Stromal Interferon Regulatory Factor 3 Can Antagonize Human Papillomavirus Replication by Supporting Epithelial-to-Mesenchymal Transition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
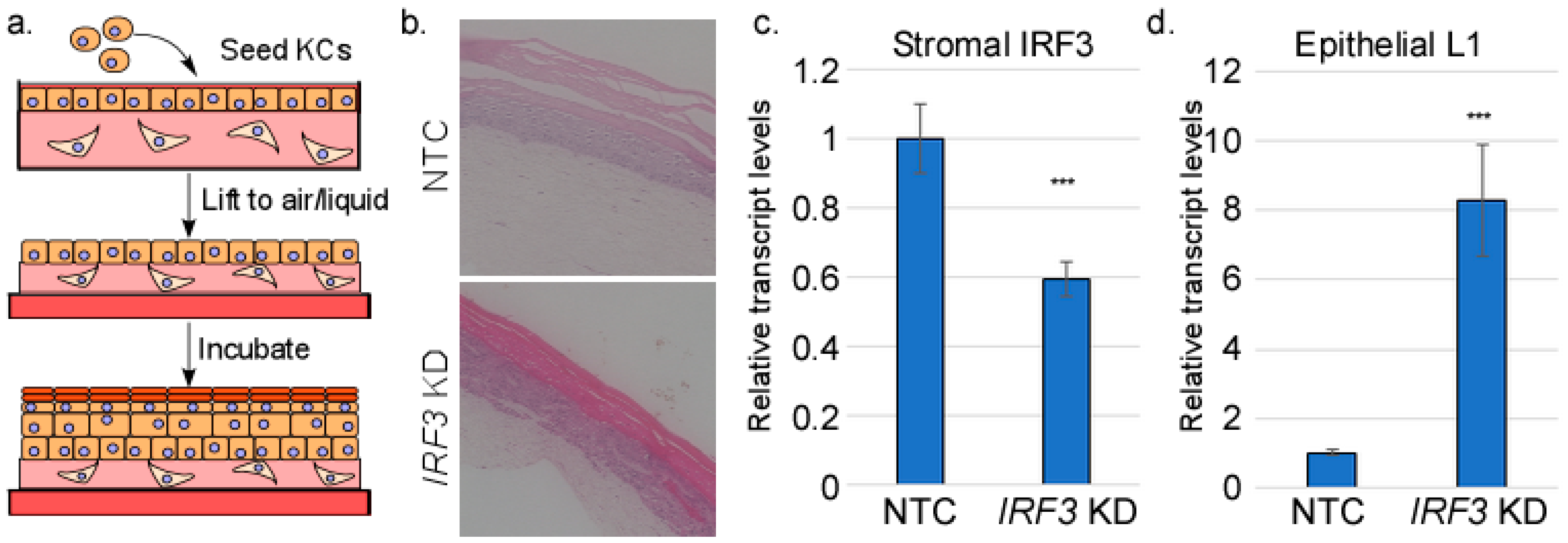
2.2. Organotypic Raft Culture
2.3. Immunofluorescence
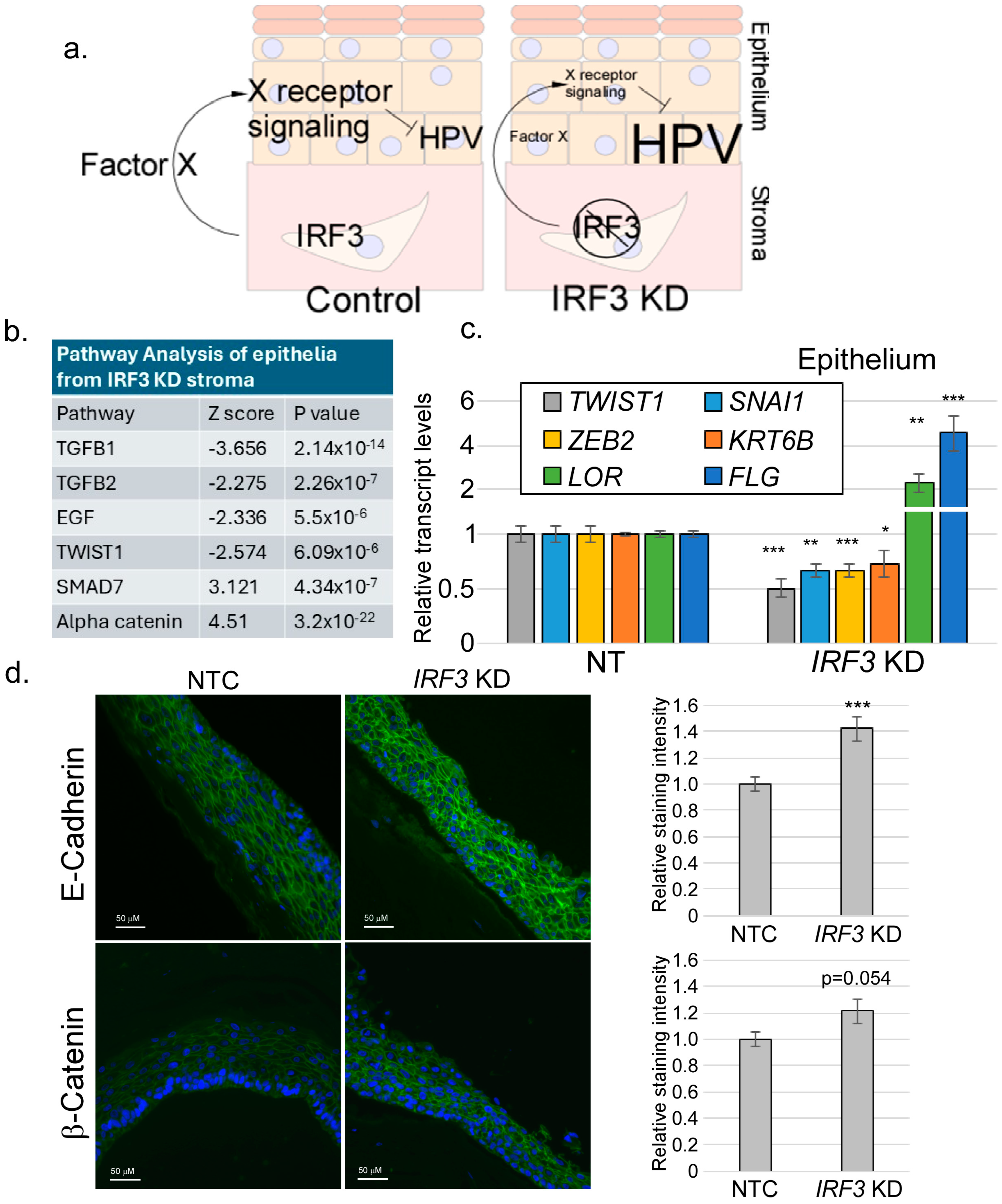
2.4. RNA-Seq
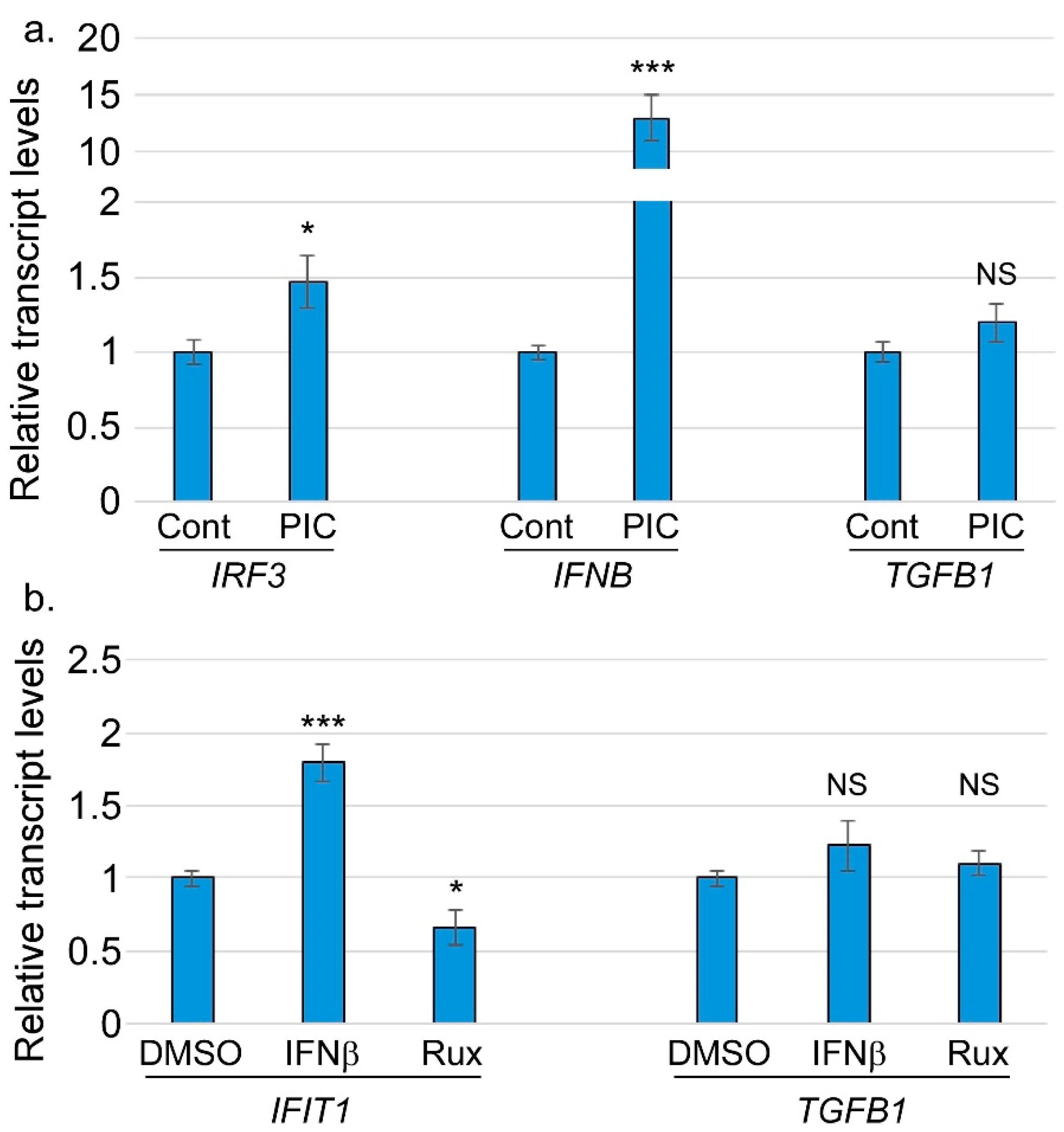
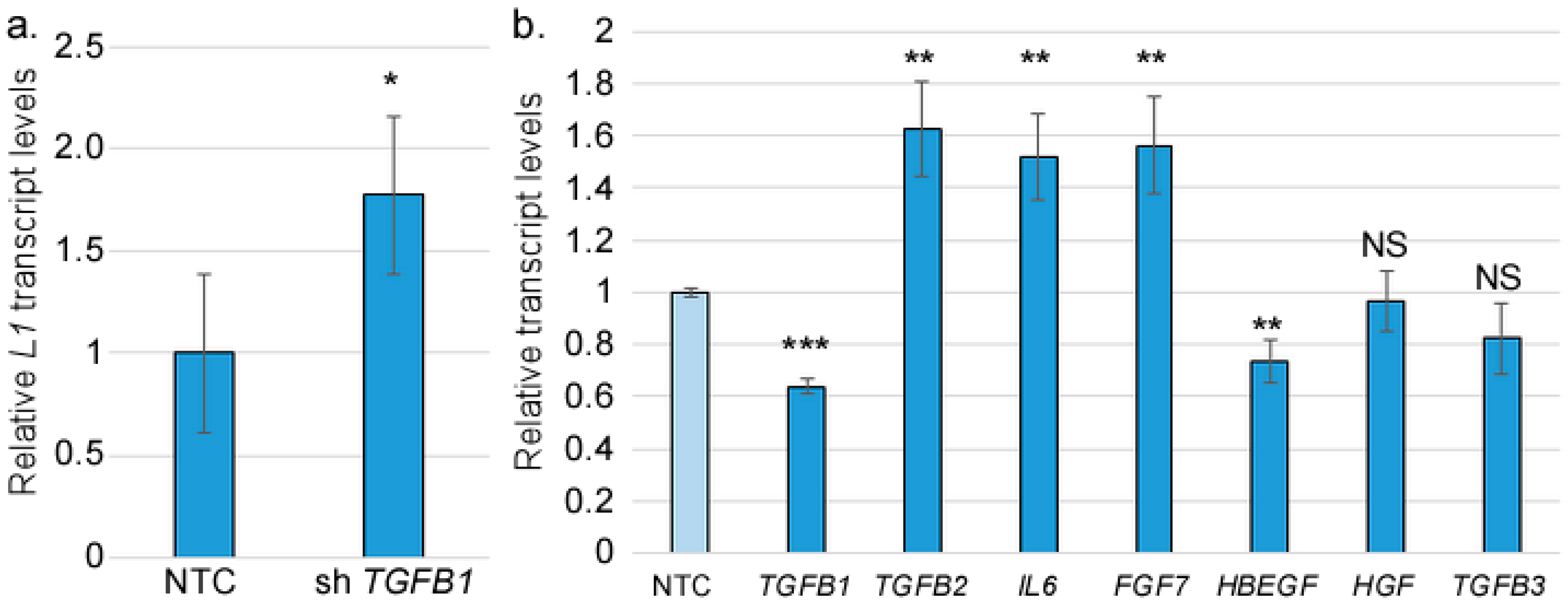
2.5. RNA Extraction and RT-qPCR
2.6. Immunoblotting
2.7. Chromatin Immunoprecipitation
2.8. siRNA Transfection
2.9. shRNA Lentivirus Transduction
2.10. Statistics
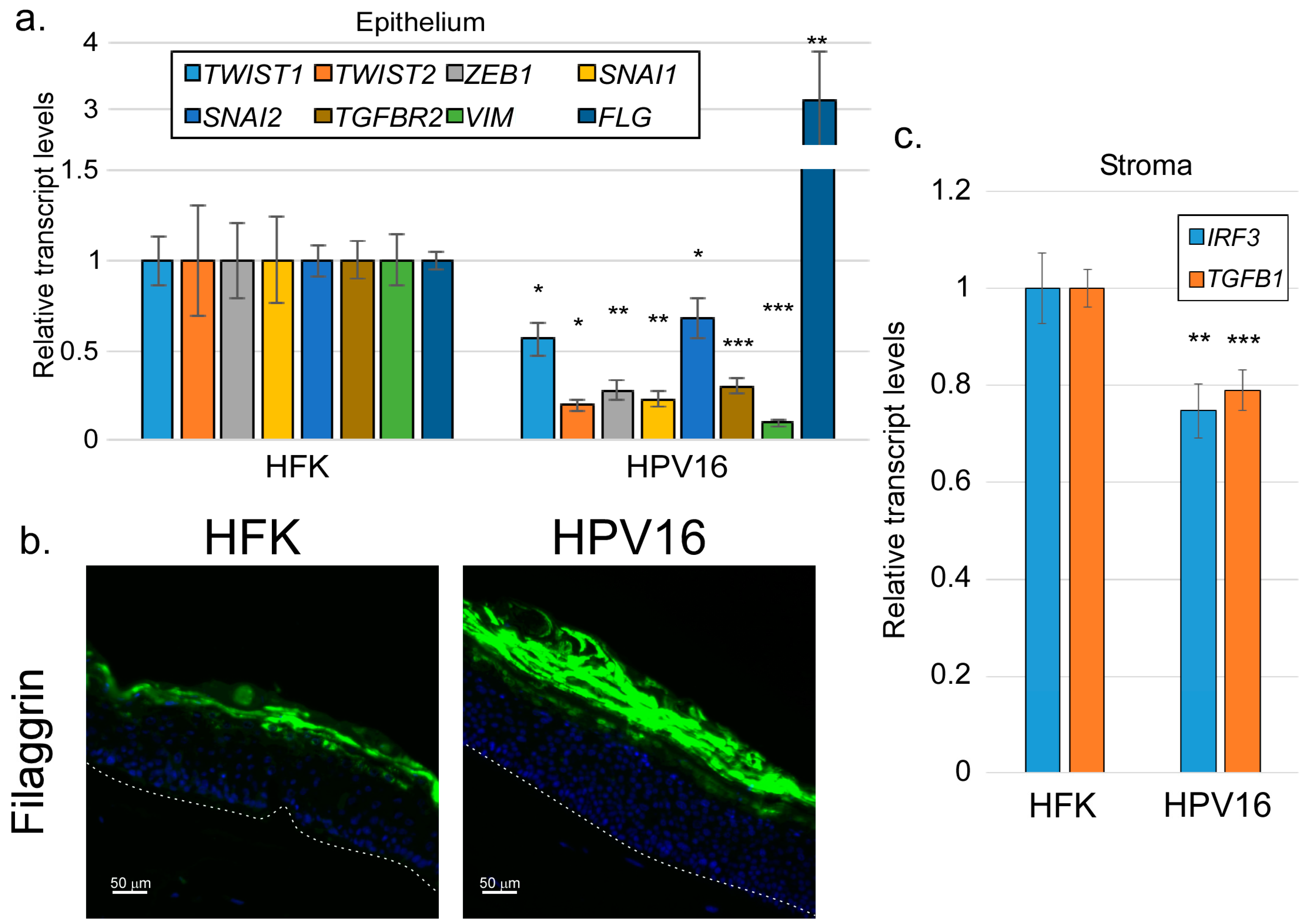
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ChIP | chromatin immunoprecipitation |
| EMT | epithelial-to-mesenchymal transition |
| FGF | fibroblast growth factor |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| HBEGF | heparin-binding epidermal growth factor |
| HFF | human foreskin fibroblast |
| HFK | human foreskin keratinocyte |
| HPV | human papillomavirus |
| IFN | interferon |
| IL6 | interleukin 6 |
| IRF3 | interferon regulatory factor 3 |
| ISG | interferon-stimulated gene |
| KD | knockdown |
| NTC | non-target control |
| PIC | poly I |
| C (PIC) | polyinosinic-polycytidylic acid |
| RT-qPCR | reverse transcriptase–quantitative polymerase chain reaction |
| TGFβ | transforming growth factor beta |
References
- Larsen, S.B.; Cowley, C.J.; Fuchs, E. Epithelial cells: Liaisons of immunity. Curr. Opin. Immunol. 2020, 62, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Filer, A.; Pitzalis, C.; Buckley, C.D. Targeting the stromal microenvironment in chronic inflammation. Curr. Opin. Pharmacol. 2006, 6, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Woodby, B.; Scott, M.; Bodily, J. The interaction between human papillomaviruses and the stromal microenvironment. Prog. Mol. Biol. Transl. Sci. 2016, 144, 169–238. [Google Scholar]
- Huet, E.; Jaroz, C.; Nguyen, H.Q.; Belkacemi, Y.; de la Taille, A.; Stavrinides, V.; Whitaker, H. Stroma in normal and cancer wound healing. FEBS J. 2019, 286, 2909–2920. [Google Scholar] [CrossRef]
- Clark, R.A. The Molecular and Cellular Biology of Wound Repair; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Tracy, L.E.; Minasian, R.A.; Caterson, E. Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Foster, D.S.; Jones, R.E.; Ransom, R.C.; Longaker, M.T.; Norton, J.A. The evolving relationship of wound healing and tumor stroma. JCI Insight 2018, 3, e99911. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial–mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef]
