, P.M.; Eriksson, U.; et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur. Heart J. 2017, 38, 2649–2662. [Google Scholar] [CrossRef]
- Kuhl, U.; Pauschinger, M.; Noutsias, M.; Seeberg, B.; Bock, T.; Lassner, D.; Poller, W.; Kandolf, R.; Schultheiss, H.P. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation 2005, 111, 887–893. [Google Scholar] [CrossRef]
- Ukimura, A.; Satomi, H.; Ooi, Y.; Kanzaki, Y. Myocarditis associated with influenza A H1N1pdm2009. Influenza Res. Treat. 2012, 2012, 351979. [Google Scholar] [CrossRef]
- Van Linthout, S.; Klingel, K.; Tschope, C. SARS-CoV2-related myocarditis-like syndroms: Shakespeare’s question: What’s in a name? Eur. J. Heart Fail. 2020, 22, 922–925. [Google Scholar] [CrossRef]
- Bozkurt, B.; Colvin, M.; Cook, J.; Cooper, L.T.; Deswal, A.; Fonarow, G.C.; Francis, G.S.; Lenihan, D.; Lewis, E.F.; McNamara, D.M.; et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: A scientific statement from the American Heart Association. Circulation 2016, 134, e579–e646. [Google Scholar] [CrossRef] [PubMed]
- Pauschinger, M.; Bowles, N.E.; Fuentes-Garcia, F.J.; Pham, V.; Kühl, U.; Schwimmbeck, P.L.; Schultheiss, H.P.; Towbin, J.A. Detection of adenoviral genome in the myocardium of adult patients with idiopathic left ventricular dysfunction. Circulation 1999, 99, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.H.; Huang, Y.C.; Chen, M.C.; Liu, C.C.; Chiu, N.C.; Lien, R.; Chang, L.Y.; Chiu, C.H.; Tsao, K.C.; Lin, T.Y. Effect of intravenous immunoglobulin for neonates with severe enteroviral infections with emphasis on the timing of administration. J. Clin. Virol. 2015, 64, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Abzug, M.J.; Michaels, M.G.; Wald, E.; Jacobs, R.F.; Romero, J.R.; Sánchez, P.J.; Wilson, G.; Krogstad, P.; Storch, G.A.; Lawrence, R.; et al. A randomized, double-blind, placebocontrolled trial of pleconaril for the treatment of neonates with enterovirus sepsis. J. Pediatr. Infect. Dis. Soc. 2016, 5, 53–62. [Google Scholar] [CrossRef]
- Amdani, S.M.; Kim, H.S.; Orvedahl, A.; John, A.O.; Said, A.; Simpson, K. Successful treatment of fulminant neonatal enteroviral myocarditis in monochorionic diamniotic twins with cardiopulmonary support, intravenous immunoglobulin and pocapavir. BMJ Case Rep. 2018, 2018, bcr2017224133. [Google Scholar] [CrossRef]
- Schultz, J.C.; Hilliard, A.A.; Cooper, L.T., Jr.; Rihal, C.S. Diagnosis and treatment of viral myocarditis. Mayo Clin. Proc. 2009, 84, 1001–1009. [Google Scholar] [CrossRef]
- Woodruff, J.F. Viral myocarditis. A review. Am. J. Pathol. 1980, 101, 425–484. [Google Scholar]
- Smith, W.G. Coxsackie B myopericarditis in adults. Am. Heart J. 1970, 80, 34–46. [Google Scholar] [CrossRef]
- Lyden, D.C.; Olszewski, J.; Feran, M.; Job, L.P.; Huber, S.A. Coxsackievirus B-3-induced myocarditis. Effect of sex steroids on viremia and infectivity of cardiocytes. Am. J. Pathol. 1987, 126, 432–438. [Google Scholar]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Weiss, P.; Murdoch, D.R. Clinical course and mortality risk of severe COVID-19. Lancet 2020, 395, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Frisancho-Kiss, S.; Davis, S.E.; Nyland, J.F.; Frisancho, J.A.; Cihakova, D.; Barrett, M.A.; Rose, N.R.; Fairweather, D. Cutting edge: Cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J. Immunol. 2007, 178, 6710–6714. [Google Scholar] [CrossRef] [PubMed]
- Frisancho-Kiss, S.; Coronado, M.J.; Frisancho, J.A.; Lau, V.M.; Rose, N.R.; Klein, S.L.; Fairweather, D. Gonadectomy of male BALB/c mice increases Tim-3(+) alternatively activated M2 macrophages, Tim-3(+) T cells, Th2 cells and Treg in the heart during acute coxsackievirus-induced myocarditis. Brain Behav. Immun. 2009, 23, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Coronado, M.J.; Bruno, K.A.; Blauwet, L.A.; Tschöpe, C.; Cunningham, M.W.; Pankuweit, S.; van Linthout, S.; Jeon, E.S.; McNamara, D.M.; Krejčí, J.; et al. Elevated sera sST2 is associated with heart failure in men ≤50 years old with myocarditis. J. Am. Heart Assoc. 2019, 8, e008968. [Google Scholar] [CrossRef]
- Aimo, A.; Januzzi, J.L., Jr.; Bayes-Genis, A.; Vergaro, G.; Sciarrone, P.; Passino, C.; Emdin, M. Clinical and Prognostic Significance of sST2 in Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 2193–2203. [Google Scholar] [CrossRef]
- Zhang, S.-F.; Tuo, J.-L.; Huang, X.-B.; Zhu, X.; Zhang, D.-M.; Zhou, K.; Yuan, L.; Luo, H.-J.; Zheng, B.-J.; Yuen, K.-Y.; et al. Epidemiology characteristics of human coronaviruses in patients with respiratory infection symptoms and phylogenetic analysis of HCoV-OC43 during 2010–2015 in Guangzhou. PLoS ONE 2018, 13, e0191789. [Google Scholar]
- Cui, J.; Li, F.; Shi, Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar]
- Zheng, Y.Y.; Ma, Y.T.; Zhang, J.Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef]
- Nappi, F.; Nappi, P.; Gambardella, I.; Avtaar Singh, S.S. Thromboembolic Disease and Cardiac Thrombotic Complication in COVID-19: A Systematic Review. Metabolites 2022, 12, 889. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Bellomo, F.; Avtaar Singh, S.S. Insights into the Role of Neutrophils and Neutrophil Extracellular Traps in Causing Cardiovascular Complications in Patients with COVID-19: A Systematic Review. J. Clin. Med. 2022, 11, 2460. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Giacinto, O.; Ellouze, O.; Nenna, A.; Avtaar Singh, S.S.; Chello, M.; Bouzguenda, A.; Copie, X. Association between COVID-19 Diagnosis and Coronary Artery Thrombosis: A Narrative Review. Biomedicines 2022, 10, 702. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar]
- Srivastava, A.; Sundararaj, S.N.; Bhatia, J.; Arya, D.S. Understanding long COVID myocarditis: A comprehensive review. Cytokine 2024, 178, 156584. [Google Scholar] [CrossRef]
- Nappi, F.; Avtaar Singh, S.S. SARS-CoV-2-Induced Myocarditis: A State-of-the-Art Review. Viruses 2023, 15, 916. [Google Scholar] [CrossRef]
- Guo, J.; Huang, Z.; Lin, L.; Lv, J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: A viewpoint on the potential influence of angiotensin converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J. Am. Heart Assoc. 2020, 9, e016219. [Google Scholar]
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santos, M.J. The ACE2/angiotensin-(1-7)/MAS axis of the renin-angiotensin system: Focus on angiotensin-(1-7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar]
- Oudit, G.Y.; Kassiri, Z.; Jiang, C.; Liu, P.P.; Poutanen, S.M.; Penninger, J.M.; Butany, J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Investig. 2009, 39, 618–625. [Google Scholar]
- Li, W. Receptor and viral determinants of SARS coronavirus adaptation to human ACE2. EMBO J. 2005, 24, 1634–1643. [Google Scholar]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Glowacka, I.; Bertram, S.; Müller, M.A.; Allen, P.; Soilleux, E.; Pfefferle, S.; Steffen, I.; Tsegaye, T.S.; He, Y.; Gnirss, K.; et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011, 85, 4122–4134. [Google Scholar] [CrossRef] [PubMed]
- Nicin, L.; Abplanalp, W.T.; Mellentin, H.; Kattih, B.; Tombor, L.; John, D.; Schmitto, J.D.; Heineke, J.; Emrich, F.; Arsalan, M.; et al. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur. Heart J. 2020, 41, 1804–1806. [Google Scholar] [CrossRef] [PubMed]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef]
- He, Y.; Chipman, P.R.; Howitt, J.; Bator, C.M.; Whitt, M.A.; Baker, T.S.; Kuhn, R.J.; Anderson, C.W.; Freimuth, P.; Rossmann, M.G. Interaction of coxsackievirus B3 with the full length coxsackievirus-adenovirus receptor. Nat. Struct. Biol. 2001, 8, 874–878. [Google Scholar] [CrossRef]
- Badorff, C.; Lee, G.H.; Lamphear, B.J.; Martone, M.E.; Campbell, K.P.; Rhoads, R.E.; Knowlton, K.U. Enteroviral protease 2A cleaves dystrophin: Evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat. Med. 1999, 5, 320–326. [Google Scholar] [CrossRef]
- Lassner, D.; Siegismund, C.S.; Kühl, U.; Rohde, M.; Stroux, A.; Escher, F.; Schultheiss, H.P. CCR5del32 genotype in human enteroviral cardiomyopathy leads to spontaneous virus clearance improved outcome compared to wild type CCR5. J. Transl. Med. 2018, 16, 249. [Google Scholar] [CrossRef]
- Kuhl, U.; Lassner, D.; von Schlippenbach, J.; Poller, W.; Schultheiss, H.P. Interferon-beta improves survival in enterovirus-associated cardiomyopathy. J. Am. Coll. Cardiol. 2012, 60, 1295–1296. [Google Scholar] [CrossRef]
- Leveque, N.; Garcia, M.; Bouin, A.; Nguyen, J.H.C.; Tran, G.P.; Andreoletti, L.; Semler, B.L. Functional consequences of RNA 5′-terminal deletions on coxsackievirus B3 RNA replication and ribonucleoprotein complex formation. J. Virol. 2017, 91, 10.1128. [Google Scholar] [CrossRef]
- Bouin, A.; Gretteau, P.A.; Wehbe, M.; Renois, F.; N’Guyen, Y.; Lévêque, N.; Vu, M.N.; Tracy, S.; Chapman, N.M.; Bruneval, P.; et al. Enterovirus persistence in cardiac cells of patients with idiopathic dilated cardiomyopathy is linked to 5′ terminal genomic RNA-deleted viral populations with viral-encoded proteinase activities. Circulation 2019, 139, 2326–2338. [Google Scholar] [CrossRef]
- Maisch, B. Cardio-immunology of myocarditis: Focus on immune mechanisms and treatment options. Front. Cardiovasc. Med. 2019, 6, 48. [Google Scholar] [CrossRef]
- Manaresi, E.; Gallinella, G. Advances in the development of antiviral strategies against parvovirus B19. Viruses 2019, 11, 659. [Google Scholar] [CrossRef]
- Duechting, A.; Tschöpe, C.; Kaiser, H.; Lamkemeyer, T.; Tanaka, N.; Aberle, S.; Lang, F.; Torresi, J.; Kandolf, R.; Bock, C.T. Human parvovirus B19 NS1 protein modulates inflammatory signaling by activation of STAT3/PIAS3 in human endothelial cells. J. Virol. 2008, 82, 7942–7952. [Google Scholar] [CrossRef]
- Van Linthout, S.; Elsanhoury, A.; Klein, O.; Sosnowski, M.; Miteva, K.; Lassner, D.; Abou-El-Enein, M.; Pieske, B.; Kühl, U.; Tschöpe, C. Telbivudine in chronic lymphocytic myocarditis and human parvovirus B19 transcriptional activity. ESC Heart Fail. 2018, 5, 818–829. [Google Scholar] [CrossRef]
- Bultmann, B.D.; Sotlar, K.; Klingel, K. Parvovirus B19. N. Engl. J. Med. 2004, 350, 2006–2007, author reply 2006-7. [Google Scholar] [CrossRef]
