A Standardised Method to Quantify the Infectious Titre of Rabbit Haemorrhagic Disease Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Rabbit Selection and Housing
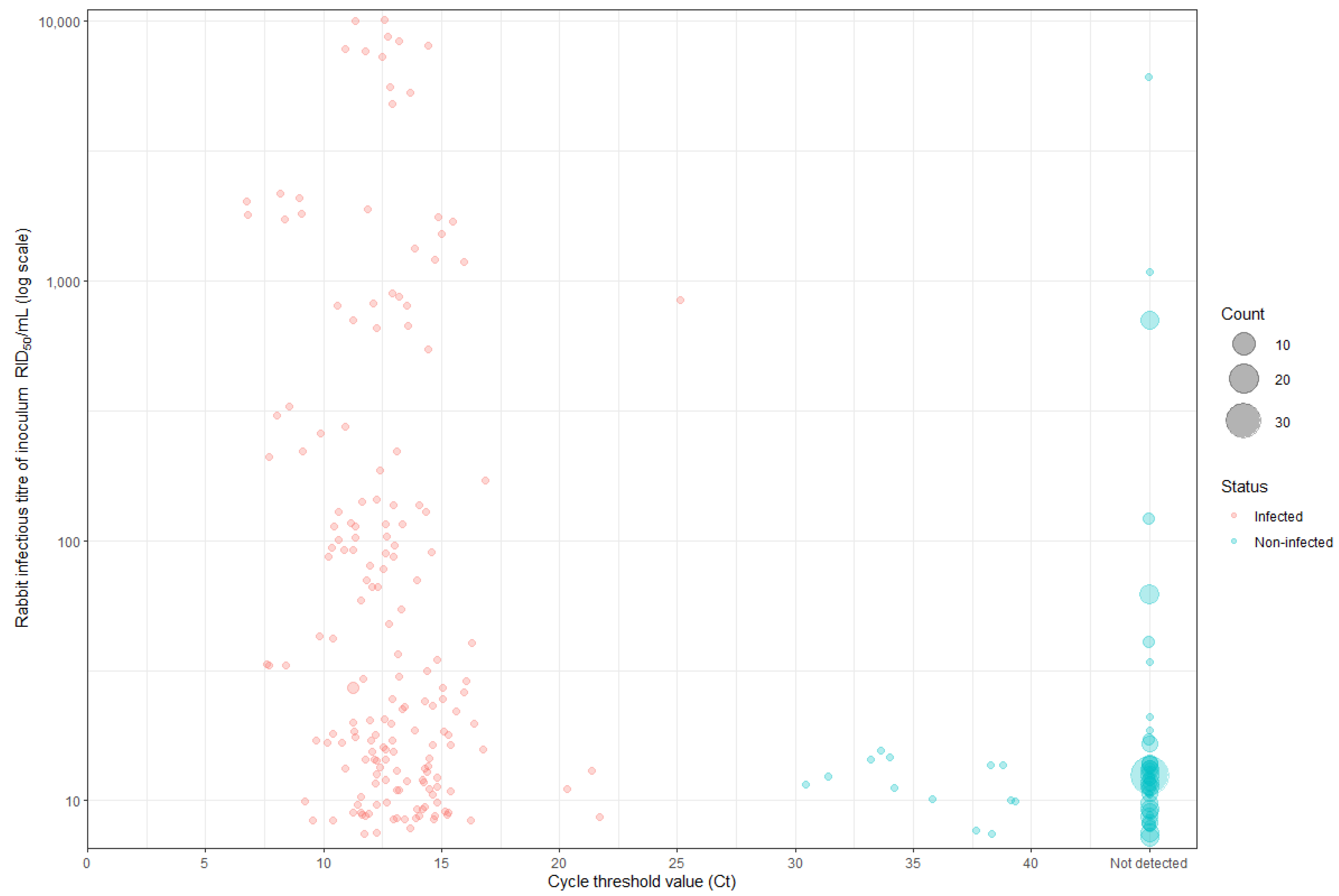
2.2. Criteria Used to Determine Infection Status
2.3. Calculating the Median Rabbit Infectious Dose and Infectious Titre of the RHDV Inoculum
2.4. Assessing the Agreement Between the Calculation Approaches for RID50
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ct | Cycle-threshold value |
| DB | Dragstedt–Behrens |
| G | Genogroup |
| ICC | Intra-class correlation coefficient |
| RHDV | Rabbit haemorrhagic disease virus |
| RID50 | Median rabbit infectious dose |
| RM | Reed–Muench |
| RT-qPCR | Reverse transcription real-time PCR |
| SK | Spearman–Kärber |
| TCID50 | Median tissue culture infectious dose |
Appendix A
| Dose | log10 Dilution Factor | Counts | Cumulative | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Infected | Non- Infected | Total | Proportion Infected (Infected/Total) | Infected | Non- Infected | Total | Proportion Infected (Infected/Total) | ||
| 1 | −4 | 0 | 6 | 6 | 0 | 0 | 13 | 13 | 0 |
| 2 | −3 | 1 | 5 | 6 | 0.167 | 1 | 7 | 8 | 0.125 |
| 3 | −2 | 4 | 2 | 6 | 0.667 | 5 | 2 | 7 | 0.714 |
| 4 | −1 | 6 | 0 | 6 | 1 | 11 | 0 | 11 | 1 |

References
- Capucci, L.; Cavadini, P.; Lavazza, A. Viral haemorrhagic disease: RHDV type 2 ten years later. World Rabbit. Sci. 2022, 30, 1–11. [Google Scholar] [CrossRef]
- Lavazza, A.; Cavadini, P.; Barbieri, I.; Tizzani, P.; Pinheiro, A.; Abrantes, J.; Esteves, P.J.; Grilli, G.; Gioia, E.; Zanoni, M.; et al. Field and experimental data indicate that the eastern cottontail (Sylvilagus floridanus) is susceptible to infection with European brown hare syndrome (EBHS) virus and not with rabbit haemorrhagic disease (RHD) virus. Vet. Res. 2015, 46, 13. [Google Scholar] [CrossRef]
- Puggioni, G.; Cavadini, P.; Maestrale, C.; Scivoli, R.; Botti, G.; Ligios, C.; Le Gall-Reculé, G.; Lavazza, A.; Capucci, L. The new French 2010 Rabbit Hemorrhagic Disease Virus causes an RHD-like disease in the Sardinian Cape hare (Lepus capensis mediterraneus). Vet. Res. 2013, 44, 96. [Google Scholar] [CrossRef] [PubMed]
- Camarda, A.; Pugliese, N.; Cavadini, P.; Circella, E.; Capucci, L.; Caroli, A.; Legretto, M.; Mallia, E.; Lavazza, A. Detection of the new emerging rabbit haemorrhagic disease type 2 virus (RHDV2) in Sicily from rabbit (Oryctolagus cuniculus) and Italian hare (Lepus corsicanus). Res. Vet. Sci. 2014, 97, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Velarde, R.; Cavadini, P.; Neimanis, A.; Cabezón, O.; Chiari, M.; Gaffuri, A.; Lavín, S.; Grilli, G.; Gavier-Widén, D.; Lavazza, A.; et al. Spillover Events of Infection of Brown Hares (Lepus europaeus) with Rabbit Haemorrhagic Disease Type 2 Virus (RHDV2) Caused Sporadic Cases of an European Brown Hare Syndrome-Like Disease in Italy and Spain. Transbound. Emerg. Dis. 2017, 64, 1750–1761. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neimanis, A.S.; Ahola, H.; Larsson Pettersson, U.; Lopes, A.M.; Abrantes, J.; Zohari, S.; Esteves, P.J.; Gavier-Widén, D. Overcoming species barriers: An outbreak of Lagovirus europaeus GI.2/RHDV2 in an isolated population of mountain hares (Lepus timidus). BMC Vet. Res. 2018, 14, 367. [Google Scholar] [CrossRef]
- Hall, R.N.; King, T.; O’Connor, T.; Read, A.J.; Arrow, J.; Trought, K.; Duckworth, J.; Piper, M.; Strive, T. Age and Infectious Dose Significantly Affect Disease Progression after RHDV2 Infection in Naïve Domestic Rabbits. Viruses 2021, 13, 1184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tu, T.; Zhou, Y.; Jiang, D.; Pang, M.; Wu, X.; Yao, X.; Luo, Y.; Yang, Z.; Ren, M.; Wang, Y. The pathogenicity comparison of Lagovirus europaeus GI.1 and GI.2 strains in China by using relative quantitative assay. Sci. Rep. 2022, 12, 20518. [Google Scholar] [CrossRef]
- Elsworth, P.; Cooke, B.D.; Kovaliski, J.; Sinclair, R.; Holmes, E.C.; Strive, T. Increased virulence of rabbit haemorrhagic disease virus associated with genetic resistance in wild Australian rabbits (Oryctolagus cuniculus). Virology 2014, 464, 415–423. [Google Scholar] [CrossRef]
- Mohamed, F.; Gidlewski, T.; Berninger, M.L.; Petrowski, H.M.; Bracht, A.J.; de Rueda, C.B.; Barrette, R.W.; Grady, M.; O’Hearn, E.S.; Lewis, C.E.; et al. Comparative susceptibility of eastern cottontails and New Zealand white rabbits to classical rabbit haemorrhagic disease virus (RHDV) and RHDV2. Transbound. Emerg. Dis. 2022, 69, e968–e978. [Google Scholar] [CrossRef]
- Droillard, C.; Lemaitre, E.; Amelot, M.; Blanchard, Y.; Keita, A.; Eterradossi, N.; Le Gall-Reculé, G. Rabbit haemorrhagic disease virus Lagovirus europaeus/GI.1d strain: Genome sequencing, in vivo virus replication kinetics, and viral dose effect. BMC Vet. Res. 2021, 17, 257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Le Gall-Reculé, G.; Lavazza, A.; Marchandeau, S.; Bertagnoli, S.; Zwingelstein, F.; Cavadini, P.; Martinelli, N.; Lombardi, G.; Guérin, J.-L.; Lemaitre, E.; et al. Emergence of a new lagovirus related to Rabbit Haemorrhagic Disease Virus. Vet. Res. 2013, 44, 81. [Google Scholar] [CrossRef] [PubMed]
- Kieran, T.J.; Sun, X.; Maines, T.R.; Beauchemin, C.A.A.; Belser, J.A. Exploring associations between viral titer measurements and disease outcomes in ferrets inoculated with 125 contemporary influenza A viruses. J. Virol. 2024, 98, e0166123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saganuwan, S.A. Application of median lethal concentration (LC(50)) of pathogenic microorganisms and their antigens in vaccine development. BMC Res. Notes 2020, 13, 289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Read, A.J.; Kirkland, P.D. Efficacy of a commercial vaccine against different strains of rabbit haemorrhagic disease virus. Aust. Vet. J. 2017, 95, 223–226. [Google Scholar] [CrossRef]