- Acloque, H.; Ocaña, O.H.; Matheu, A.; Rizzoti, K.; Wise, C.; Lovell-Badge, R.; Nieto, M.A. Reciprocal repression between Sox3 and snail transcription factors defines embryonic territories at gastrulation. Dev. Cell 2011, 21, 546–558. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The type-2 EMT in wound healing, tissue regeneration and organ fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef]
- Suarez-Carmona, M.; Lesage, J.; Cataldo, D.; Gilles, C. EMT and inflammation: Inseparable actors of cancer progression. Mol. Oncol. 2017, 11, 805–823. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-mesenchymal transition in cancer: A historical overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef] [PubMed]
- David, C.J.; Massagué, J. Contextual determinants of TGFβ action in development, immunity and cancer. Nat. Rev. Mol. Cell Biol. 2018, 19, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Trugilo, K.P.; Cebinelli, G.C.M.; Castilha, E.P.; da Silva, M.R.; Berti, F.C.B.; de Oliveira, K.B. The role of transforming growth factor β in cervical carcinogenesis. Cytokine Growth Factor Rev. 2024, 80, 12–23. [Google Scholar] [CrossRef]
- Massagué, J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef]
- Kubiczkova, L.; Sedlarikova, L.; Hajek, R.; Sevcikova, S. TGF-β—An excellent servant but a bad master. J. Transl. Med. 2012, 10, 183. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Derynck, R.; Akhurst, R.J. Differentiation plasticity regulated by TGF-β family proteins in development and disease. Nat. Cell Biol. 2007, 9, 1000–1004. [Google Scholar] [CrossRef]
- Calon, A.; Espinet, E.; Palomo-Ponce, S.; Tauriello, D.V.; Iglesias, M.; Céspedes, M.V.; Sevillano, M.; Nadal, C.; Jung, P.; Zhang, X.H.-F. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell 2012, 22, 571–584. [Google Scholar] [CrossRef]
- Costanza, B.; Umelo, I.A.; Bellier, J.; Castronovo, V.; Turtoi, A. Stromal Modulators of TGF-β in Cancer. J. Clin. Med. 2017, 6, 7. [Google Scholar] [CrossRef]
- Massagué, J. TGF-b signaling in development and disease. Febs Lett. 2012, 586, 1833. [Google Scholar] [CrossRef] [PubMed]