- Kindermann, I.; Kindermann, M.; Kandolf, R.; Klingel, K.; Bültmann, B.; Müller, T.; Lindinger, A.; Böhm, M. Predictors of outcome in patients with suspected myocarditis. Circulation 2008, 118, 639–648. [Google Scholar] [CrossRef]
- Hjalmarsson, C.; Liljeqvist, J.Å.; Lindh, M.; Karason, K.; Bollano, E.; Oldfors, A.; Andersson, B. Parvovirus B19 in endomyocardial biopsy of patients with idiopathic dilated cardiomyopathy: Foe or bystander? J. Card. Fail. 2019, 25, 60–63. [Google Scholar] [CrossRef]
- Schenk, T.; Enders, M.; Pollak, S.; Hahn, R.; Huzly, D. High prevalence of human parvovirus B19 DNA in myocardial autopsy samples from subjects without myocarditis or dilative cardiomyopathy. J. Clin. Microbiol. 2009, 47, 106–110. [Google Scholar] [CrossRef]
- Lotze, U.; Egerer, R.; Glück, B.; Zell, R.; Sigusch, H.; Erhardt, C.; Heim, A.; Kandolf, R.; Bock, T.; Wutzler, P.; et al. Low level myocardial parvovirus B19 persistence is a frequent finding in patients with heart disease but unrelated to ongoing myocardial injury. J. Med. Virol. 2010, 82, 1449–1457. [Google Scholar] [CrossRef]
- Koepsell, S.A.; Anderson, D.R.; Radio, S.J. Parvovirus B19 is a bystander in adult myocarditis. Cardiovasc. Pathol. 2012, 21, 476–481. [Google Scholar] [CrossRef]
- Bock, C.T.; Klingel, K.; Kandolf, R. Human parvovirus B19-associated myocarditis. N. Engl. J. Med. 2010, 362, 1248–1249. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.T.; Düchting, A.; Utta, F.; Brunner, E.; Sy, B.T.; Klingel, K.; Lang, F.; Gawaz, M.; Felix, S.B.; Kandolf, R. Molecular phenotypes of human parvovirus B19 in patients with myocarditis. World J. Cardiol. 2014, 6, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Dennert, R.; van Paassen, P.; Wolffs, P.; Bruggeman, C.; Velthuis, S.; Felix, S.; van Suylen, R.J.; Crijns, H.J.; Cohen Tervaert, J.W.; Heymans, S. Differences in virus prevalence and load in the hearts of patients with idiopathic dilated cardiomyopathy with and without immune-mediated inflammatory diseases. Clin. Vaccine Immunol. 2012, 19, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, U.; Lassner, D.; Dorner, A.; Rohde, M.; Escher, F.; Seeberg, B.; Hertel, E.; Tschope, C.; Skurk, C.; Gross, U.M.; et al. A distinct subgroup of cardiomyopathy patients characterized by transcriptionally active cardiotropic erythrovirus and altered cardiac gene expression. Basic Res. Cardiol. 2013, 108, 372. [Google Scholar] [CrossRef]
- Adamson-Small, L.A.; Ignatovich, I.V.; Laemmerhirt, M.G.; Hobbs, J.A. Persistent parvovirus B19 infection in non-erythroid tissues: Possible role in the inflammatory and disease process. Virus Res. 2014, 190, 8–16. [Google Scholar] [CrossRef]
- Richter, J.; Quintanilla-Martinez, L.; Bienemann, K.; Zeus, T.; Germing, U.; Sander, O.; Kandolf, R.; Häussinger, D.; Klingel, K. An unusual presentation of a common infection. Infection 2013, 41, 565–569. [Google Scholar] [CrossRef]
- Kaufer, B.B.; Flamand, L. Chromosomally integrated HHV-6: Impact on virus, cell and organismal biology. Curr. Opin. Virol. 2014, 9, 111–118. [Google Scholar] [CrossRef]
- Barbaro, G. HIV-associated cardiomyopathy etiopathogenesis and clinical aspects. Herz 2005, 30, 486–492. [Google Scholar] [CrossRef]
- Sanchez, M.J.; Bergasa, N.V. Hepatitis C associated cardiomyopathy: Potential pathogenic mechanisms and clinical implications. Med. Sci. Monit. 2008, 14, RA55–RA63. [Google Scholar]
- Kumar, K.; Guirgis, M.; Zieroth, S.; Lo, E.; Menkis, A.H.; Arora, R.C.; Freed, D.H. Influenza myocarditis and myositis: Case presentation and review of the literature. Can. J. Cardiol. 2011, 27, 514–522. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M. Cardioimmunology: The immune system in cardiac homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Pollack, A.; Kontorovich, A.R.; Fuster, V.; Dec, G.W. Viral myocarditis—Diagnosis, treatment options, and current controversies. Nat. Rev. Cardiol. 2015, 12, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Tschöpe, C.; Müller, I.; Xia, Y.; Savvatis, K.; Pappritz, K.; Pinkert, S.; Lassner, D.; Heimesaat, M.M.; Spillmann, F.; Miteva, K.; et al. NOD2 (Nucleotide-Binding Oligomerization Domain 2) Is a Major Pathogenic Mediator of Coxsackievirus B3-Induced Myocarditis. Circ. Heart Fail. 2017, 10, e003870. [Google Scholar] [CrossRef]
- Miteva, K.; Pappritz, K.; Sosnowski, M.; El-Shafeey, M.; Müller, I.; Dong, F.; Savvatis, K.; Ringe, J.; Tschöpe, C.; Van Linthout, S. Mesenchymal stromal cells inhibit NLRP3 inflammasome activation in a model of Coxsackievirus B3-induced inflammatory cardiomyopathy. Sci. Rep. 2018, 8, 2820. [Google Scholar] [CrossRef]
- Müller, I.; Vogl, T.; Kühl, U.; Krannich, A.; Banks, A.; Trippel, T.; Noutsias, M.; Maisel, A.S.; van Linthout, S.; Tschöpe, C. Serum alarmin S100A8/S100A9 levels and its potential role as biomarker in myocarditis. ESC Heart Fail. 2020, 7, 1442–1451. [Google Scholar] [CrossRef]
- Heymans, S.; Eriksson, U.; Lehtonen, J.; Cooper, L.T., Jr. The Quest for New Approaches in Myocarditis and Inflammatory Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 68, 2348–2364. [Google Scholar] [CrossRef]
- Huang, C.H.; Vallejo, J.G.; Kollias, G.; Mann, D.L. Role of the innate immune system in acute viral myocarditis. Basic Res. Cardiol. 2009, 104, 228–237. [Google Scholar] [CrossRef]
- Libby, P.; Nahrendorf, M.; Swirski, F.K. Leukocytes Link Local and Systemic Inflammation in Ischemic Cardiovascular Disease: An Expanded “Cardiovascular Continuum”. J. Am. Coll. Cardiol. 2016, 67, 1091–1103. [Google Scholar] [CrossRef]
- Leuschner, F.; Rauch, P.J.; Ueno, T.; Gorbatov, R.; Marinelli, B.; Lee, W.W.; Dutta, P.; Wei, Y.; Robbins, C.; Iwamoto, Y.; et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J. Exp. Med. 2012, 209, 123–137. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Ismahil, M.A.; Hamid, T.; Bansal, S.S.; Patel, B.; Kingery, J.R.; Prabhu, S.D. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: Critical importance of the cardiosplenic axis. Circ. Res. 2014, 114, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, F.; Courties, G.; Dutta, P.; Mortensen, L.J.; Gorbatov, R.; Sena, B.; Novobrantseva, T.I.; Borodovsky, A.; Fitzgerald, K.; Koteliansky, V.; et al. Silencing of CCR2 in myocarditis. Eur. Heart J. 2015, 36, 1478–1488. [Google Scholar] [CrossRef]
- Miteva, K.; Pappritz, K.; El-Shafeey, M.; Dong, F.; Ringe, J.; Tschöpe, C.; Van Linthout, S. Mesenchymal Stromal Cells Modulate Monocytes Trafficking in Coxsackievirus B3-Induced Myocarditis. Stem Cells Transl. Med. 2017, 6, 1249–1261. [Google Scholar] [CrossRef]
- Cooper, L.T., Jr.; Fairweather, D. Nano-scale treatment for a macro-scale disease: Nanoparticle-delivered siRNA silences CCR2 and treats myocarditis. Eur. Heart J. 2015, 36, 1434–1436. [Google Scholar]
- Pappritz, K.; Savvatis, K.; Miteva, K.; Kerim, B.; Dong, F.; Fechner, H.; Müller, I.; Brandt, C.; Lopez, B.; González, A.; et al. Immunomodulation by adoptive regulatory T-cell transfer improves Coxsackievirus B3-induced myocarditis. FASEB J. 2018, 32, fj201701408R. [Google Scholar] [CrossRef]
- Müller, I.; Pappritz, K.; Savvatis, K.; Puhl, K.; Dong, F.; El-Shafeey, M.; Hamdani, N.; Hamman, I.; Noutsias, M.; Infante-Duarte, C.; et al. CX3CR1 knockout aggravates Coxsackievirus B3-induced myocarditis. PLoS ONE 2017, 12, e0182643. [Google Scholar]
- Klingel, K.; Stephan, S.; Sauter, M.; Zell, R.; McManus, B.M.; Bültmann, B.; Kandolf, R. Pathogenesis of murine enterovirus myocarditis: Virus dissemination and immune cell targets. J. Virol. 1996, 70, 8888–8895. [Google Scholar]
- Hofmann, P.; Schmidtke, M.; Stelzner, A.; Gemsa, D. Suppression of proinflammatory cytokines and induction of IL-10 in human monocytes after Coxsackievirus B3 infection. J. Med. Virol. 2001, 64, 487–498. [Google Scholar]
- Kandolf, R.; Sauter, M.; Aepinus, C.; Schnorr, J.J.; Selinka, H.C.; Klingel, K. Mechanisms and consequences of enterovirus persistence in cardiac myocytes and cells of the immune system. Virus Res. 1999, 62, 149–158. [Google Scholar]
- Savvatis, K.; van Linthout, S.; Miteva, K.; Pappritz, K.; Westermann, D.; Schefold, J.C.; Fusch, G.; Weithäuser, A.; Rauch, U.; Becher, P.-M.; et al. Mesenchymal stromal cells but not cardiac fibroblasts exert beneficial systemic immunomodulatory effects in experimental myocarditis. PLoS ONE 2012, 7, e41047. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Frisancho-Kiss, S.; Gatewood, S.; Njoku, D.; Steele, R.; Barrett, M.; Rose, N.R. Mast cells and innate cytokines are associated with susceptibility to autoimmune heart disease following coxsackievirus B3 infection. Autoimmunity 2004, 37, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Klingel, K.; Fabritius, C.; Sauter, M.; Göldner, K.; Stauch, D.; Kandolf, R.; Ettischer, N.; Gahlen, S.; Schönberger, T.; Ebner, S.; et al. The activating receptor NKG2D of natural killer cells promotes resistance against enterovirus-mediated inflammatory cardiomyopathy. J. Pathol. 2014, 234, 164–177. [Google Scholar] [PubMed]
- Yuan, J.; Liu, Z.; Lim, T.; Zhang, H.; He, J.; Walker, E.; Shier, C.; Wang, Y.; Su, Y.; Sall, A.; et al. CXCL10 inhibits viral replication through recruitment of natural killer cells in coxsackievirus B3-induced myocarditis. Circ. Res. 2009, 104, 628–638. [Google Scholar] [CrossRef]
- Clemente-Casares, X.; Hosseinzadeh, S.; Barbu, I.; Dick, S.A.; Macklin, J.A.; Wang, Y.; Momen, A.; Kantores, C.; Aronoff, L.; Farno, M.; et al. A CD103(+) conventional dendritic cell surveillance system prevents development of overt heart failure during subclinical viral myocarditis. Immunity 2017, 47, 974–989.e8. [Google Scholar] [CrossRef]
- Eriksson, U.; Ricci, R.; Hunziker, L.; Kurrer, M.O.; Oudit, G.Y.; Watts, T.H.; Sonderegger, I.; Bachmaier, K.; Kopf, M.; Penninger, J.M. Dendritic cell-induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nat. Med. 2003, 9, 1484–1490. [Google Scholar] [CrossRef]
- Xu, D.; Wang, P.; Yang, J.; Qian, Q.; Li, M.; Wei, L.; Xu, W. Gr-1+ cells other than Ly6G+ neutrophils limit virus replication and promote myocardial inflammation and fibrosis following coxsackievirus B3 infection of mice. Front. Cell Infect. Microbiol. 2018, 8, 157. [Google Scholar] [CrossRef]
- Rivadeneyra, L.; Charó, N.; Kviatcovsky, D.; de la Barrera, S.; Gómez, R.M.; Schattner, M. Role of neutrophils in CVB3 infection and viral myocarditis. J. Mol. Cell Cardiol. 2018, 125, 149–161. [Google Scholar] [CrossRef]