- Armitage, P.; Allen, I. Methods of estimating the LD 50 in quantal response data. Epidemiol. Infect. 1950, 48, 298–322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Finney, D. Statistical Method in Biological Assay, 2nd ed; Griffin: London, UK, 1964. [Google Scholar]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Behrens, B. Zur Auswertung der Digitalisblätter im Froschversuch. Naunyn-Schmiedebergs Arch. Für Exp. Pathol. Und Pharmakologie 1929, 140, 237–256. [Google Scholar] [CrossRef]
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Spearman, C. The method of “right and wrong cases” (constant stimuli) without Gauss’s formula. Br. J. Psychol. 1908, 2, 227–242. [Google Scholar] [CrossRef]
- Miller, R.G. Nonparametric Estimators of the Mean Tolerance in Bioassay. Biometrika 1973, 60, 535–542. [Google Scholar] [CrossRef]
- Ramakrishnan, M.A. Determination of 50% endpoint titer using a simple formula. World J. Virol. 2016, 5, 85–86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Labarre, D.D.; Lowy, R.J. Improvements in methods for calculating virus titer estimates from TCID50 and plaque assays. J. Virol. Methods 2001, 96, 107–126. [Google Scholar] [CrossRef]
- Bryan, W.R. Interpretation of host response in quantitative studies on animal viruses. Ann. N. Y. Acad. Sci. 1957, 69, 698–728. [Google Scholar] [CrossRef]
- Cresta, D.; Warren, D.C.; Quirouette, C.; Smith, A.P.; Lane, L.C.; Smith, A.M.; Beauchemin, C.A.A. Time to revisit the endpoint dilution assay and to replace the TCID50 as a measure of a virus sample’s infection concentration. PLoS Comput. Biol. 2021, 17, e1009480. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Dhanavelu, M. Influence of Reed-Muench Median Dose Calculation Method in Virology in the Millennium. Antivir. Res. 2018, 28, 16–18. [Google Scholar]
- O’Connor, T.W.; Read, A.J.; Hall, R.N.; Strive, T.; Kirkland, P.D. Immunological Cross-Protection between Different Rabbit Hemorrhagic Disease Viruses—Implications for Rabbit Biocontrol and Vaccine Development. Vaccines 2022, 10, 666. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Animal Research Review Panel. Guidelines for the Care and Management of Rabbits in Scientific Institutions in: Department of Primary Industries; New South Wales Government: Orange, Australia, 2023. [Google Scholar]
- Teixeira, L.; Marques, R.M.; Águas, A.P.; Ferreira, P.G. A simple and rapid method for isolation of caliciviruses from liver of infected rabbits. Res. Vet. Sci. 2011, 91, 164–166. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health (WOAH). Terrestrial Manual, Chapter 3.7.2 Rabbit Haemorrahgic Disease 2023. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.07.02_RHD.pdf (accessed on 28 February 2025).