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Condon, M.S. The role of the stromal microenvironment in prostate cancer. Semin. Cancer Biol. 2005, 15, 132–137. [Google Scholar] [CrossRef]
- Hu, M.; Huang, L. Strategies targeting tumor immune and stromal microenvironment and their clinical relevance. Adv. Drug Deliv. Rev. 2022, 183, 114137. [Google Scholar] [CrossRef]
- Ligorio, M.; Sil, S.; Malagon-Lopez, J.; Nieman, L.T.; Misale, S.; Di Pilato, M.; Ebright, R.Y.; Karabacak, M.N.; Kulkarni, A.S.; Liu, A. Stromal microenvironment shapes the intratumoral architecture of pancreatic cancer. Cell 2019, 178, 160–175.e127. [Google Scholar] [CrossRef]
- Wild, C.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; IARC Press: Lyon, France, 2020. [Google Scholar]
- Doorbar, J. The papillomavirus life cycle. J. Clin. Virol. 2005, 32, 7–15. [Google Scholar] [CrossRef]
- Pyeon, D.; Pearce, S.M.; Lank, S.M.; Ahlquist, P.; Lambert, P.F. Establishment of human papillomavirus infection requires cell cycle progression. PLoS Pathog. 2009, 5, e1000318. [Google Scholar] [CrossRef]
- Fernandes, J.V.; De Medeiros Fernandes, T.A.A.; De Azevedo, J.C.V.; Cobucci, R.N.O.; De Carvalho, M.G.F.; Andrade, V.S.; De Araujo, J.M.G. Link between chronic inflammation and human papillomavirus-induced carcinogenesis. Oncol. Lett. 2015, 9, 1015–1026. [Google Scholar] [CrossRef]
- Maglennon, G.A.; McIntosh, P.; Doorbar, J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression. Virology 2011, 414, 153–163. [Google Scholar] [CrossRef]
- Ozbun, M.A. Human papillomavirus type 31b infection of human keratinocytes and the onset of early transcription. J. Virol. 2002, 76, 11291–11300. [Google Scholar] [CrossRef]
- Wilson, V.G.; West, M.; Woytek, K.; Rangasamy, D. Papillomavirus E1 proteins: Form, function, and features. Virus Genes 2002, 24, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.T.; Broker, T.R.; Steinberg, B.M. The natural history of human papillomavirus infections of the mucosal epithelia. Apmis 2010, 118, 422–449. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.; Munger, K. The papillomavirus E7 proteins. Virology 2013, 445, 138–168. [Google Scholar] [CrossRef] [PubMed]
- Pol, S.B.V.; Klingelhutz, A.J. Papillomavirus E6 oncoproteins. Virology 2013, 445, 115–137. [Google Scholar]