- Weckbach, L.T.; Grabmaier, U.; Uhl, A.; Gess, S.; Boehm, F.; Zehrer, A.; Pick, R.; Salvermoser, M.; Czermak, T.; Pircher, J.; et al. Midkine drives cardiac inflammation by promoting neutrophil trafficking and NETosis in myocarditis. J. Exp. Med. 2019, 216, 350–368. [Google Scholar] [CrossRef]
- Afanasyeva, M.; Georgakopoulos, D.; Belardi, D.F.; Ramsundar, A.C.; Barin, J.G.; Kass, D.A.; Rose, N.R. Quantitative analysis of myocardial inflammation by flow cytometry in murine autoimmune myocarditis: Correlation with cardiac function. Am. J. Pathol. 2004, 164, 807–815. [Google Scholar]
- Müller, I.; Vogl, T.; Pappritz, K.; Miteva, K.; Savvatis, K.; Rohde, D.; Most, P.; Lassner, D.; Pieske, B.; Kühl, U.; et al. Pathogenic role of the damageassociated molecular patterns S100A8 and S100A9 in coxsackievirus B3-induced myocarditis. Circ. Heart Fail. 2017, 10, e004125. [Google Scholar] [CrossRef] [PubMed]
- Tahto, E.; Jadric, R.; Pojskic, L.; Kicic, E. Neutrophilto-lymphocyte ratio and its relation with markers of inflammation and myocardial necrosis in patients with acute coronary syndrome. Med. Arch. 2017, 71, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Swirski, F.K. Monocyte and macrophage heterogeneity in the heart. Circ. Res. 2013, 112, 1624–1633. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Yu, C.; Yang, X.F.; Wang, H. Monocyte and macrophage differentiation: Circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark. Res. 2014, 2, 1. [Google Scholar] [CrossRef]
- Pappritz, K.; Savvatis, K.; Koschel, A.; Miteva, K.; Tschöpe, C.; Van Linthout, S. Cardiac (myo)fibroblasts modulate the migration of monocyte subsets. Sci. Rep. 2018, 8, 5575. [Google Scholar] [CrossRef]
- Hou, X.; Chen, G.; Bracamonte-Baran, W.; Choi, H.S.; Diny, N.L.; Sung, J.; Hughes, D.; Won, T.; Wood, M.K.; Talor, M.V.; et al. The cardiac microenvironment instructs divergent monocyte fates and functions in myocarditis. Cell Rep. 2019, 28, 172–189.e7. [Google Scholar] [CrossRef]
- Liu, P.; Aitken, K.; Kong, Y.Y.; Opavsky, M.A.; Martino, T.; Dawood, F.; Wen, W.H.; Kozieradzki, I.; Bachmaier, K.; Straus, D.; et al. The tyrosine kinase p56lck is essential in coxsackievirus B3-mediated heart disease. Nat. Med. 2000, 6, 429–434. [Google Scholar] [CrossRef]
- Baldeviano, G.C.; Barin, J.G.; Talor, M.V.; Srinivasan, S.; Bedja, D.; Zheng, D.; Gabrielson, K.; Iwakura, Y.; Rose, N.R.; Cihakova, D. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ. Res. 2010, 106, 1646–1655. [Google Scholar] [CrossRef]
- Shi, Y.; Fukuoka, M.; Li, G.; Liu, Y.; Chen, M.; Konviser, M.; Chen, X.; Opavsky, M.A.; Liu, P.P. Regulatory T cells protect mice against coxsackievirus-induced myocarditis through the transforming growth factor β-coxsackie-adenovirus receptor pathway. Circulation 2010, 121, 2624–2634. [Google Scholar] [CrossRef]
- Anzai, A.; Mindur, J.E.; Halle, L.; Sano, S.; Choi, J.L.; He, S.; McAlpine, C.S.; Chan, C.T.; Kahles, F.; Valet, C.; et al. Self-reactive CD4(+) IL-3(+) T cells amplify autoimmune inflammation in myocarditis by inciting monocyte chemotaxis. J. Exp. Med. 2019, 216, 369–383. [Google Scholar] [CrossRef]
- Opavsky, M.A.; Penninger, J.; Aitken, K.; Wen, W.H.; Dawood, F.; Mak, T.; Liu, P. Susceptibility to myocarditis is dependent on the response of alphabeta T lymphocytes to coxsackieviral infection. Circ. Res. 1999, 85, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Klingel, K.; Schnorr, J.J.; Sauter, M.; Szalay, G.; Kandolf, R. β2-Microglobulin-associated regulation of interferon-γ and virus-specific immunoglobulin G confer resistance against the development of chronic coxsackievirus myocarditis. Am. J. Pathol. 2003, 162, 1709–1720. [Google Scholar] [CrossRef] [PubMed]
- Rangachari, M.; Mauermann, N.; Marty, R.R.; Dirnhofer, S.; Kurrer, M.O.; Komnenovic, V.; Penninger, J.M.; Eriksson, U. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J. Exp. Med. 2006, 203, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.M.; Cooper, L.T.; Kem, D.C.; Stavrakis, S.; Kosanke, S.D.; Shevach, E.M.; Fairweather, D.; Stoner, J.A.; Cox, C.J.; Cunningham, M.W. Cardiac myosin-Th17 responses promote heart failure in human myocarditis. JCI Insight 2016, 1, e85851. [Google Scholar] [CrossRef]
- Ahern, P.P.; Schiering, C.; Buonocore, S.; McGeachy, M.J.; Cua, D.J.; Maloy, K.J.; Powrie, F. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity 2010, 33, 279–288. [Google Scholar] [CrossRef]
- Wu, L.; Diny, N.L.; Ong, S.; Barin, J.G.; Hou, X.; Rose, N.R.; Talor, M.V.; Čiháková, D. Pathogenic IL-23 signaling is required to initiate GM-CSF-driven autoimmune myocarditis in mice. Eur. J. Immunol. 2016, 46, 582–592. [Google Scholar] [CrossRef]
- Kaya, Z.; Leib, C.; Katus, H.A. Autoantibodies in heart failure and cardiac dysfunction. Circ. Res. 2012, 110, 145–158. [Google Scholar] [CrossRef]
- Weber, M.S.; Prod’homme, T.; Patarroyo, J.C.; Molnarfi, N.; Karnezis, T.; Lehmann-Horn, K.; Danilenko, D.M.; Eastham-Anderson, J.; Slavin, A.J.; Linington, C.; et al. B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann. Neurol. 2010, 68, 369–383. [Google Scholar] [CrossRef]
- Zouggari, Y.; Ait-Oufella, H.; Bonnin, P.; Simon, T.; Sage, A.P.; Guérin, C.; Vilar, J.; Caligiuri, G.; Tsiantoulas, D.; Laurans, L.; et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat. Med. 2013, 19, 1273–1280. [Google Scholar] [CrossRef]
- Tschope, C.; Van Linthout, S.; Spillmann, F.; Posch, M.G.; Reinke, P.; Volk, H.D.; Elsanhoury, A.; Kühl, U. Targeting CD20+ B-lymphocytes in inflammatory dilated cardiomyopathy with rituximab improves clinical course: A case series. Eur. Heart J. Case Rep. 2019, 3, ytz131. [Google Scholar] [CrossRef]
- Diny, N.; Baldeviano, G.C.L.; Talor, M.V.; Barin, J.G.; Ong, S.; Bedja, D.; Hays, A.G.; Gilotra, N.A.; Coppens, I.; Rose, N.R.; et al. Eosinophil-derived IL-4 drives progression of myocarditis to inflammatory dilated cardiomyopathy. J. Exp. Med. 2017, 214, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.C.; Ackerman, S.J.; Spry, C.J.; Dunnette, S.; Olsen, E.G.; Gleich, G.J. Deposits of eosinophil granule proteins in cardiac tissues of patients with eosinophilic endomyocardial disease. Lancet 1987, 1, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Thambidorai, S.K.; Korlakunta, H.L.; Arouni, A.J.; Hunter, W.J.; Holmberg, M.J. Acute eosinophilic myocarditis mimicking myocardial infarction. Tex. Heart Inst. J. 2009, 36, 355–357. [Google Scholar] [PubMed]
- Song, T.; Jones, D.M.; Homsi, Y. Therapeutic effect of anti-IL-5 on eosinophilic myocarditis with large pericardial effusion. BMJ Case Rep. 2017, 2017, bcr2016218992. [Google Scholar] [CrossRef]
- Mahon, N.G.; Madden, B.P.; Caforio, A.L.; Elliott, P.M.; Haven, A.J.; Keogh, B.E.; Davies, M.J.; McKenna, W.J. Immunohistologic evidence of myocardial disease in apparently healthy relatives of patients with dilated cardiomyopathy. J. Am. Coll. Cardiol. 2002, 39, 455–462. [Google Scholar] [CrossRef]
- Caforio, A.L.; Keeling, P.J.; Zachara, E.; Mestroni, L.; Camerini, F.; Mann, J.M.; Bottazzo, G.F.; McKenna, W.J. Evidence from family studies for autoimmunity in dilated cardiomyopathy. Lancet 1994, 344, 773–777. [Google Scholar] [CrossRef]
- Caforio, A.L.; Mahon, N.G.; Baig, M.K.; Tona, F.; Murphy, R.T.; Elliott, P.M.; McKenna, W.J. Prospective familial assessment in dilated cardiomyopathy: Cardiac autoantibodies predict disease development in asymptomatic relatives. Circulation 2007, 115, 76–83. [Google Scholar] [CrossRef]
- Mestroni, L.; Rocco, C.; Gregori, D.; Sinagra, G.; Di Lenarda, A.; Miocic, S.; Vatta, M.; Pinamonti, B.; Muntoni, F.; Caforio, A.L.; et al. Familial dilated cardiomyopathy: Evidence for genetic and phenotypic heterogeneity. J. Am. Coll. Cardiol. 1999, 34, 181–190. [Google Scholar] [CrossRef]
- Neu, N.; Rose, N.R.; Beisel, K.W.; Herskowitz, A.; Gurri-Glass, G.; Craig, S.W. Cardiac myosin induces myocarditis in genetically predisposed mice. J. Immunol. 1987, 139, 3630–3636. [Google Scholar] [CrossRef]
- Smith, S.C.; Allen, P.M. Myosin-induced acute myocarditis is a T cell-mediated disease. J. Immunol. 1991, 147, 2141–2147. [Google Scholar] [CrossRef]
- Li, Y.; Heuser, J.S.; Cunningham, L.C.; Kosanke, S.D.; Cunningham, M.W. Mimicry and antibody-mediated cell signaling in autoimmune myocarditis. J. Immunol. 2006, 177, 8234–8240. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Chimenti, C.; Calabrese, F.; Pieroni, M.; Thiene, G.; Maseri, A. Immunosuppressive therapy for active lymphocytic myocarditis: Virological and immunologic profile of responders versus nonresponders. Circulation 2003, 107, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Russo, M.A.; Chimenti, C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: The TIMIC study. Eur. Heart J. 2009, 30, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Escher, F.; Kühl, U.; Lassner, D.; Poller, W.; Westermann, D.; Pieske, B.; Tschöpe, C.; Schultheiss, H.P. Long-term outcome of patients with virus-negative chronic myocarditis or inflammatory cardiomyopathy after immunosuppressive therapy. Clin. Res. Cardiol. 2016, 105, 1011–1020. [Google Scholar] [CrossRef]
- Caforio, A.L.; Bonifacio, E.; Stewart, J.T.; Neglia, D.; Parodi, O.; Bottazzo, G.F.; McKenna, W.J. Novel organ-specific circulating cardiac autoantibodies in dilated cardiomyopathy. J. Am. Coll. Cardiol. 1990, 15, 1527–1534. [Google Scholar] [CrossRef]
- Caforio, A.L.; Calabrese, F.; Angelini, A.; Tona, F.; Vinci, A.; Bottaro, S.; Ramondo, A.; Carturan, E.; Iliceto, S.; Thiene, G.; et al. A prospective study of biopsy-proven myocarditis: Prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur. Heart J. 2007, 28, 1326–1333. [Google Scholar] [CrossRef]
- Caforio, A.L.; Grazzini, M.; Mann, J.M.; Keeling, P.J.; Bottazzo, G.F.; McKenna, W.J.; Schiaffino, S. Identification of alpha-and beta-cardiac myosin heavy chain isoforms as major autoantigens in dilated cardiomyopathy. Circulation 1992, 85, 1734–1742. [Google Scholar] [CrossRef]
- Schulze, K.; Becker, B.F.; Schultheiss, H.P. Antibodies to the ADP/ATP carrier, an autoantigen in myocarditis and dilated cardiomyopathy, penetrate into myocardial cells and disturb energy metabolism in vivo. Circ. Res. 1989, 64, 179–192. [Google Scholar] [CrossRef]