- Gall, A.; Hoffmann, B.; Teifke, J.P.; Lange, B.; Schirrmeier, H. Persistence of viral RNA in rabbits which overcome an experimental RHDV infection detected by a highly sensitive multiplex real-time RT-PCR. Vet. Microbiol. 2007, 120, 17–32. [Google Scholar] [CrossRef]
- Hall, R.N.; Mahar, J.E.; Read, A.J.; Mourant, R.; Piper, M.; Huang, N.; Strive, T. A strain-specific multiplex RT-PCR for Australian rabbit haemorrhagic disease viruses uncovers a new recombinant virus variant in rabbits and hares. Transbound. Emerg. Dis. 2018, 65, e444–e456. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Siev, D.; Vendettuoli, M.; Kent, T. skrmdb: Package to estimate ED50. 4.5.0. 2024. Available online: https://github.com/ABS-dev/skrmdb (accessed on 18 October 2024).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: New York, NY, USA, 2002. [Google Scholar]
- Chang, W.; Cheng, J.; Allaire, J.J.; Sievert, C.; Schloerke, B.; Xie, Y.; Allen, J.; McPherson, J.; Dipert, A.; Borges, B. Shiny: Web Application Framework for R. 1.10.0. 2025. Available online: https://github.com/rstudio/shiny (accessed on 18 October 2024).
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Gamer, M.; Lemon, J.; Fellows, I.; Singh, P. irr: Various Coefficients of Interrater Reliability and Agreement. 0.84.1. 2019. Available online: https://CRAN.R-project.org/package=irr (accessed on 18 October 2024).
- Bartko, J. The intraclass correlation coefficient as a measure of reliability. Psychol. Rep. 1966, 19, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Šmíd, B.; Valíček, L.; Rodak, L.; Štěpánek, J.; Jurak, E.J.V.M. Rabbit haemorrhagic disease: An investigation of some properties of the virus and evaluation of an inactivated vaccine. Veter-Microbiol. 1991, 26, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Marcato, P.; Benazzi, C.; Vecchi, G.; Galeotti, M.; Della Salda, L.; Sarli, G.; Lucidi, P. Clinical and pathological features of viral haemorrhagic disease of rabbits and the European brown hare syndrome. Rev. Sci. Tech. l’OIE 1991, 10, 371–392. [Google Scholar] [CrossRef]
- Dalton, K.P.; Nicieza, I.; Balseiro, A.; Muguerza, M.A.; Rosell, J.M.; Casais, R.; Álvarez, Á.L.; Parra, F. Variant rabbit hemorrhagic disease virus in young rabbits, Spain. Emerg. Infect. Dis. 2012, 18, 2009–2012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cooke, B.D.; McPhee, S.; Robinson, A.J.; Capucci, L. Rabbit haemorrhagic disease: Does a pre-existing RHDV-like virus reduce the effectiveness of RHD as a biological control in Australia? Wildl. Res. 2002, 29, 673–682. [Google Scholar] [CrossRef]