- Bodily, J.; Laimins, L.A. Persistence of human papillomavirus infection: Keys to malignant progression. Trends Microbiol. 2011, 19, 33–39. [Google Scholar] [CrossRef]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The biology and life-cycle of human papillomaviruses. Vaccine 2012, 30, F55–F70. [Google Scholar] [CrossRef]
- Pestka, S.; Krause, C.D.; Walter, M.R. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 2004, 202, 8–32. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Sato, M.; Suemori, H.; Hata, N.; Asagiri, M.; Ogasawara, K.; Nakao, K.; Nakaya, T.; Katsuki, M.; Noguchi, S.; Tanaka, N. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-α/β gene induction. Immunity 2000, 13, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Yanai, H.; Savitsky, D.; Taniguchi, T. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 2008, 26, 535–584. [Google Scholar] [CrossRef]
- Jefferies, C.A. Regulating IRFs in IFN Driven Disease. Front. Immunol. 2019, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Takaoka, A.; Taniguchi, T. Type I inteferon gene induction by the interferon regulatory factor family of transcription factors. Immunity 2006, 25, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Menachery, V.D.; Pasieka, T.J.; Leib, D.A. Interferon regulatory factor 3-dependent pathways are critical for control of herpes simplex virus type 1 central nervous system infection. J. Virol. 2010, 84, 9685–9694. [Google Scholar] [CrossRef] [PubMed]
- Petro, T.M. IFN Regulatory Factor 3 in Health and Disease. J. Immunol. 2020, 205, 1981–1989. [Google Scholar] [CrossRef]
- Cheng, T.-F.; Brzostek, S.; Ando, O.; Van Scoy, S.; Kumar, K.P.; Reich, N.C. Differential activation of IFN regulatory factor (IRF)-3 and IRF-5 transcription factors during viral infection. J. Immunol. 2006, 176, 7462–7470. [Google Scholar] [CrossRef]
- Sharif-Askari, E.; Nakhaei, P.; Oliere, S.; Tumilasci, V.; Hernandez, E.; Wilkinson, P.; Lin, R.; Bell, J.; Hiscott, J. Bax-dependent mitochondrial membrane permeabilization enhances IRF3-mediated innate immune response during VSV infection. Virology 2007, 365, 20–33. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Yamashita, M.; Zhang, Y.; Sen, G.C. The IRF-3/Bax-mediated apoptotic pathway, activated by viral cytoplasmic RNA and DNA, inhibits virus replication. J. Virol. 2011, 85, 3708–3716. [Google Scholar] [CrossRef]
- Günthner, R.; Anders, H.-J. Interferon-regulatory factors determine macrophage phenotype polarization. Mediat. Inflamm. 2013, 2013, 731023. [Google Scholar] [CrossRef]