- Caforio, A.L.; Angelini, A.; Blank, M.; Shani, A.; Kivity, S.; Goddard, G.; Doria, A.; Schiavo, A.; Testolina, M.; Bottaro, S.; et al. Passive transfer of affinity-purified anti-heart autoantibodies (AHA) from sera of patients with myocarditis induces experimental myocarditis in mice. Int. J. Cardiol. 2015, 179, 166–177. [Google Scholar] [CrossRef]
- Zwacka, R.M.; Zhou, W.; Zhang, Y.; Darby, C.J.; Dudus, L.; Halldorson, J.; Oberley, L.; Engelhardt, J.F. Redox gene therapy for ischemia/reperfusion injury of the liver reduces AP1 and NF-kappaB activation. Nat. Med. 1998, 4, 698–704. [Google Scholar] [CrossRef]
- Jahns, R.; Boivin, V.; Hein, L.; Triebel, S.; Angermann, C.E.; Ertl, G.; Lohse, M.J. Direct evidence for a beta 1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J. Clin. Invest. 2004, 113, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Meder, B.; Rühle, F.; Weis, T.; Homuth, G.; Keller, A.; Franke, J.; Peil, B.; Lorenzo Bermejo, J.; Frese, K.; Huge, A.; et al. A genome-wide association study identifies 6p21 as novel risk locus for dilated cardiomyopathy. Eur. Heart J. 2014, 35, 1069–1077. [Google Scholar] [CrossRef]
- Arbustini, E.; Narula, N.; Dec, G.W.; Reddy, K.S.; Greenberg, B.; Kushwaha, S.; Marwick, T.; Pinney, S.; Bellazzi, R.; Favalli, V.; et al. The MOGE(S) classification for a phenotype-genotype nomenclature of cardiomyopathy: Endorsed by the World Heart Federation. J. Am. Coll. Cardiol. 2013, 62, 2046–2072. [Google Scholar] [CrossRef]
- Hazebroek, M.R.; Moors, S.; Dennert, R.; van den Wijngaard, A.; Krapels, I.; Hoos, M.; Verdonschot, J.; Merken, J.J.; de Vries, B.; Wolffs, P.F.; et al. Prognostic Relevance of Gene-Environment Interactions in Patients with Dilated Cardiomyopathy: Applying the MOGE(S) Classification. J. Am. Coll. Cardiol. 2015, 66, 1313–1323. [Google Scholar] [CrossRef]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, J.; de Groote, P.; Imazio, M.; et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef]
- Gil-Cruz, C.; Perez-Shibayama, C.; De Martin, A.; Ronchi, F.; van der Borght, K.; Niederer, R.; Onder, L.; Lütge, M.; Novkovic, M.; Nindl, V.; et al. Microbiota-derived peptide mimics drive lethal inflammatory cardiomyopathy. Science 2019, 366, 881–886. [Google Scholar] [CrossRef]
- Corrado, D.; Basso, C.; Thiene, G. Sudden cardiac death in young people with apparently normal heart. Cardiovasc. Res. 2001, 50, 399–408. [Google Scholar] [CrossRef]
- Aquaro, G.D.; Perfetti, M.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Pepe, A.; Todiere, G.; Lanzillo, C.; Scatteia, A.; et al. Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY study. J. Am. Coll. Cardiol. 2017, 70, 1977–1987. [Google Scholar] [CrossRef]
- Kasner, M.; Aleksandrov, A.; Escher, F.; Al-Saadi, N.; Makowski, M.; Spillmann, F.; Genger, M.; Schultheiss, H.P.; Kühl, U.; Pieske, B.; et al. Multimodality imaging approach in the diagnosis of chronic myocarditis with preserved left ventricular ejection fraction (MCpEF): The role of 2D speckle-tracking echocardiography. Int. J. Cardiol. 2017, 243, 374–378. [Google Scholar] [CrossRef]
- Ammirati, E.; Veronese, G.; Cipriani, M.; Moroni, F.; Garascia, A.; Brambatti, M.; Adler, E.D.; Frigerio, M. Acute and fulminant myocarditis: A pragmatic clinical approach to diagnosis and 61treatment. Curr. Cardiol. Rep. 2018, 20, 114. [Google Scholar] [CrossRef]
- Tschope, C.; Cooper, L.T.; Torre-Amione, G.; Van Linthout, S. Management of myocarditis-related cardiomyopathy in adults. Circ. Res. 2019, 124, 1568–1583. [Google Scholar] [CrossRef]
- Merlo, M.; Ammirati, E.; Gentile, P.; Artico, J.; Cannatà, A.; Finocchiaro, G.; Barbati, G.; Sormani, P.; Varrenti, M.; Perkan, A.; et al. Persistent left ventricular dysfunction after acute lymphocytic myocarditis: Frequency and predictors. PLoS ONE 2019, 14, e0214616. [Google Scholar] [CrossRef]
- McMurray, J.J.; Adamopoulos, S.; Anker, S.D.; Auricchio, A.; Böhm, M.; Dickstein, K.; Falk, V.; Filippatos, G.; Fonseca, C.; Gomez-Sanchez, M.A.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2012, 33, 1787–1847. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: Expert recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Luetkens, J.A.; Faron, A.; Isaak, A.; Dabir, D.; Kuetting, D.; Feisst, A.; Schmeel, F.C.; Sprinkart, A.M.; Thomas, D. Comparison of original and 2018 Lake Louise criteria for diagnosis of acute myocarditis: Results of a validation cohort. Radiol. Cardiothorac. Imaging 2019, 1, e190010. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Walls, M.; Giri, S.; Verhaert, D.; Rajagopalan, S.; Moore, S.; Simonetti, O.P.; Raman, S.V. Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping. Circ. Cardiovasc. Imaging 2012, 5, 102–110. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar] [CrossRef]
- Bohnen, S.; Radunski, U.K.; Lund, G.K.; Kandolf, R.; Stehning, C.; Schnackenburg, B.; Adam, G.; Blankenberg, S.; Muellerleile, K. Performance of T1 and T2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circ. Cardiovasc. Imaging 2015, 8, e003073. [Google Scholar] [CrossRef]
- Radunski, U.K.; Lund, G.K.; Säring, D.; Bohnen, S.; Stehning, C.; Schnackenburg, B.; Avanesov, M.; Tahir, E.; Adam, G.; Blankenberg, S.; et al. T1 and T2 mapping cardiovascular magnetic resonance imaging techniques reveal unapparent myocardial injury in patients with myocarditis. Clin. Res. Cardiol. 2017, 106, 10–17. [Google Scholar] [CrossRef]
- Lurz, P.; Luecke, C.; Eitel, I.; Föhrenbach, F.; Frank, C.; Grothoff, M.; de Waha, S.; Rommel, K.P.; Lurz, J.A.; Klingel, K.; et al. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: The MyoRacer-trial. J. Am. Coll. Cardiol. 2016, 67, 1800–1811. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Zeiher, A.M.; Nagel, E. T1 and T2 mapping in myocarditis: Seeing beyond the horizon ofLake Louise criteria and histopathology. Expert Rev. Cardiovasc. Ther. 2018, 16, 319–330. [Google Scholar] [CrossRef]
- Francone, M.; Chimenti, C.; Galea, N.; Scopelliti, F.; Verardo, R.; Galea, R.; Carbone, I.; Catalano, C.; Fedele, F.; Frustaci, A. CMR sensitivity varies with clinical presentation and extent of cell necrosis in biopsyproven acute myocarditis. JACC Cardiovasc. Imaging 2014, 7, 254–263. [Google Scholar] [CrossRef]
- Tanacli, R.; Hashemi, D.; Lapinskas, T.; Edelmann, F.; Gebker, R.; Pedrizzetti, G.; Schuster, A.; Nagel, E.; Pieske, B.; Düngen, H.D.; et al. Range variability in CMR feature tracking multilayer strain across different stages of heart failure. Sci. Rep. 2019, 9, 16478. [Google Scholar] [CrossRef]
- Escher, F.; Westermann, D.; Gaub, R.; Pronk, J.; Bock, T.; Al-Saadi, N.; Kühl, U.; Schultheiss, H.P.; Tschöpe, C. Development of diastolic heart failure in a 6-year follow-up study in patients after acute myocarditis. Heart 2011, 97, 709–714. [Google Scholar] [CrossRef]
- Bohnen, S.; Radunski, U.K.; Lund, G.K.; Ojeda, F.; Looft, Y.; Senel, M.; Radziwolek, L.; Avanesov, M.; Tahir, E.; Stehning, C.; et al. Tissue characterization by T1 and T2 mapping cardiovascular magnetic resonance imaging to monitor myocardial inflammation in healing myocarditis. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 744–751. [Google Scholar] [CrossRef]
- Heidecker, B.; Ruedi, G.; Baltensperger, N.; Gresser, E.; Kottwitz, J.; Berg, J.; Manka, R.; Landmesser, U.; Lüscher, T.F.; Patriki, D. Systematic use of cardiac magnetic resonance imaging in MINOCA led to a five-fold increase in the detection rate of myocarditis: A retrospective study. Swiss Med. Wkly. 2019, 149, w20098. [Google Scholar] [CrossRef]
- Patriki, D.; Gresser, E.; Manka, R.; Emmert, M.Y.; Lüscher, T.F.; Heidecker, B. Approximation of the incidence of myocarditis by systematic screening with cardiac magnetic resonance imaging. JACC Heart Fail. 2018, 6, 573–579. [Google Scholar] [CrossRef]
- Jessup, M.; Lindenfeld, J. Light at the end of the myocarditis tunnel. JACC Heart Fail. 2018, 6, 580–582. [Google Scholar] [CrossRef]
- Schneider, J.E.; Stojanovic, I. Economic evaluation of cardiac magnetic resonance with fast-SENC in the diagnosis and management of early heart failure. Health Econ. Rev. 2019, 9, 13. [Google Scholar] [CrossRef]
- Ge, Y.; Pandya, A.; Steel, K.; Bingham, S.; Jerosch-Herold, M.; Chen, Y.Y.; Mikolich, J.R.; Arai, A.E.; Bandettini, W.P.; Patel, A.R.; et al. Cost-effectiveness analysis of stress cardiovascular magnetic resonance imaging for stable chest pain syndromes. JACC Cardiovasc. Imaging 2020, 13, 1505–1517. [Google Scholar] [CrossRef]
- Petrov, G.; Kelle, S.; Fleck, E.; Wellnhofer, E. Incremental cost-effectiveness of dobutamine stress cardiac magnetic resonance imaging in patients at intermediate risk for coronary artery disease. Clin. Res. Cardiol. 2015, 104, 401–409. [Google Scholar] [CrossRef]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.N.; Narula, J.; Starling, R.C.; Towbin, J.; et al. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. J. Am. Coll. Cardiol. 2007, 50, 1914–1931. [Google Scholar] [CrossRef]
- Backhaus, S.J.; Lange, T.; Beuthner, B.E.; Topci, R.; Wang, X.; Kowallick, J.T.; Lotz, J.; Seidler, T.; Toischer, K.; Zeisberg, E.M.; et al. Real-time cardiovascular magnetic resonance T1 and extracellular volume fraction mapping for tissue characterisation in aortic stenosis. Cardiovasc. Magn. Reson. 2020, 22, 46. [Google Scholar] [CrossRef]
- Zhang, S.; Joseph, A.A.; Voit, D.; Schaetz, S.; Merboldt, K.D.; Unterberg-Buchwald, C.; Hennemuth, A.; Lotz, J.; Frahm, J. Real-time magnetic resonance imaging of cardiac function and flow—Recent progress. Quant. Imaging Med. Surg. 2014, 4, 313–329. [Google Scholar] [CrossRef]
- Lurz, P.; Eitel, I.; Adam, J.; Steiner, J.; Grothoff, M.; Desch, S.; Fuernau, G.; de Waha, S.; Sareban, M.; Luecke, C.; et al. Diagnostic performance of CMR imaging compared with EMB in patients with suspected myocarditis. JACC Cardiovasc. Imaging 2012, 5, 513–524. [Google Scholar] [CrossRef]
- Gräni, C.; Eichhorn, C.; Bière, L.; Murthy, V.L.; Agarwal, V.; Kaneko, K.; Cuddy, S.; Aghayev, A.; Steigner, M.; Blankstein, R.; et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J. Am. Coll. Cardiol. 2017, 70, 1964–1976. [Google Scholar] [CrossRef]
