TREM-1-Linked Inflammatory Cargo in SARS-CoV-2-Stimulated Macrophage Extracellular Vesicles Drives Cellular Senescence and Impairs Antibacterial Defense
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Macrophage Differentiation In Vitro
2.2. Immunofluorescence for the SARS-CoV-2 N Protein and NLRP3
2.3. Isolation and Purification of Mφ-EVs
2.4. Characterization of Mφ-EVs by NTA, Zeta Sizer, and Transmission Electron Microscopy (TEM)
2.5. Atomic Force Microscopy (AFM)
2.6. Phenotypic Analysis of Mφ-EVs by Flow Cytometry
2.7. Prophylactic Treatment of Cells with Different Populations of EVs
2.8. Cytokines Quantification
2.9. Bacteria Killing Assay
2.10. Protein Concentration and Proteome Membrane-Based Immunoassay of EVs
2.11. RNA Extraction and qPCR
2.12. Flex Real-Time PCR for miRNA
2.13. Statistical Analysis
3. Results
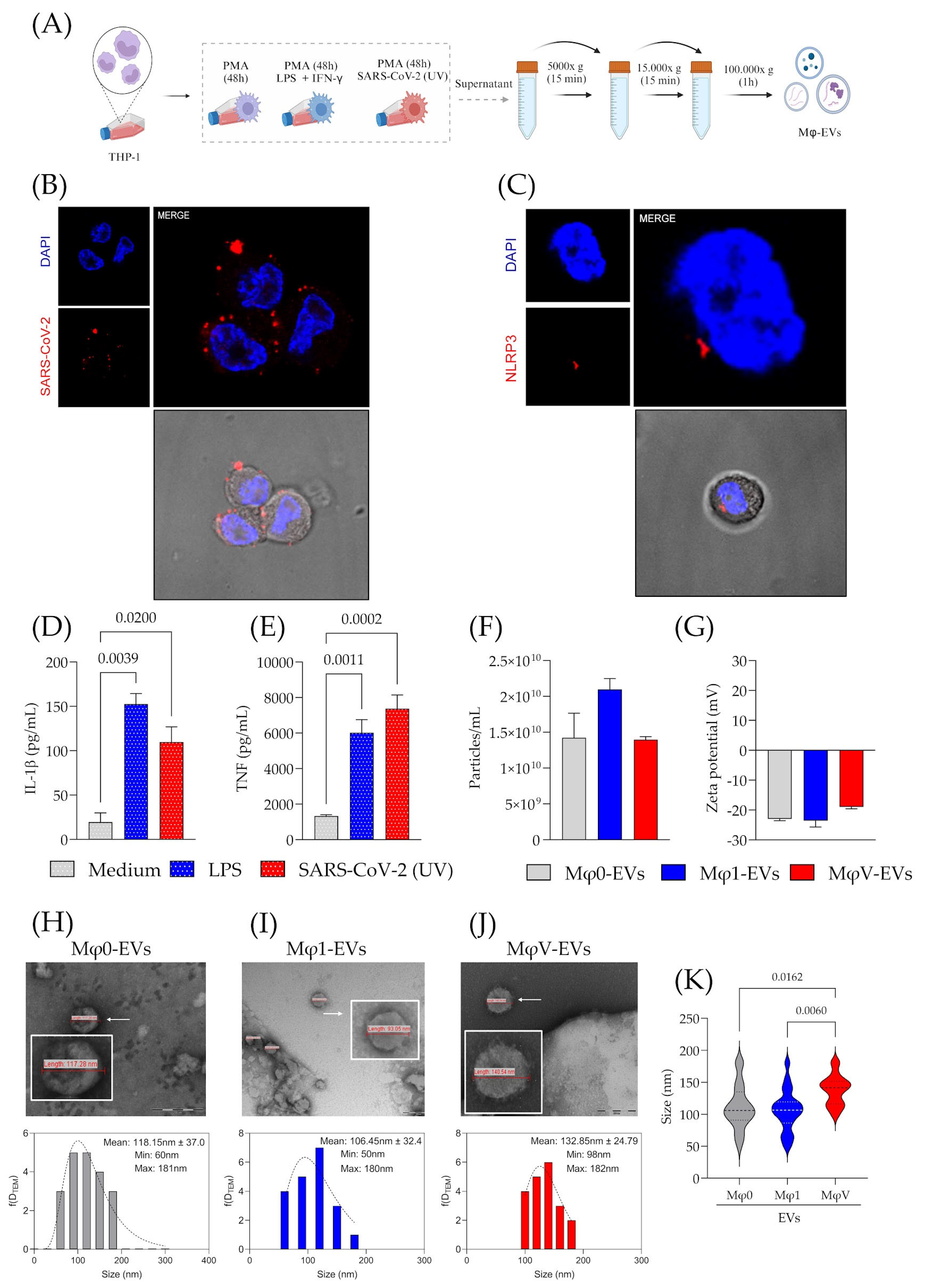
3.1. Inactivated SARS-CoV-2 Particles Modulate Macrophage Immune Response and Macrophage-Derived EVs Size