- Satoh, T.; Takeuchi, O.; Vandenbon, A.; Yasuda, K.; Tanaka, Y.; Kumagai, Y.; Miyake, T.; Matsushita, K.; Okazaki, T.; Saitoh, T. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat. Immunol. 2010, 11, 936–944. [Google Scholar] [CrossRef]
- Negishi, H.; Fujita, Y.; Yanai, H.; Sakaguchi, S.; Ouyang, X.; Shinohara, M.; Takayanagi, H.; Ohba, Y.; Taniguchi, T.; Honda, K. Evidence for licensing of IFN-γ-induced IFN regulatory factor 1 transcription factor by MyD88 in Toll-like receptor-dependent gene induction program. Proc. Natl. Acad. Sci. USA 2006, 103, 15136–15141. [Google Scholar] [CrossRef]
- Krausgruber, T.; Blazek, K.; Smallie, T.; Alzabin, S.; Lockstone, H.; Sahgal, N.; Hussell, T.; Feldmann, M.; Udalova, I.A. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat. Immunol. 2011, 12, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Blazek, K.; Byrne, A.J.; Perocheau, D.P.; Udalova, I.A. IRF5 is a specific marker of inflammatory macrophages in vivo. Mediat. Inflamm. 2013, 2013, 245804. [Google Scholar] [CrossRef] [PubMed]
- Raikhy, G.; Woodby, B.L.; Scott, M.L.; Shin, G.; Myers, J.E.; Scott, R.S.; Bodily, J.M. Suppression of Stromal Interferon Signaling by Human Papillomavirus 16. J. Virol. 2019, 93, e00458-19. [Google Scholar] [CrossRef]
- Trammel, J.; Amusan, O.; Hultgren, A.; Raikhy, G.; Bodily, J.M. Epidermal growth factor receptor-dependent stimulation of differentiation by human papillomavirus type 16 E5. Virology 2024, 590, 109952. [Google Scholar] [CrossRef]
- Pickard, A.; McDade, S.S.; McFarland, M.; McCluggage, W.G.; Wheeler, C.M.; McCance, D.J. HPV16 Down-Regulates the Insulin-Like Growth Factor Binding Protein 2 to Promote Epithelial Invasion in Organotypic Cultures. PLoS Pathog. 2015, 11, e1004988. [Google Scholar] [CrossRef]
- Wilson, R.; Fehrmann, F.; Laimins, L.A. Role of the E1∧ E4 protein in the differentiation-dependent life cycle of human papillomavirus type 31. J. Virol. 2005, 79, 6732–6740. [Google Scholar] [CrossRef]
- Genther, S.M.; Sterling, S.; Duensing, S.; Münger, K.; Sattler, C.; Lambert, P.F. Quantitative role of the human papillomavirus type 16 E5 gene during the productive stage of the viral life cycle. J. Virol. 2003, 77, 2832–2842. [Google Scholar] [CrossRef]