- Schelbert, E.B.; Piehler, K.M.; Zareba, K.M.; Moon, J.C.; Ugander, M.; Messroghli, D.R.; Valeti, U.S.; Chang, C.C.; Shroff, S.G.; Diez, J.; et al. Myocardial fibrosis quantified by extracellular volume is associated with subsequent hospitalization for heart failure, death, or both across the spectrum of ejection fraction and heart failure stage. J. Am. Heart Assoc. 2015, 4, e002613. [Google Scholar] [CrossRef]
- Mewton, N.; Liu, C.Y.; Croisille, P.; Bluemke, D.; Lima, J.A. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2011, 57, 891–903. [Google Scholar] [CrossRef]
- Berg, J.; Kottwitz, J.; Baltensperger, N.; Kissel, C.K.; Lovrinovic, M.; Mehra, T.; Scherff, F.; Schmied, C.; Templin, C.; Lüscher, T.F.; et al. Cardiac magnetic resonance imaging in myocarditis reveals persistent disease activity despite normalization of cardiac enzymes and inflammatory parameters at 3-month follow-up. Circ. Heart Fail. 2017, 10, e004262. [Google Scholar] [CrossRef]
- Murtagh, G.; Laffin, L.J.; Beshai, J.F.; Maffessanti, F.; Bonham, C.A.; Patel, A.V.; Yu, Z.; Addetia, K.; Mor-Avi, V.; Moss, J.D.; et al. Prognosis of myocardial damage in sarcoidosis patients with preserved left ventricular ejection fraction: Risk stratification using cardiovascular magnetic resonance. Circ. Cardiovasc. Imaging 2016, 9, e003738. [Google Scholar] [CrossRef]
- Nensa, F.; Bamberg, F.; Rischpler, C.; Menezes, L.; Poeppel, T.D.; la Fougère, C.; Beitzke, D.; Rasul, S.; Loewe, C.; Nikolaou, K.; et al. European Society of Cardiovascular Radiology (ESCR); European Association of Nuclear Medicine (EANM) Cardiovascular Committee. Hybrid cardiac imaging using PET/MRI: A joint position statement by the European Society of Cardiovascular Radiology (ESCR) and the European Association of Nuclear Medicine (EANM). Eur. Radiol. 2018, 28, 4086–4101. [Google Scholar] [CrossRef]
- Lapinskas, T.; Pedrizzetti, G.; Stoiber, L.; Düngen, H.D.; Edelmann, F.; Pieske, B.; Kelle, S. The Intraventricular hemodynamic forces estimated using routine CMR cine images: A new marker of the failing heart. JACC Cardiovasc. Imaging 2019, 12, 377–379. [Google Scholar] [CrossRef]
- Frey, N.; Meder, B.; Katus, H.A. Left ventricular biopsy in the diagnosis of myocardial diseases. Circulation 2018, 137, 993–995. [Google Scholar] [CrossRef]
- Nakayama, T.; Murai, S.; Ohte, N. Dilated cardiomyopathy with eosinophilic granulomatosis with polyangiitis in which active myocardial inflammation was only detected by endomyocardial biopsy. Intern. Med. 2018, 57, 2675–2679. [Google Scholar] [CrossRef]
- Raafs, A.G.; Verdonschot, J.A.J.; Henkens, M.T.H.M.; Adriaans, B.P.; Wang, P.; Derks, K.; Abdul Hamid, M.A.; Knackstedt, C.; van Empel, V.P.M.; Díez, J.; et al. The combination of carboxy-terminal propeptide of procollagen type I blood levels and late gadolinium enhancement at cardiac magnetic resonance provides additional prognostic information in idiopathic dilated cardiomyopathy—A multilevel assessment of myocardial fibrosis in dilated cardiomyopathy. Eur. J. Heart Fail. 2021, 23, 933–944. [Google Scholar] [CrossRef]
- Leone, O.; Veinot, J.P.; Angelini, A.; Baandrup, U.T.; Basso, C.; Berry, G.; Bruneval, P.; Burke, M.; Butany, J.; Calabrese, F.; et al. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc. Pathol. 2012, 21, 245–274. [Google Scholar] [CrossRef]
- Katzmann, J.L.; Schlattmann, P.; Rigopoulos, A.G.; Noutsias, E.; Bigalke, B.; Pauschinger, M.; Tschope, C.; Sedding, D.; Schulze, P.C.; Noutsias, M. Meta-analysis on the immunohistological detection of inflammatory cardiomyopathy in endomyocardial biopsies. Heart Fail. Rev. 2020, 25, 277–294. [Google Scholar] [CrossRef]
- Baughman, K.L. Diagnosis of myocarditis: Death of Dallas criteria. Circulation 2006, 113, 593–595. [Google Scholar] [CrossRef]
- Andreoletti, L.; Leveque, N.; Boulagnon, C.; Brasselet, C.; Fornes, P. Viral causes of human myocarditis. Arch. Cardiovasc. Dis. 2009, 102, 559–568. [Google Scholar] [CrossRef]
- Badorff, C.; Knowlton, K.U. Dystrophin disruption in enterovirus-induced myocarditis and dilated cardiomyopathy: From bench to bedside. Med. Microbiol. Immunol. 2004, 193, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Spieker, M.; Haberkorn, S.; Gastl, M.; Behm, P.; Katsianos, S.; Horn, P.; Jacoby, C.; Schnackenburg, B.; Reinecke, P.; Kelm, M.; et al. Abnormal T2 mapping cardiovascular magnetic resonance correlates with adverse clinical outcome in patients with suspected acute myocarditis. J. Cardiovasc. Magn. Reson. 2017, 19, 38. [Google Scholar] [PubMed]
- Unterberg-Buchwald, C.; Ritter, C.O.; Reupke, V.; Wilke, R.N.; Stadelmann, C.; Steinmetz, M.; Schuster, A.; Hasenfuß, G.; Lotz, J.; Uecker, M. Targeted endomyocardial biopsy guided by real-time cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2017, 19, 45. [Google Scholar] [PubMed]
- Casella, M.; Pizzamiglio, F.; Dello Russo, A.; Carbucicchio, C.; Al-Mohani, G.; Russo, E.; Notarstefano, P.; Pieroni, M.; D’Amati, G.; Sommariva, E.; et al. Feasibility of combined unipolar and bipolar voltage maps to improve sensitivity of endomyocardial biopsy. Circ. Arrhythm. Electrophysiol. 2015, 8, 625–632. [Google Scholar] [CrossRef]
- Liang, J.J.; Hebl, V.B.; DeSimone, C.V.; Madhavan, M.; Nanda, S.; Kapa, S.; Maleszewski, J.J.; Edwards, W.D.; Reeder, G.; Cooper, L.T.; et al. Electrogram guidance: A method to increase the precision and diagnostic yield of endomyocardial biopsy for suspected cardiac sarcoidosis and myocarditis. JACC Heart Fail. 2014, 2, 466–473. [Google Scholar]
- Konecny, T.; Noseworthy, P.A.; Kapa, S.; Cooper, L.T.; Mulpuru, S.K.; Sandhu, G.S.; Asirvatham, S. Endomyocardial biopsy-integrating electrode at the bioptome tip. Ther. Adv. Cardiovasc. Dis. 2015, 9, 66–69. [Google Scholar]
- Vaidya, V.R.; Abudan, A.A.; Vasudevan, K.; Shantha, G.; Cooper, L.T.; Kapa, S.; Noseworthy, P.A.; Cha, Y.-M.; Asirvatham, S.J.; Deshmukh, A.J. The efficacy and safety of electroanatomic mapping-guided endomyocardial biopsy: A systematic review. J. Interv. Card. Electrophysiol. 2018, 53, 63–71. [Google Scholar]
- Omote, K.; Naya, M.; Koyanagawa, K.; Aikawa, T.; Manabe, O.; Nagai, T.; Kamiya, K.; Kato, Y.; Komoriyama, H.; Kuzume, M.; et al. 18F-FDG uptake of the right ventricle is an important predictor of histopathologic diagnosis by endomyocardial biopsy in patients with cardiac sarcoidosis. J. Nucl. Cardiol. 2019, 27, 2135–2143. [Google Scholar] [CrossRef]
- Van Linthout, S.; Tschope, C. Viral myocarditis: A prime example for endomyocardial biopsy-guided diagnosis and therapy. Curr. Opin. Cardiol. 2018, 33, 325–333. [Google Scholar]
- Lassner, D.; Kühl, U.; Siegismund, C.S.; Rohde, M.; Elezkurtaj, S.; Escher, F.; Tschöpe, C.; Gross, U.M.; Poller, W.; Schultheiss, H.-P. Improved diagnosis of idiopathic giant cell myocarditis and cardiac sarcoidosis by myocardial gene expression profiling. Eur. Heart J. 2014, 35, 2186–2195. [Google Scholar]
- Hammer, E.; Darm, K.; Volker, U. Characterization of the human myocardial proteome in dilated cardiomyopathy by label-free quantitative shotgun proteomics of heart biopsies. Methods Mol. Biol. 2013, 1005, 67–76. [Google Scholar] [PubMed]
- Van Linthout, S.; Tschope, C. Lost in markers? Time for phenomics and phenomapping in dilated cardiomyopathy. Eur. J. Heart Fail. 2017, 19, 499–501. [Google Scholar]
- Soler-Botija, C.; Galvez-Monton, C.; Bayes-Genis, A. Epigenetic biomarkers in cardiovascular diseases. Front. Genet. 2019, 10, 950. [Google Scholar]
- Halliday, B.P.; Cleland JG, F.; Goldberger, J.J.; Prasad, S.K. Personalizing risk stratification for sudden death in dilated cardiomyopathy: The past, present, and future. Circulation 2017, 136, 215–231. [Google Scholar]
- Takeuchi, S.; Kawada, J.I.; Okuno, Y.; Horiba, K.; Suzuki, T.; Torii, Y.; Yasuda, K.; Numaguchi, A.; Kato, T.; Takahashi, Y.; et al. Identification of potential pathogenic viruses in patients with acute myocarditis using next generation sequencing. J. Med. Virol. 2018, 90, 1814–1821. [Google Scholar]
- Reichl, K.; Kreykes, S.E.; Martin, C.M.; Shenoy, C. Desmoplakin variant-associated arrhythmogenic cardiomyopathy presenting as acute myocarditis. Circ. Genom. Precis. Med. 2018, 11, e002373. [Google Scholar]
- Calabrese, F.; Basso, C.; Carturan, E.; Valente, M.; Thiene, G. Arrhythmogenic right ventricular cardiomyopathy/dysplasia: Is there a role for viruses? Cardiovasc. Pathol. 2006, 15, 11–17. [Google Scholar]
- Lopez-Ayala, J.M.; Pastor-Quirante, F.; Gonzalez-Carrillo, J.; Lopez-Cuenca, D.; Sanchez-Munoz, J.J.; Oliva-Sandoval, M.J.; Gimeno, J.R. Genetics of myocarditis in arrhythmogenic right ventricular dysplasia. Heart Rhythm 2015, 12, 766–773. [Google Scholar]
- Protonotarios, A.; Wicks, E.; Ashworth, M.; Stephenson, E.; Guttmann, O.; Savvatis, K.; Sekhri, N.; Mohiddin, S.A.; Syrris, P.; Menezes, L.; et al. Prevalence of 18Ffluorodeoxyglucose positron emission tomography abnormalities in patients with arrhythmogenic right ventricular cardiomyopathy. Int. J. Cardiol. 2019, 284, 99–104. [Google Scholar]
- Hata, Y.; Hirono, K.; Yamaguchi, Y.; Ichida, F.; Oku, Y.; Nishida, N. Minimal inflammatory foci of unknown etiology may be a tentative sign of early stage inherited cardiomyopathy. Mod. Pathol. 2019, 32, 1281–1290. [Google Scholar]
- Belkaya, S.; Kontorovich, A.R.; Byun, M.; Mulero-Navarro, S.; Bajolle, F.; Cobat, A.; Josowitz, R.; Itan, Y.; Quint, R.; Lorenzo, L.; et al. Autosomal recessive cardiomyopathy presenting as acute myocarditis. J. Am. Coll. Cardiol. 2017, 69, 1653–1665. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Iervolino, A.; Avtaar Singh, S.S.; Chello, M. MicroRNAs in Valvular Heart Diseases: Biological Regulators, Prognostic Markers and Therapeutical Targets. Int. J. Mol. Sci. 2021, 22, 12132. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F. Non-Coding RNA-Targeted Therapy: A State-of-the-Art Review. Int. J. Mol. Sci. 2024, 25, 3630. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Avtaar Singh, S.S.; Jitendra, V.; Alzamil, A.; Schoell, T. The Roles of microRNAs in the Cardiovascular System. Int. J. Mol. Sci. 2023, 24, 14277. [Google Scholar] [CrossRef]
- Nenna, A.; Loreni, F.; Giacinto, O.; Chello, C.; Nappi, P.; Chello, M.; Nappi, F. miRNAs in Cardiac Myxoma: New Pathologic Findings for Potential Therapeutic Opportunities. Int. J. Mol. Sci. 2022, 23, 3309. [Google Scholar] [CrossRef]