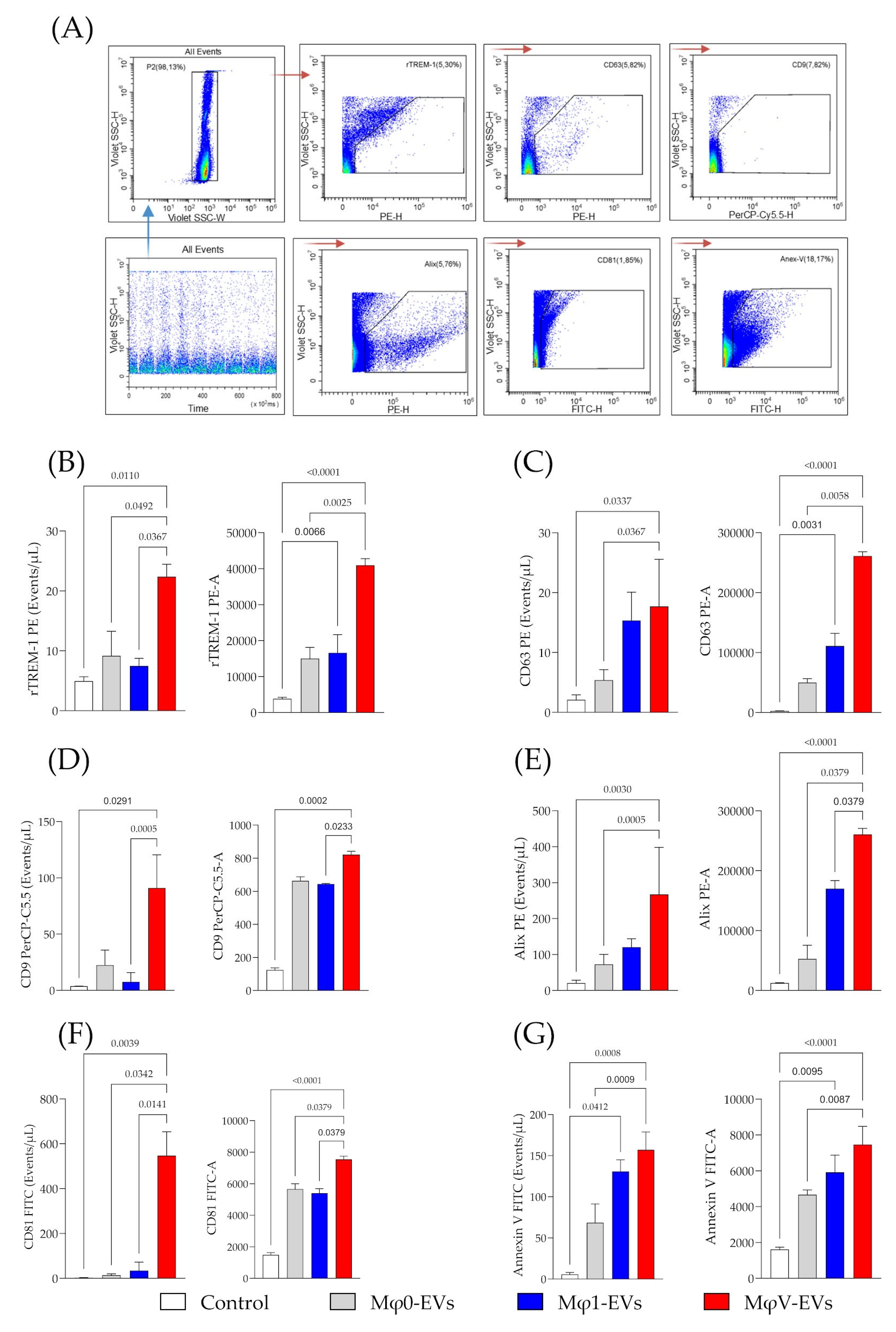
3.2. Determination of Classical Markers and TREM-1 Expression in Macrophage-Derived EVs Stimulated by SARS-CoV-2 Particles
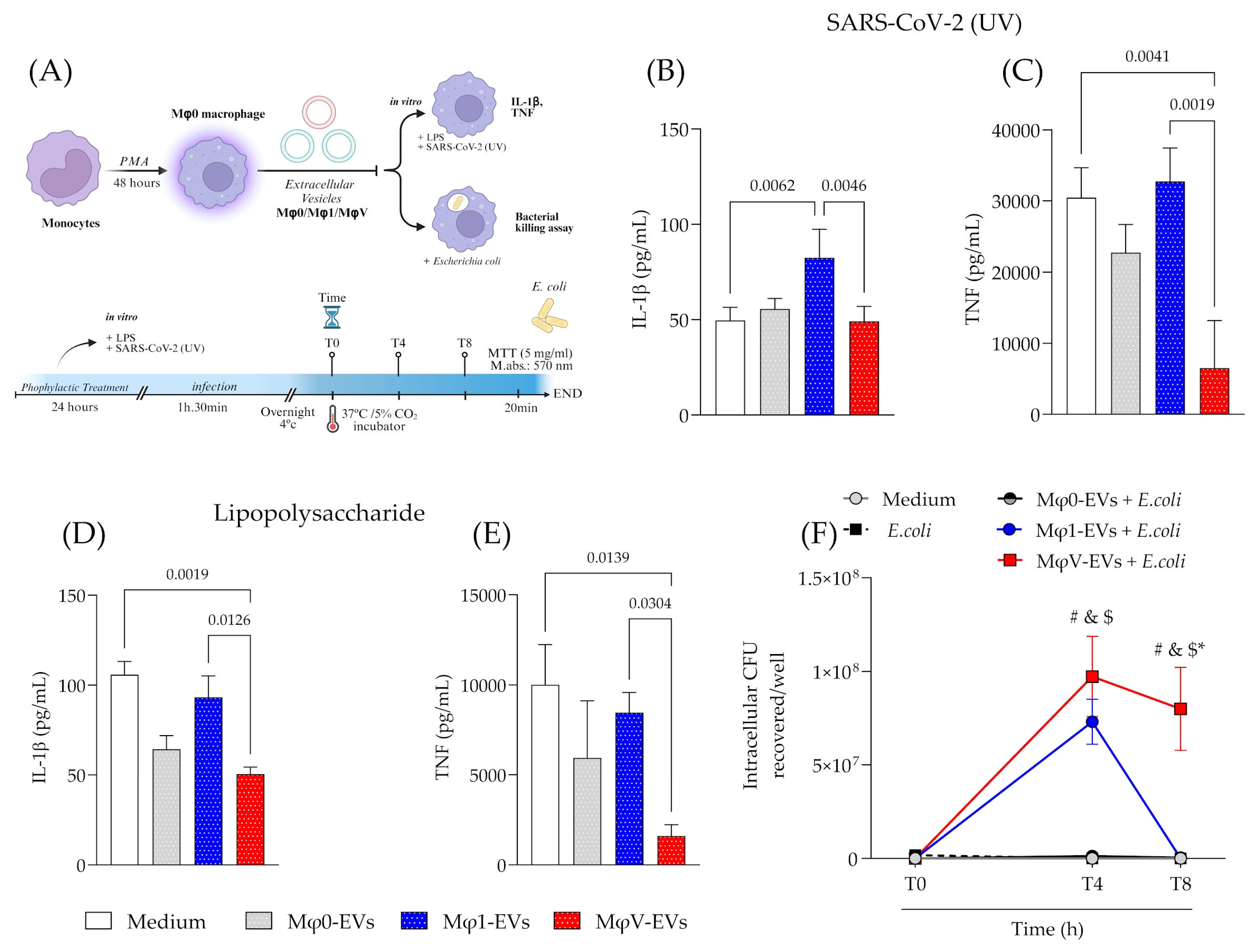
3.3. Prophylactic Treatment with EVs Modulates the Inflammatory Response and Bacterial Killing Activity of Macrophages
3.4. EVs from SARS-CoV-2-Stimulated Macrophage Display a Distinct Proteomic Profile and Enhanced Expression of Specific miRNA
3.5. Treatment with MφV-EVs Markedly Affected the Expression of Genes Involved in Senescence Pathways, Particularly CDKN2A and TP53, in Macrophages
4. Discussion
5. Conclusions
Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA-J. Am. Med. Assoc. 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-Acute COVID-19 Syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Bartleson, J.M.; Radenkovic, D.; Covarrubias, A.J.; Furman, D.; Winer, D.A.; Verdin, E. SARS-CoV-2, COVID-19 and the Aging Immune System. Nat. Aging 2021, 1, 769–782. [Google Scholar] [CrossRef]