- Banerjee, N.S.; Moore, D.W.; Broker, T.R.; Chow, L.T. Vorinostat, a pan-HDAC inhibitor, abrogates productive HPV-18 DNA amplification. Proc. Natl. Acad. Sci. USA 2018, 115, E11138–E11147. [Google Scholar] [CrossRef]
- Ozbun, M.A.; Patterson, N.A. Using organotypic (raft) epithelial tissue cultures for the biosynthesis and isolation of infectious human papillomaviruses. Curr. Protoc. Microbiol. 2014, 34, 14B.13.11–14B.13.18. [Google Scholar] [CrossRef]
- Bodily, J.M.; Mehta, K.P.; Cruz, L.; Meyers, C.; Laimins, L.A. The E7 open reading frame acts in cis and in trans to mediate differentiation-dependent activities in the human papillomavirus type 16 life cycle. J. Virol. 2011, 85, 8852–8862. [Google Scholar] [CrossRef]
- Bodily, J.M.; Meyers, C. Genetic analysis of the human papillomavirus type 31 differentiation-dependent late promoter. J. Virol. 2005, 79, 3309–3321. [Google Scholar] [CrossRef] [PubMed]
- Meyers, C.; Mayer, T.J.; Ozbun, M.A. Synthesis of infectious human papillomavirus type 18 in differentiating epithelium transfected with viral DNA. J. Virol. 1997, 71, 7381–7386. [Google Scholar] [CrossRef] [PubMed]
- Woodby, B.L.; Songock, W.K.; Scott, M.L.; Raikhy, G.; Bodily, J.M. Induction of interferon kappa in human papillomavirus 16 infection by transforming growth factor beta-induced promoter demethylation. J. Virol. 2018, 92, e01714-17. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.; Kim, S.-m.; Rodriguez, C.; Songock, W.; Raikhy, G.; Lopez, R.; Henderson, L.; Yusufji, A.; Bodily, J. Suppression of a subset of interferon-induced genes by human papillomavirus type 16 E7 via a cyclin dependent kinase 8-dependent mechanism. Viruses 2020, 12, 311. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, S.; Xi, L.; Wu, S.; Chen, G.; Zhao, Y.; Wu, Y.; Ma, D. Effects of human papillomavirus type 16 E7 protein on the growth of cervical carcinoma cells and immuno-escape through the TGF-β1 signaling pathway. Gynecol. Oncol. 2006, 101, 132–139. [Google Scholar] [CrossRef]
- Torres-Poveda, K.; Bahena-Román, M.; Madrid-González, C.; Burguete-García, A.I.; Bermúdez-Morales, V.H.; Peralta-Zaragoza, O.; Madrid-Marina, V. Role of IL-10 and TGF-β1 in local immunosuppression in HPV-associated cervical neoplasia. World J. Clin. Oncol. 2014, 5, 753. [Google Scholar] [CrossRef]