- Nappi, F.; Alzamil, A.; Avtaar Singh, S.S.; Spadaccio, C.; Bonnet, N. Current Knowledge on the Interaction of Human Cytomegalovirus Infection, Encoded miRNAs, and Acute Aortic Syndrome. Viruses 2023, 15, 2027. [Google Scholar] [CrossRef]
- Loreni, F.; Nenna, A.; Nappi, F.; Ferrisi, C.; Chello, C.; Lusini, M.; Vincenzi, B.; Tonini, G.; Chello, M. miRNAs in the diagnosis and therapy of cardiac and mediastinal tumors: A new dawn for cardio-oncology? Future Cardiol. 2024, 20, 795–806. [Google Scholar] [CrossRef]
- Small, E.M.; Olson, E.N. Pervasive roles of microRNAs in cardiovascular biology. Nature 2011, 469, 336–342. [Google Scholar] [CrossRef]
- Corsten, M.F.; Papageorgiou, A.; Verhesen, W.; Carai, P.; Lindow, M.; Obad, S.; Summer, G.; Coort, S.L.; Hazebroek, M.; van Leeuwen, R.; et al. MicroRNA profiling identifies microRNA-155 as an adverse mediator of cardiac injury and dysfunction during acute viral myocarditis. Circ. Res. 2012, 111, 415–425. [Google Scholar] [CrossRef]
- Kuehl, U.; Lassner, D.; Gast, M.; Stroux, A.; Rohde, M.; Siegismund, C.; Wang, X.; Escher, F.; Gross, M.; Skurk, C.; et al. Differential cardiac microRNA expression predicts the clinical course in human enterovirus cardiomyopathy. Circ. Heart Fail. 2015, 8, 605–618. [Google Scholar] [CrossRef]
- Navarro, I.C.; Ferreira, F.M.; Nakaya, H.I.; Baron, M.A.; Vilar-Pereira, G.; Pereira, I.R.; Silva, A.M.; Real, J.M.; De Brito, T.; Chevillard, C.; et al. MicroRNA transcriptome profiling in heart of Trypanosoma cruzi-infected mice: Parasitological and cardiological outcomes. PLoS Negl. Trop. Dis. 2015, 9, e0003828. [Google Scholar] [CrossRef] [PubMed]
- Corsten, M.F.; Dennert, R.; Jochems, S.; Kuznetsova, T.; Devaux, Y.; Hofstra, L.; Wagner, D.R.; Staessen, J.A.; Heymans, S.; Schroen, B. Circulating microRNA-208b and microRNA-499 reflect myocardial damage in cardiovascular disease. Circ. Cardiovasc. Genet. 2010, 3, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, L.; Tirosh-Wagner, T.; Vardi, A.; Abbas, H.; Pillar, N.; Shomron, N.; Nevo-Caspi, Y.; Paret, G. Circulating microRNAs: A potential biomarker for cardiac damage, inflammatory response, and left ventricular function recovery in pediatric viral myocarditis. J. Cardiovasc. Transl. Res. 2018, 11, 319–328. [Google Scholar] [CrossRef]
- Devaux, Y.; Vausort, M.; Goretti, E.; Nazarov, P.V.; Azuaje, F.; Gilson, G.; Corsten, M.F.; Schroen, B.; Lair, M.L.; Heymans, S.; et al. Use of circulating microRNAs to diagnose acute myocardial infarction. Clin. Chem. 2012, 58, 559–567. [Google Scholar] [CrossRef]
- Heidecker, B.; Kittleson, M.M.; Kasper, E.K.; Wittstein, I.S.; Champion, H.C.; Russell, S.D.; Hruban, R.H.; Rodriguez, E.R.; Baughman, K.L.; Hare, J.M. Transcriptomic biomarkers for the accurate diagnosis of myocarditis. Circulation 2011, 123, 1174–1184. [Google Scholar] [CrossRef]
- Nie, X.; Li, H.; Wang, J.; Cai, Y.; Fan, J.; Dai, B.; Chen, C.; Wang, D.W. Expression Profiles and Potential Functions of Long Non-Coding RNAs in the Heart of Mice with Coxsackie B3 Virus-Induced Myocarditis. Front. Cell. Infect. Microbiol. 2021, 11, 704919. [Google Scholar] [CrossRef]
- Chen, P.; Baldeviano, G.C.; Ligons, D.L.; Talor, M.V.; Barin, J.G.; Rose, N.R.; Cihakova, D. Susceptibility to autoimmune myocarditis is associated with intrinsic differences in CD4(+) T cells. Clin. Exp. Immunol. 2012, 169, 79–88. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Wang, S.; Zhu, H.; Ye, P.; Xie, A.; Shen, B.; Liu, C.; Guo, C.; Fu, Q.; et al. The Treg/Th17 imbalance in patients with idiopathic dilated cardiomyopathy. Scand. J. Immunol. 2010, 71, 298–303. [Google Scholar] [CrossRef]
- Benincasa, G.; Mansueto, G.; Napoli, C. Fluid-based assays and precision medicine of cardiovascular diseases: The ‘hope’ for Pandora’s box? J. Clin. Pathol. 2019, 72, 785–799. [Google Scholar] [CrossRef]
- Kennel, P.J.; Saha, A.; Maldonado, D.A.; Givens, R.; Brunjes, D.L.; Castillero, E.; Zhang, X.; Ji, R.; Yahi, A.; George, I.; et al. Serum exosomal protein profiling for the non-invasive detection of cardiac allograft rejection. J. Heart Lung Transpl. 2018, 37, 409–417. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef] [PubMed]
- Peretto, G.; Sala, S.; Rizzo, S.; Palmisano, A.; Esposito, A.; De Cobelli, F.; Campochiaro, C.; De Luca, G.; Foppoli, L.; Dagna, L.; et al. Ventricular arrhythmias in myocarditis: Characterization and relationships with myocardial inflammation. J. Am. Coll. Cardiol. 2020, 75, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Baksi, A.J.; Kanaganayagam, G.S.; Prasad, S.K. Arrhythmias in viral myocarditis and pericarditis. Card. Electrophysiol. Clin. 2015, 7, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.T., Jr.; Berry, G.J.; Shabetai, R. Idiopathic giant-cell myocarditis—Natural history and treatment. N. Engl. J. Med. 1997, 336, 1860–1866. [Google Scholar]
- Birnie, D.H.; Sauer, W.H.; Bogun, F.; Cooper, J.M.; Culver, D.A.; Duvernoy, C.S.; Judson, M.A.; Kron, J.; Mehta, D.; Cosedis Nielsen, J.; et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014, 11, 1305–1323. [Google Scholar] [CrossRef]
- Imazio, M.; Trinchero, R. Myopericarditis: Etiology, management, and prognosis. Myopericarditis: Etiology, management, and prognosis. Int. J. Cardiol. 2008, 127, 17–26. [Google Scholar] [CrossRef]
- Adegbala, O.; Olagoke, O.; Akintoye, E.; Adejumo, A.C.; Oluwole, A.; Jara, C.; Williams, K.; Briasoulis, A.; Afonso, L. Predictors, burden, and the impact of arrhythmia on patients admitted for acute myocarditis. Am. J. Cardiol. 2019, 123, 139–144. [Google Scholar] [CrossRef]
- Maron, B.J.; Udelson, J.E.; Bonow, R.O.; Nishimura, R.A.; Ackerman, M.J.; Estes, N.A., 3rd; Cooper, L.T., Jr.; Link, M.S.; Maron, M.S. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 3: Hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: A scientific statement from the American Heart Association and American College of Cardiology. Circulation 2015, 132, e273–e280. [Google Scholar] [CrossRef]
- Zorzi, A.; Perazzolo Marra, M.; Rigato, I.; De Lazzari, M.; Susana, A.; Niero, A.; Pilichou, K.; Migliore, F.; Rizzo, S.; Giorgi, B.; et al. Nonischemic left ventricular scar as a substrate of life-threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ. Arrhythm. Electrophysiol. 2016, 9, e004229. [Google Scholar] [CrossRef]
- Steinke, K. Coxsackievirus B3 modulates cardiac ion channels Coxsackievirus B3 modulates cardiac ion channels. FASEB J. 2013, 27, 4108–4121. [Google Scholar] [CrossRef]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace 2015, 17, 1601–1687. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, R.; Mather, P.J.; Alexis, J.D.; Starling, R.C.; Boehmer, J.P.; Thohan, V.; Pauly, D.F.; Markham, D.W.; Zucker, M.; Kip, K.E.; et al. Implantable cardiac defibrillators and sudden death in recent onset nonischemiccardiomyopathy: Results from IMAC2. J. Card. Fail. 2012, 18, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K. The role of the wearable cardioverter defibrillator in clinical practice. Cardiol. Clin. 2014, 32, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Halle, M.; Binzenhöfer, L.; Mahrholdt, H.; Johannes Schindler, M.; Esefeld, K.; Tschöpe, C. Myocarditis in athletes: A clinical perspective. Eur. J. Prev. Cardiol. 2021, 28, 1050–1057. [Google Scholar] [CrossRef]
- Wojnicz, R.; Nowalany-Kozielska, E.; Wojciechowska, C.; Glanowska, G.; Wilczewski, P.; Niklewski, T.; Zembala, M.; Polonski, L.; Rozek, M.M.; Wodniecki, J. Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: Two-year follow-up results. Circulation 2001, 104, 39–45. [Google Scholar] [CrossRef]
- Merken, J.; Hazebroek, M.; Van Paassen, P.; Verdonschot, J.; Van Empel, V.; Knackstedt, C.; Abdul Hamid, M.; Seiler, M.; Kolb, J.; Hoermann, P.; et al. Immunosuppressive therapy improves both short-and long-term prognosis in patients with virus-negative nonfulminant inflammatory cardiomyopathy. Circ. Heart Fail. 2018, 11, e004228. [Google Scholar] [CrossRef]
- Kleinert, S.; Weintraub, R.G.; Wilkinson, J.L.; Chow, C.W. Myocarditis in children with dilated cardiomyopathy: Incidence and outcome after dual therapy immunosuppression. J. Heart Lung Transpl. 1997, 16, 1248–1254. [Google Scholar]
- De Luca, G.; Campochiaro, C.; Sartorelli, S.; Peretto, G.; Sala, S.; Palmisano, A.; Esposito, A.; Candela, C.; Basso, C.; Rizzo, S.; et al. Efficacy and safety of mycophenolate mofetil in patients with virus-negative lymphocytic myocarditis: A prospective cohort study. J. Autoimmun. 2020, 106, 102330. [Google Scholar] [CrossRef]
- Felix, S.B.; Staudt, A.; Dörffel, W.V.; Stangl, V.; Merkel, K.; Pohl, M.; Döcke, W.D.; Morgera, S.; Neumayer, H.H.; Wernecke, K.D.; et al. Hemodynamic effects of immunoadsorption and subsequent immunoglobulin substitution in dilated cardiomyopathy: Three-month results from a randomized study. J. Am. Coll. Cardiol. 2000, 35, 1590–1598. [Google Scholar] [CrossRef]
- Trimpert, C.; Herda, L.R.; Eckerle, L.G.; Pohle, S.; Müller, C.; Landsberger, M.; Felix, S.B.; Staudt, A. Immunoadsorption in dilated cardiomyopathy: Long-term reduction of cardiodepressant antibodies. Eur. J. Clin. Invest. 2010, 40, 685–691. [Google Scholar] [CrossRef]
- Dandel, M.; Wallukat, G.; Englert, A.; Lehmkuhl, H.B.; Knosalla, C.; Hetzer, R. Long-term benefits of immunoadsorption in β(1)-adrenoceptor autoantibody-positive transplant candidates with dilated cardiomyopathy. Eur. J. Heart Fail. 2012, 14, 1374–1388. [Google Scholar] [CrossRef] [PubMed]
- Kronbichler, A.; Brezina, B.; Quintana, L.F.; Jayne, D.R. Efficacy of plasma exchange and immunoadsorption in systemic lupus erythematosus and antiphospholipid syndrome: A systematic review. Autoimmun. Rev. 2016, 15, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, K. Immunoadsorption for collagen and rheumatic diseases. Transfus. Apher. Sci. 2017, 56, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Staudt, A.; Schäper, F.; Stangl, V.; Plagemann, A.; Böhm, M.; Merkel, K.; Wallukat, G.; Wernecke, K.D.; Stangl, K.; Baumann, G.; et al. Immunohistological changes in dilated cardiomyopathy induced by immunoadsorption therapy and subsequent immunoglobulin substitution. Circulation 2001, 103, 2681–2686. [Google Scholar] [CrossRef]
- US National Library of Medicine. ClinicalTrials.gov. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT00558584 (accessed on 20 March 2025).