- Baratelli, M.; Molist-Badiola, J.; Puigredon-Fontanet, A.; Pascual, M.; Boix, O.; Mora-Igual, F.X.; Woodward, M.; Lavazza, A.; Capucci, L. Characterization of the Maternally Derived Antibody Immunity against Rhdv-2 after Administration in Breeding Does of an Inactivated Vaccine. Vaccines 2020, 8, 484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elsworth, P.G.; Kovaliski, J.; Cooke, B.D. Rabbit haemorrhagic disease: Are Australian rabbits (Oryctolagus cuniculus) evolving resistance to infection with Czech CAPM 351 RHDV? Epidemiol. Infect. 2012, 140, 1972–1981. [Google Scholar] [CrossRef]
- Schwensow, N.; Pederson, S.; Peacock, D.; Cooke, B.; Cassey, P. Adaptive changes in the genomes of wild rabbits after 16 years of viral epidemics. Mol. Ecol. 2020, 29, 3777–3794. [Google Scholar] [CrossRef]
- Crespo, I.; Miguel, B.S.; Laliena, A.; Alvarez, M.; Culebras, J.M.; González-Gallego, J.; Tuñón, M.J. Melatonin prevents the decreased activity of antioxidant enzymes and activates nuclear erythroid 2-related factor 2 signaling in an animal model of fulminant hepatic failure of viral origin. J. Pineal Res. 2010, 49, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xue, H.; Pu, B.; Qian, N. A new viral disease in rabbits. Anim. Husb. Vet. Med. 1984, 16, 253–255. [Google Scholar]
- Bustin, S.A. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 2000, 25, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): Trends and problems. J. Mol. Endocrinol. 2002, 29, 23–39. [Google Scholar] [CrossRef]
- Cooke, B. Rabbit haemorrhagic disease: Field epidemiology and the management of wild rabbit populations. Rev. Sci. et Tech. de l’OIE 2002, 21, 347–358. [Google Scholar] [CrossRef]
- Abrantes, J.; van Der Loo, W.; Le Pendu, J.; Esteves, P.J. Rabbit haemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): A review. Vet. Res. 2012, 43, 1–19. [Google Scholar] [CrossRef]
- Matthaei, M.; Kerr, P.J.; Read, A.J.; Hick, P.; Haboury, S.; Wright, J.D.; Strive, T. Comparative quantitative monitoring of rabbit haemorrhagic disease viruses in rabbit kittens. Virol. J. 2014, 11, 109. [Google Scholar] [CrossRef]
- Lavazza, A.; Capucci, L. How Many Caliciviruses Are There in Rabbits? A Review on RHDV and Correlated Viruses, Lagomorph Biology: Evolution, Ecology, and Conservation; Springer: Berlin, Germany, 2008; pp. 263–278. [Google Scholar]
- Shien, J.H.; Shieh, H.K.; Lee, L.H. Experimental infections of rabbits with rabbit haemorrhagic disease virus monitored by polymerase chain reaction. Res. Vet. Sci. 2000, 68, 255–259. [Google Scholar] [CrossRef]
- Guittré, C.; Ruvoen-Clouet, N.; Barraud, L.; Cherel, Y.; Baginski, I.; Prave, M.; Ganiere, J.P.; Trépo, C.; Cova, L. Early Stages of Rabbit Haemorrhagic Disease Virus Infection Monitored by Polymerase Chain Reaction. J. Vet. Med. 1996, 43, 109–118. [Google Scholar] [CrossRef]