- Peralta-Zaragoza, O.; Bermúdez-Morales, V.; Gutiérrez-Xicotencatl, L.; Alcocer-González, J.; Recillas-Targa, F.; Madrid-Marina, V. E6 and E7 oncoproteins from human papillomavirus type 16 induce activation of human transforming growth factor β 1 promoter throughout Sp1 recognition sequence. Viral Immunol. 2006, 19, 468–480. [Google Scholar] [CrossRef]
- Scott, M.L.; Woodby, B.L.; Ulicny, J.; Raikhy, G.; Orr, A.W.; Songock, W.K.; Bodily, J.M. Human papillomavirus 16 E5 inhibits interferon signaling and supports episomal viral maintenance. J. Virol. 2020, 94, e01582-19. [Google Scholar] [CrossRef]
- Mansfield, K.; Naik, S. Unraveling Immune-Epithelial Interactions in Skin Homeostasis and Injury. Yale J. Biol. Med. 2020, 93, 133–143. [Google Scholar]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Arnold, K.M.; Opdenaker, L.M.; Flynn, D.; Sims-Mourtada, J. Wound healing and cancer stem cells: Inflammation as a driver of treatment resistance in breast cancer. Cancer Growth Metastasis 2015, 8, CGM-S11286. [Google Scholar] [CrossRef]
- Al Hamrashdi, M.; Brady, G. Regulation of IRF3 activation in human antiviral signaling pathways. Biochem. Pharmacol. 2022, 200, 115026. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Chiba, S.; Hangai, S.; Kometani, K.; Inoue, A.; Kimura, Y.; Abe, T.; Kiyonari, H.; Nishio, J.; Taguchi-Atarashi, N.; et al. Revisiting the role of IRF3 in inflammation and immunity by conditional and specifically targeted gene ablation in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 5253–5258. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.-m.; Xu, T.; Wang, Y.-r.; He, Y.-h.; Zhou, Q.; Huang, C.; Meng, X.-m.; Li, J. Inhibition of IRF3 expression reduces TGF-β1-induced proliferation of hepatic stellate cells. J. Physiol. Biochem. 2016, 72, 9–23. [Google Scholar] [CrossRef]
- Xu, P.; Bailey-Bucktrout, S.; Xi, Y.; Xu, D.; Du, D.; Zhang, Q.; Xiang, W.; Liu, J.; Melton, A.; Sheppard, D.; et al. Innate Antiviral Host Defense Attenuates TGF-β Function through IRF3-Mediated Suppression of Smad Signaling. Mol. Cell 2014, 56, 723–737. [Google Scholar] [PubMed]
- Andrilenas, K.K.; Ramlall, V.; Kurland, J.; Leung, B.; Harbaugh, A.G.; Siggers, T. DNA-binding landscape of IRF3, IRF5 and IRF7 dimers: Implications for dimer-specific gene regulation. Nucleic Acids Res. 2018, 46, 2509–2520. [Google Scholar] [CrossRef]