- Dungen, H.D.; Dordevic, A.; Felix, S.B.; Pieske, B.; Voors, A.A.; McMurray, J.J.V.; Butler, J. β1-Adrenoreceptor autoantibodies in heart failure: Physiology and therapeutic implications. Circ. Heart Fail. 2020, 13, e006155. [Google Scholar] [CrossRef]
- Schultheiss, H.P.; Piper, C.; Sowade, O.; Waagstein, F.; Kapp, J.F.; Wegscheider, K.; Groetzbach, G.; Pauschinger, M.; Escher, F.; Arbustini, E.; et al. Betaferon in chronic viral cardiomyopathy (BICC) trial: Effects of interferon-β treatment in patients with chronic viral cardiomyopathy. Clin. Res. Cardiol. 2016, 105, 763–773. [Google Scholar] [CrossRef]
- Kuhl, U.; Lassner, D.; Wallaschek, N.; Gross, U.M.; Krueger, G.R.; Seeberg, B.; Kaufer, B.B.; Escher, F.; Poller, W.; Schultheiss, H.P. Chromosomally integrated human herpesvirus 6 in heart failure: Prevalence and treatment. Eur. J. Heart Fail. 2015, 17, 9–19. [Google Scholar] [CrossRef]
- Tschope, C.; Elsanhoury, A.; Schlieker, S.; Van Linthout, S.; Kuhl, U. Immunosuppression in inflammatory cardiomyopathy and parvovirus B19 persistence. Eur. J. Heart Fail. 2019, 21, 1468–1469. [Google Scholar] [CrossRef]
- Ameling, S.; Bhardwaj, G.; Hammer, E.; Beug, D.; Steil, L.; Reinke, Y.; Weitmann, K.; Grube, M.; Trimpert, C.; Klingel, K.; et al. Changes of myocardial gene expression and protein composition in patients with dilated cardiomyopathy after immunoadsorption with subsequent immunoglobulin substitution. Basic Res. Cardiol. 2016, 111, 53. [Google Scholar] [CrossRef]
- McNamara, D.M.; Holubkov, R.; Starling, R.C.; Dec, G.W.; Loh, E.; Torre-Amione, G.; Gass, A.; Janosko, K.; Tokarczyk, T.; Kessler, P.; et al. Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy. Circulation 2001, 103, 2254–2259. [Google Scholar] [CrossRef]
- Maisch, B.; Hufnagel, G.; Kölsch, S.; Funck, R.; Richter, A.; Rupp, H.; Herzum, M.; Pankuweit, S. Treatment of inflammatory dilated cardiomyopathy and (peri)myocarditis with immunosuppression and i.v. immunoglobulins. Herz 2004, 29, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Sudano, I.; Spieker, L.E.; Noll, G.; Corti, R.; Weber, R.; Lüscher, T.F. Cardiovascular disease in HIV infection. Am. Heart J. 2006, 151, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.H.; Jeong, H.S.; Kim, S.J.; Yoon, Y.K.; Sohn, J.W.; Kim, M.J. A case of influenza associated fulminant myocarditis successfully treated with intravenous peramivir. Infect. Chemother. 2015, 47, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Sato, M.; Momoi, N.; Aoyagi, Y.; Endo, K.; Chishiki, M.; Kawasaki, Y.; Hosoya, M. Influenza A H1N1 pdm09-associated myocarditis during zanamivir therapy. Pediatr. Int. 2015, 57, 1172–1174. [Google Scholar] [CrossRef]
- Sanders, J.M.; Monogue, M.L.; Jodlowski, T.Z.; Cutrell, J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review. JAMA 2020, 323, 1824–1836. [Google Scholar] [CrossRef]
- Mann, D.L. Targeted anticytokine therapy in patients with chronic heart failure: Results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 2004, 109, 1594–1602. [Google Scholar] [CrossRef]
- Tschöpe, C.; Van Linthout, S.; Jäger, S.; Arndt, R.; Trippel, T.; Müller, I.; Elsanhoury, A.; Rutschow, S.; Anker, S.D.; Schultheiss, H.P.; et al. Modulation of the acute defence reaction by eplerenone prevents cardiac disease progression in viral myocarditis. ESC Heart Fail. 2020, 7, 2838–2852. [Google Scholar] [CrossRef]
- Lee, W.S.; Erdelyi, K.; Matyas, C.; Mukhopadhyay, P.; Varga, Z.V.; Liaudet, L.; Haskú, G.; Čiháková, D.; Mechoulam, R.; Pacher, P. Cannabidiol limits T cell-mediated chronic autoimmune myocarditis: Implications to autoimmune disorders and organ transplantation. Mol. Med. 2016, 22, 136–146. [Google Scholar] [CrossRef]
- Branchereau, M.; Burcelin, R.; Heymes, C. The gut microbiome and heart failure: A better gut for a better heart. Rev. Endocr. Metab. Disord. 2019, 20, 407–414. [Google Scholar] [CrossRef]
- Pinkert, S.; Westermann, D.; Wang, X.; Klingel, K.; Dörner, A.; Savvatis, K.; Grössl, T.; Krohn, S.; Tschöpe, C.; Zeichhardt, H.; et al. Prevention of cardiac dysfunction in acute coxsackievirus B3 cardiomyopathy by inducible expression of a soluble coxsackievirus-adenovirus receptor. Circulation 2009, 120, 2358–2366. [Google Scholar] [CrossRef]
- Pinkert, S.; Dieringer, B.; Klopfleisch, R.; Savvatis, K.; Van Linthout, S.; Pryshliak, M.; Tschöpe, C.; Klingel, K.; Kurreck, J.; Beling, A.; et al. Early treatment of coxsackievirus B3-infected animals with soluble coxsackievirusadenovirus receptor inhibits development of chronic coxsackievirus B3 cardiomyopathy. Circ. Heart Fail. 2019, 12, e005250. [Google Scholar] [CrossRef]
- Kraft Erdenesukh, T.; Sauter, M.; Tschope, C.; Klingel, K. Blocking the IL-1β signalling pathway prevents chronic viral myocarditis and cardiac remodeling. Basic Res. Cardiol. 2019, 114, 11. [Google Scholar] [CrossRef]
- Brucato, A.; Imazio, M.; Gattorno, M.; Lazaros, G.; Maestroni, S.; Carraro, M.; Finetti, M.; Cumetti, D.; Carobbio, A.; Ruperto, N.; et al. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: The AIRTRIP randomized clinical trial. JAMA 2016, 316, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.C.; Hajela, V.; Hawkins, P.N.; Lachmann, H.J. A case series and systematic literature review of anakinra and immunosuppression in idiopathic recurrent pericarditis. J. Cardiol. Cases 2011, 4, e93–e97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodriguez-Gonzalez, M.; Ruiz-Gonzalez, E.; Castellano-Martinez, A. Anakinra as rescue therapy for steroid-dependent idiopathic recurrent pericarditis in children: Case report and literature review. Cardiol. Young 2019, 29, 241–243. [Google Scholar]
- US National Library of Medicine. ClinicalTrials.gov. 2024. Available online: https://clinicaltrials.gov/ct2/show/NCT03018834 (accessed on 20 March 2025).
- US National Library of Medicine. ClinicalTrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT03737110 (accessed on 20 March 2025).
- Li, Z.; Yue, Y.; Xiong, S. Distinct Th17 inductions contribute to the gender bias in CVB3-induced myocarditis. Cardiovasc. Pathol. 2013, 22, 373–382. [Google Scholar] [CrossRef]
- Abou-El-Enein, M.; Volk, H.D.; Reinke, P. Clinical development of cell therapies: Setting the stage for academic success. Clin. Pharmacol. Ther. 2017, 101, 35–38. [Google Scholar] [CrossRef]
- Koch, M.; Savvatis, K.; Scheeler, M.; Dhayat, S.; Bonaventura, K.; Pohl, T.; Riad, A.; Bulfone-Paus, S.; Schultheiss, H.P.; Tschöpe, C. Immunosuppression with an interleukin-2 fusion protein leads to improved LV function in experimental ischemic cardiomyopathy. Int. Immunopharmacol. 2010, 10, 207–212. [Google Scholar]
- Fan, M.Y.; Low, J.S.; Tanimine, N.; Finn, K.K.; Priyadharshini, B.; Germana, S.K.; Kaech, S.M.; Turka, L.A. Differential roles of IL-2 signaling in developing versus mature Tregs. Cell Rep. 2018, 25, 1204–1213.e4. [Google Scholar] [CrossRef]