- Lei, C.; Yang, J.; Hu, J.; Sun, X. On the Calculation of TCID(50) for Quantitation of Virus Infectivity. Virol. Sin. 2021, 36, 141–144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Finney, D. Probit Analysis; Cambridge University Press: London, UK, 1971. [Google Scholar]

| Experiment | Reed–Muench | Dragstedt–Behrens | Spearman–Kärber | 95% CI | Probit | 95% CI |
|---|---|---|---|---|---|---|
| E1 | 3.60 | 3.62 | 3.67 | 2.84–4.50 | 4.01 | −∞ to +∞ |
| E2 | 4.97 | 4.93 | 5.00 | 4.60–5.40 | 5.19 | 4.65–5.47 |
| E3 | 4.20 | 4.21 | 4.37 | 3.79–4.94 | 4.23 | 1.85–5.06 |
| E4 | 4.63 | 4.58 | 4.51 | 3.81–5.22 | 6.35 | −∞ to +∞ |
| E5 | 4.00 | 4.00 | 4.00 | 3.55–4.45 | 4.00 | 3.60–5.43 |
| E6 | NA | NA | 4.17 | 3.75–4.59 | 4.07 | 3.76 to +∞ |
| E7 | 4.38 | 4.38 | 4.33 | 3.65–5.02 | 4.46 | 3.72–6.51 |
| E8 | 6.09 | 6.20 | 6.08 | 5.25–6.92 | 5.35 | 4.90–7.01 |
| E9 | 3.00 | 3.00 | 3.00 | 2.55–3.45 | 3.00 | 2.60–3.40 |
| E10 | 5.83 | 5.83 | 5.83 | 5.07–6.59 | 5.96 | 4.84–8.84 |
| E11 | 3.50 | 3.50 | 3.50 | 2.90–4.10 | 3.50 | 2.94–4.06 |
| E12 | 6.50 | 6.50 | 6.50 | 5.90–7.10 | 6.82 | 5.93 to +∞ |
| E13 | 3.50 | 3.50 | 3.50 | 3.03–3.97 | 3.50 | 2.90–3.96 |
| E14 | 3.50 | 3.50 | 3.50 | 2.90–4.10 | 3.45 | 1.99–4.06 |
| E15 | 3.33 | 3.36 | 3.33 | 2.80–3.87 | 3.31 | 2.05–3.84 |
| E16 | 6.47 | 6.48 | 6.47 | 5.95–6.99 | 6.47 | 5.92–7.11 |
| E17 | 6.43 | 6.45 | 6.50 | 5.80–7.20 | 6.53 | 5.65–7.38 |
| E18 | 3.57 | 3.59 | 3.67 | 2.92–4.41 | 2.69 | −∞ to +∞ |
| E19 | 3.71 | 3.69 | 3.67 | 3.05–4.28 | 3.61 | 2.16–4.31 |
| E20 | 4.71 | 4.68 | 4.70 | 4.07–5.33 | 4.76 | 4.12–7.93 |
| E21 | 3.13 | 3.17 | 3.10 | 2.61–3.59 | 3.04 | 2.12–3.51 |
| E22 | 6.50 | 6.45 | 6.33 | 5.56–7.11 | 9.43 | −∞ to +∞ |
| E23 | 4.00 | 4.00 | 4.00 | 3.55–4.45 | 4.00 | 3.60–4.40 |
| E24 | 4.00 | 4.00 | 4.00 | 3.55–4.45 | 4.00 | 3.60–4.40 |
| E25 | 3.70 | 3.70 | 3.83 | 3.20–4.47 | 3.78 | −∞ to +∞ |
| E26 | NA | NA | 7.33 | 7.00–7.67 | 7.17 | 6.95 to +∞ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Connor, T.W.; Collins, D.; Read, A.J.; Hick, P.M.; Kirkland, P.D. A Standardised Method to Quantify the Infectious Titre of Rabbit Haemorrhagic Disease Virus. Viruses 2025, 17, 609. https://doi.org/10.3390/v17050609
O’Connor TW, Collins D, Read AJ, Hick PM, Kirkland PD. A Standardised Method to Quantify the Infectious Titre of Rabbit Haemorrhagic Disease Virus. Viruses. 2025; 17(5):609. https://doi.org/10.3390/v17050609
Chicago/Turabian StyleO’Connor, Tiffany W., Damian Collins, Andrew J. Read, Paul M. Hick, and Peter D. Kirkland. 2025. "A Standardised Method to Quantify the Infectious Titre of Rabbit Haemorrhagic Disease Virus" Viruses 17, no. 5: 609. https://doi.org/10.3390/v17050609
APA StyleO’Connor, T. W., Collins, D., Read, A. J., Hick, P. M., & Kirkland, P. D. (2025). A Standardised Method to Quantify the Infectious Titre of Rabbit Haemorrhagic Disease Virus. Viruses, 17(5), 609. https://doi.org/10.3390/v17050609