- Lin, R.; Heylbroeck, C.; Genin, P.; Pitha, P.M.; Hiscott, J. Essential role of interferon regulatory factor 3 in direct activation of RANTES chemokine transcription. Mol. Cell Biol. 1999, 19, 959–966. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Yiu, J.H.C.; Lam, J.K.W.; Wong, C.-M.; Dorweiler, B.; Xu, A.; Woo, C.W. TRIF-dependent Toll-like receptor signaling suppresses Scd1 transcription in hepatocytes and prevents diet-induced hepatic steatosis. Sci. Signal. 2017, 10, eaal3336. [Google Scholar] [CrossRef]
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal 2014, 7, re8. [Google Scholar] [CrossRef]
- Abaurrea, A.; Araujo, A.M.; Caffarel, M.M. The Role of the IL-6 Cytokine Family in Epithelial-Mesenchymal Plasticity in Cancer Progression. Int. J. Mol. Sci. 2021, 22, 8334. [Google Scholar] [CrossRef]
- Dahler, A.L.; Cavanagh, L.L.; Saunders, N.A. Suppression of Keratinocyte Growth and Differentiation by Transforming Growth Factor β1 Involves Multiple Signaling Pathways. J. Investig. Dermatol. 2001, 116, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Hatta, M.; Miyake, Y.; Uchida, K.; Yamazaki, J. Keratin 13 gene is epigenetically suppressed during transforming growth factor-β1-induced epithelial-mesenchymal transition in a human keratinocyte cell line. Biochem. Biophys. Res. Commun. 2018, 496, 381–386. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amusan, O.T.; Lopez, R.; Burks, E.; Trammel, J.; Raikhy, G.; Guo, H.; Bodily, J. Stromal Interferon Regulatory Factor 3 Can Antagonize Human Papillomavirus Replication by Supporting Epithelial-to-Mesenchymal Transition. Viruses 2025, 17, 598. https://doi.org/10.3390/v17050598
Amusan OT, Lopez R, Burks E, Trammel J, Raikhy G, Guo H, Bodily J. Stromal Interferon Regulatory Factor 3 Can Antagonize Human Papillomavirus Replication by Supporting Epithelial-to-Mesenchymal Transition. Viruses. 2025; 17(5):598. https://doi.org/10.3390/v17050598
Chicago/Turabian StyleAmusan, Oluwamuyiwa T., Rebecca Lopez, Elijah Burks, Jessica Trammel, Gaurav Raikhy, Hongyan Guo, and Jason Bodily. 2025. "Stromal Interferon Regulatory Factor 3 Can Antagonize Human Papillomavirus Replication by Supporting Epithelial-to-Mesenchymal Transition" Viruses 17, no. 5: 598. https://doi.org/10.3390/v17050598
APA StyleAmusan, O. T., Lopez, R., Burks, E., Trammel, J., Raikhy, G., Guo, H., & Bodily, J. (2025). Stromal Interferon Regulatory Factor 3 Can Antagonize Human Papillomavirus Replication by Supporting Epithelial-to-Mesenchymal Transition. Viruses, 17(5), 598. https://doi.org/10.3390/v17050598