- Van Linthout, S.; Savvatis, K.; Miteva, K.; Peng, J.; Ringe, J.; Warstat, K.; Schmidt-Lucke, C.; Sittinger, M.; Schultheiss, H.P.; Tschöpe, C. Mesenchymal stem cells improve murine acute coxsackievirus B3-induced myocarditis. Eur. Heart J. 2011, 32, 2168–2178. [Google Scholar] [CrossRef]
- Hare, J.M.; DiFede, D.L.; Rieger, A.C.; Florea, V.; Landin, A.M.; El-Khorazaty, J.; Khan, A.; Mushtaq, M.; Lowery, M.H.; Byrnes, J.J.; et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDONDCM trial. J. Am. Coll. Cardiol. 2017, 69, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Rieger, A.C.; Myerburg, R.J.; Florea, V.; Tompkins, B.A.; Natsumeda, M.; Premer, C.; Khan, A.; Schulman, I.H.; Vidro-Casiano, M.; DiFede, D.L.; et al. Genetic determinants of responsiveness to mesenchymal stem cell injections in non-ischemic dilated cardiomyopathy. EBioMedicine 2019, 48, 377–385. [Google Scholar] [CrossRef]
- Lorusso, R.; Centofanti, P.; Gelsomino, S.; Barili, F.; Di Mauro, M.; Orlando, P.; Botta, L.; Milazzo, F.; Actis Dato, G.; Casabona, R.; et al. Venoarterial extracorporeal membrane oxygenation for acute fulminant myocarditis in adult patients: A 5-year multi-institutional experience. Ann. Thorac. Surg. 2016, 101, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.K.; Davila, C.D.; Jumean, M.F. Integrating interventional cardiology and heart failure management for cardiogenic shock. Interv. Cardiol. Clin. 2017, 6, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, S.; Li, C.; Ran, X.; Cui, G.; He, M.; Miao, K.; Zhao, C.; Yan, J.; Hui, R.; et al. A life support-based comprehensive treatment regimen dramatically lowers the in-hospital mortality of patients with fulminant myocarditis: A multiple center study. Sci. China Life Sci. 2019, 62, 369–380. [Google Scholar] [CrossRef]
- Kapur, N.K.; Esposito, M.L.; Bader, Y.; Morine, K.J.; Kiernan, M.S.; Pham, D.T.; Burkhoff, D. Mechanical circulatory support devices for acute right ventricular failure. Circulation 2017, 136, 314–326. [Google Scholar] [CrossRef]
- Annamalai, S.K.; Esposito, M.L.; Jorde, L.; Schreiber, T.; AHall, S.; O’Neill, W.W.; Kapur, N.K. The Impella microaxial flow catheter is safe and effective for treatment of myocarditis complicated by cardiogenic shock: An analysis from the global cVAD registry. J. Card. Fail. 2018, 24, 706–710. [Google Scholar] [CrossRef]
- Spillmann, F. Mode-of-action of the PROPELLA concept in fulminant myocarditis. Mode-of-action of the PROPELLA concept in fulminant myocarditis. Eur. Heart J. 2019, 40, 2164–2169. [Google Scholar] [CrossRef]
- Sun, M.; Ishii, R.; Okumura, K.; Krauszman, A.; Breitling, S.; Gomez, O.; Hinek, A.; Boo, S.; Hinz, B.; Connelly, K.A.; et al. Experimental right ventricular hypertension induces regional β1-integrin-mediated transduction of hypertrophic and profibrotic right and left ventricular signaling. J. Am. Heart Assoc. 2018, 7, e007928. [Google Scholar] [CrossRef]
- Lindner, D. Cardiac fibroblasts support cardiac inflammation in heart failure. Basic Res. Cardiol. 2014, 109, 428. [Google Scholar] [CrossRef]
- Levin, H.R.; Oz, M.C.; Chen, J.M.; Packer, M.; Rose, E.A.; Burkhoff, D. Reversal of chronic ventricular dilation in patients with end-stage cardiomyopathy by prolonged mechanical unloading. Circulation 1995, 91, 2717–2720. [Google Scholar] [CrossRef] [PubMed]
- Hata, J.A.; Williams, M.L.; Schroder, J.N.; Lima, B.; Keys, J.R.; Blaxall, B.C.; Petrofski, J.A.; Jakoi, A.; Milano, C.A.; Koch, W.J. Lymphocyte levels of GRK2 (βARK1) mirror changes in the LVAD-supported failing human heart: Lower GRK2 associated with improved β-adrenergic signaling after mechanical unloading. J. Card. Fail. 2006, 12, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Tschope, C.; Van Linthout, S.; Klein, O.; Mairinger, T.; Krackhardt, F.; Potapov, E.V.; Schmidt, G.; Burkhoff, D.; Pieske, B.; Spillmann, F. Mechanical unloading by fulminant myocarditis: LV-IMPELLA, ECMELLA, BI-PELLA, and PROPELLA concepts. J Cardiovasc. Transl. Res. 2019, 12, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, S.V.; Badheka, A.; Marzouka, G.R.; Tanawuttiwat, T.; Ahmed, F.; Sacher, V.; Pham, S.M. Combined use of Impella left ventricular assist device and extracorporeal membrane oxygenation as a bridge to recovery in fulminant myocarditis. ASAIO J. 2012, 58, 285–287. [Google Scholar] [CrossRef]
- Pappalardo, F.; Schulte, C.; Pieri, M.; Schrage, B.; Contri, R.; Soeffker, G.; Greco, T.; Lembo, R.; Müllerleile, K.; Colombo, A.; et al. Concomitant implantation of Impella((R)) on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur. J. Heart Fail. 2017, 19, 404–412. [Google Scholar] [CrossRef]
- Pappalardo, F.; Scandroglio, A.M.; Latib, A. Full percutaneous biventricular support with two Impella pumps: The Bi-Pella approach. ESC Heart Fail. 2018, 5, 368–371. [Google Scholar] [CrossRef]
- Tominaga, Y.; Toda, K.; Miyagawa, S.; Yoshioka, D.; Kainuma, S.; Kawamura, T.; Kawamura, A.; Nakamoto, K.; Sakata, Y.; Sawa, Y. Total percutaneous biventricular assist device implantation for fulminant myocarditis. J. Artif. Organs 2021, 24, 254–257. [Google Scholar] [CrossRef]
- Fiedler, A. Uber akute interstitielle Myokarditis. Zentralblatt Inn. Med. 1900, 21, 212–213. [Google Scholar]
- Sakakibara, S.; Konno, S. Endomyocardial biopsy. Jpn. Heart J. 1962, 3, 537–543. [Google Scholar] [CrossRef]
- Laser, J.A.; Fowles, R.E.; Mason, J.W. Endomyocardial biopsy. Cardiovasc. Clin. 1985, 15, 141–163. [Google Scholar]
- Mason, J.W.; O’Connell, J.B. Clinical merit of endomyocardial biopsy. Circulation 1989, 79, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, A.; Carturan, E.; Rizzo, S.; Basso, C.; Thiene, G. Story telling of myocarditis. Int. J. Cardiol. 2019, 294, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Thiene, G. Storytelling of Myocarditis. Biomedicines 2024, 12, 832. [Google Scholar] [CrossRef] [PubMed]
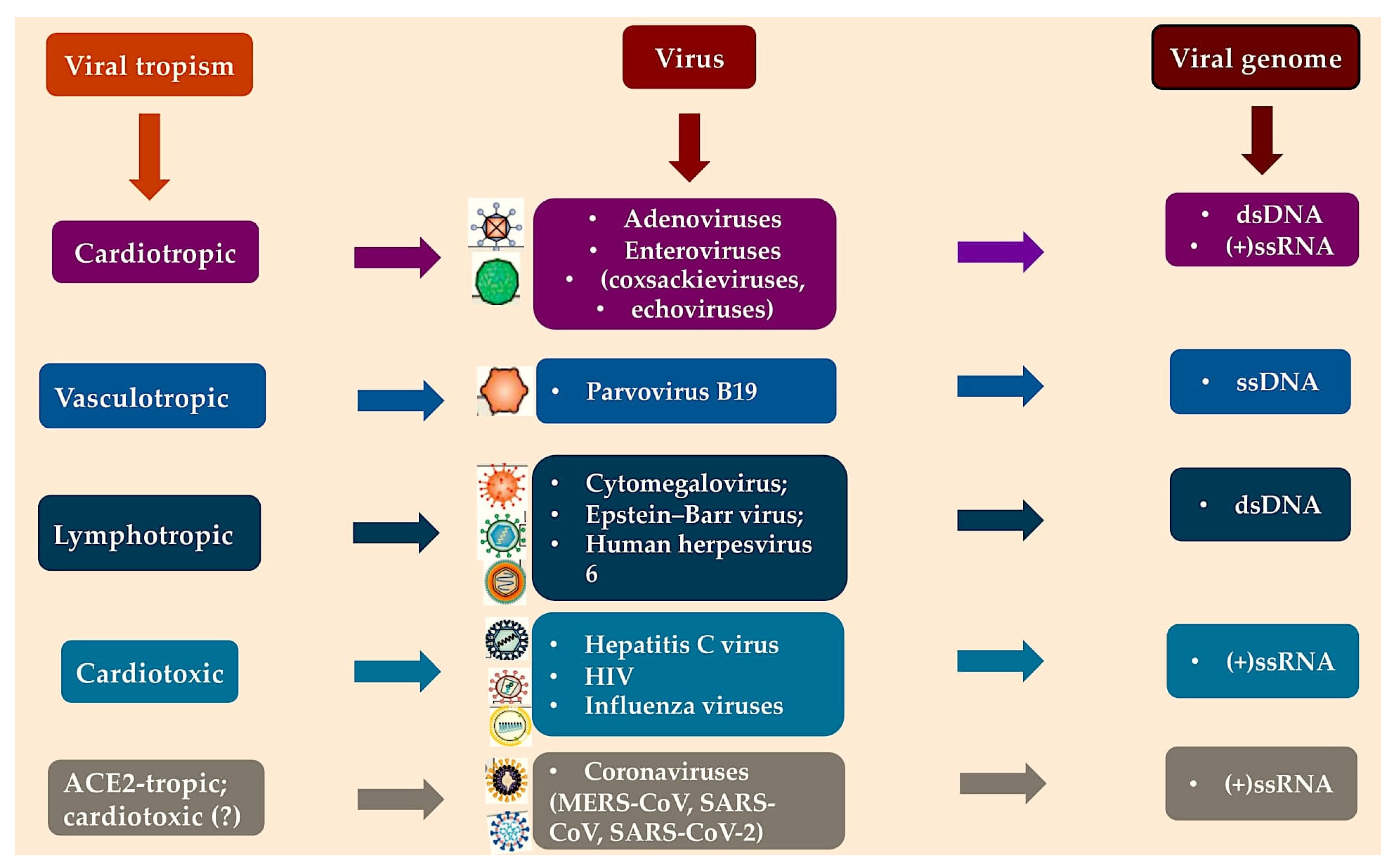
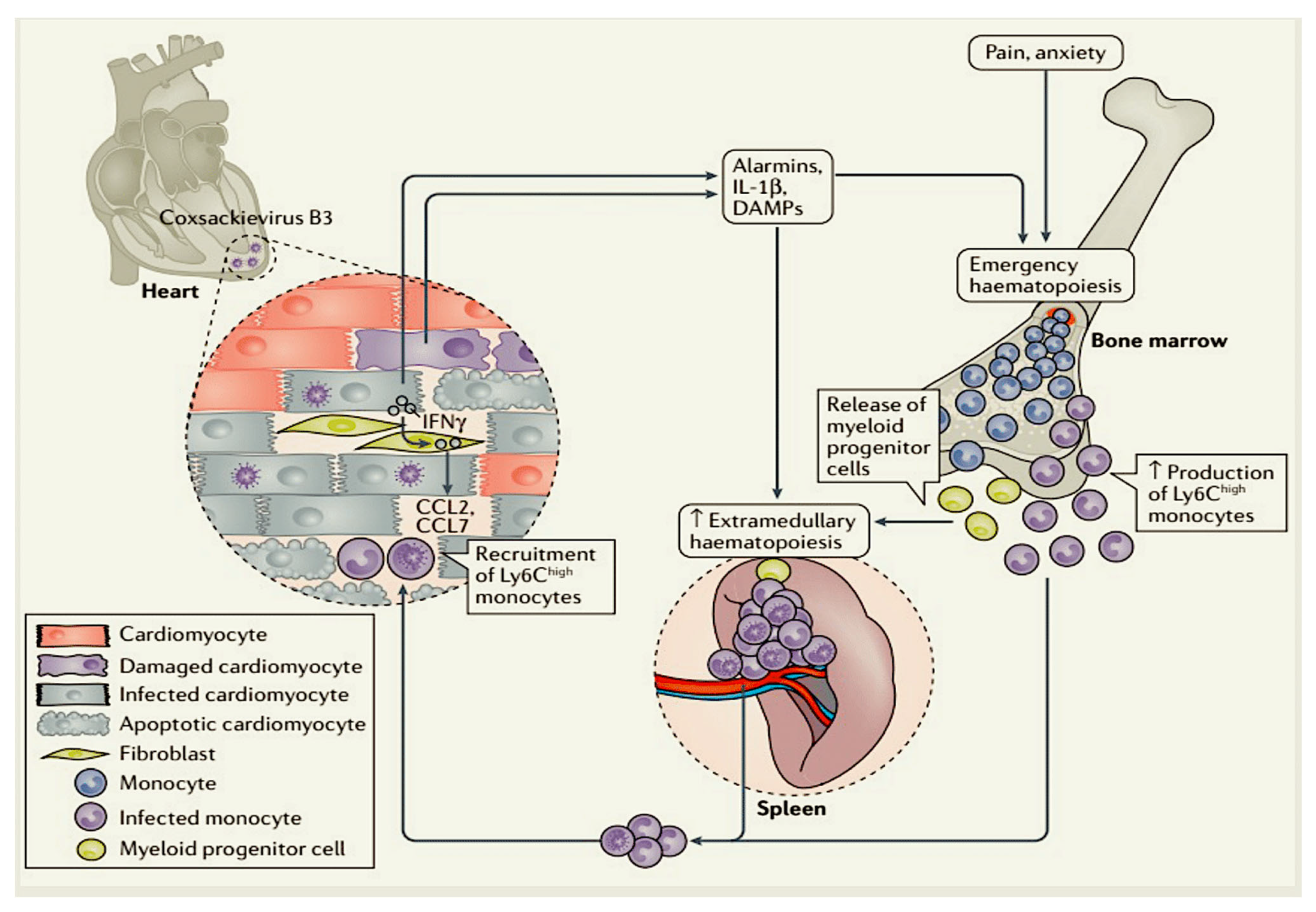
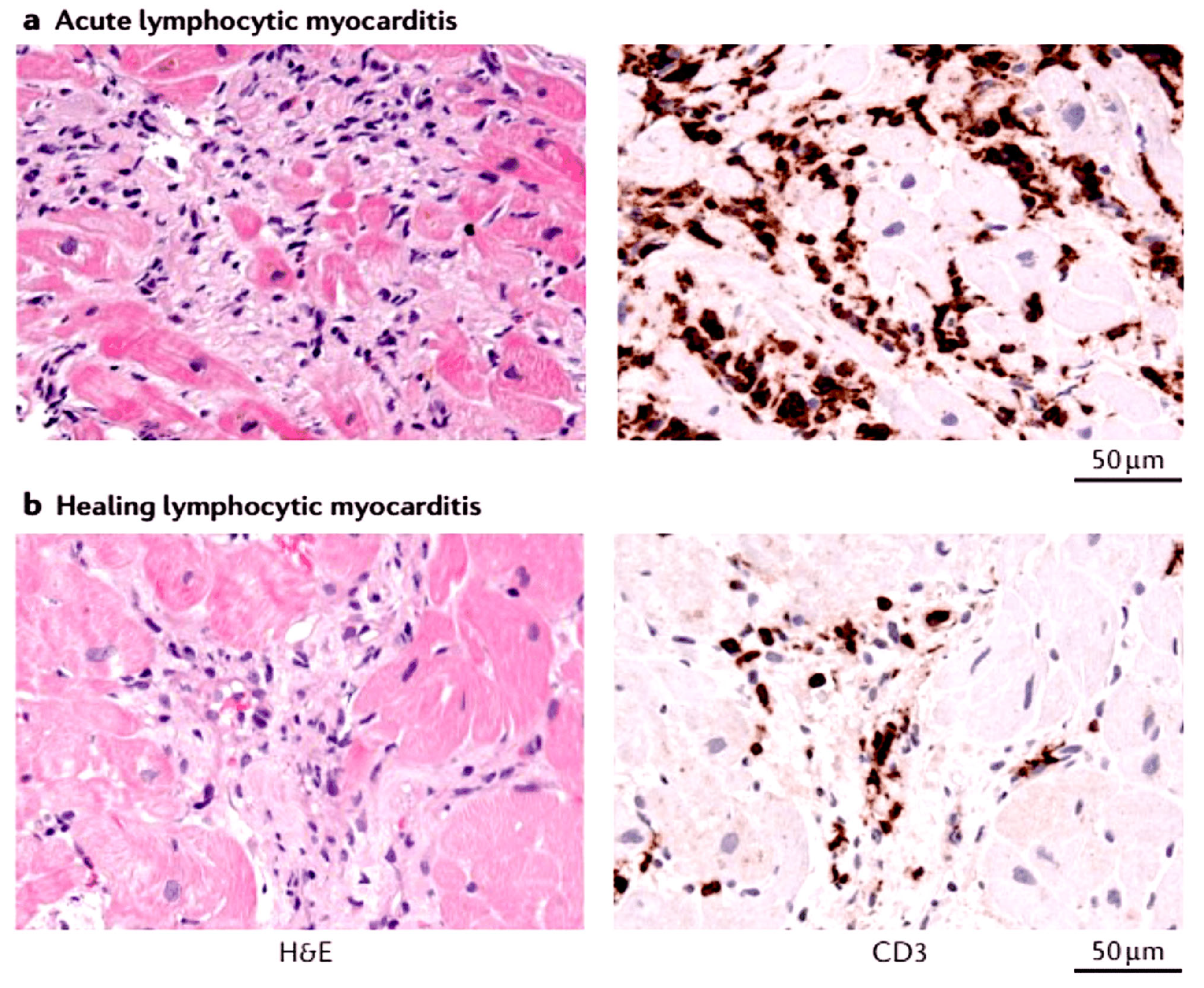
| Proinflammatory and Immunomodulatory Cell Phenotype | How It Works | Findings |
|---|---|---|
| Monocytes [85,96,113,114,115,116] |
|
|
| Neutrophils [86,93,94,95,96,97,98,99,100,107,108,109,110,111,112] |
|
|
| Mast cells, natural killer cells and dendritic cells [102,103,104,105,106] |
|
|
| T cells and B cells [81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,117,118,119,120,121,122,123,124,125,126] |
|
|
| Eosinophils [131,132,133,134] |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nappi, F. Myocarditis and Inflammatory Cardiomyopathy in Dilated Heart Failure. Viruses 2025, 17, 484. https://doi.org/10.3390/v17040484
Nappi F. Myocarditis and Inflammatory Cardiomyopathy in Dilated Heart Failure. Viruses. 2025; 17(4):484. https://doi.org/10.3390/v17040484
Chicago/Turabian StyleNappi, Francesco. 2025. "Myocarditis and Inflammatory Cardiomyopathy in Dilated Heart Failure" Viruses 17, no. 4: 484. https://doi.org/10.3390/v17040484
APA StyleNappi, F. (2025). Myocarditis and Inflammatory Cardiomyopathy in Dilated Heart Failure. Viruses, 17(4), 484. https://doi.org/10.3390/v17040484