- Tammaro, A.; Derive, M.; Gibot, S.; Leemans, J.C.; Florquin, S.; Dessing, M.C. TREM-1 and Its Potential Ligands in Non-Infectious Diseases: From Biology to Clinical Perspectives. Pharmacol. Ther. 2017, 177, 81–95. [Google Scholar] [CrossRef]
- Bouchon, A.; Dietrich, J.; Colonna, M. Cutting Edge: Inflammatory Responses Can Be Triggered by TREM-1, a Novel Receptor Expressed on Neutrophils and Monocytes. J. Immunol. 2000, 164, 4991–4995. [Google Scholar] [CrossRef]
- Kerget, F.; Kerget, B.; İba Yılmaz, S.; Kızıltunç, A. Evaluation of the Relationship between TREM-1/TREM-2 Ratio and Clinical Course in COVID-19 Pneumonia. Int. J. Clin. Pract. 2021, 75, e14697. [Google Scholar] [CrossRef]
- de Oliveira, Y.L.M.; de Sá Resende, A.; Martins-Filho, P.R.; de Moura, T.R. Role of Triggering Receptor Expressed on Myeloid Cells-1 (TREM-1) in COVID-19 and Other Viral Pneumonias: A Systematic Review and Meta-Analysis of Clinical Studies. SSRN Electron. J. 2022, 30, 1037–1045. [Google Scholar] [CrossRef]
- da Silva-Neto, P.V.; de Carvalho, J.C.S.; Pimentel, V.E.; Pérez, M.M.; Toro, D.M.; Fraga-Silva, T.F.C.; Fuzo, C.A.; Oliveira, C.N.S.; Rodrigues, L.C.; Argolo, J.G.M.; et al. STREM-1 Predicts Disease Severity and Mortality in COVID-19 Patients: Involvement of Peripheral Blood Leukocytes and MMP-8 Activity. Viruses 2021, 13, 2521. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Cheng, Z.; Huang, Z.; Yang, Y.; Sun, N.; Hu, B.; Hou, P.; Liu, B.; Huang, C.; Liu, S. TREM-1, TREM-2 and Their Association with Disease Severity in Patients with COVID-19. Ann. Med. 2023, 55, 2269558. [Google Scholar] [CrossRef] [PubMed]
- Leyfman, Y.; Gohring, G.; Joshi, M.; Menon, G.P.; Van de Kieft, A.; del Rivero, T.; Bellio, M.A.; Mitrani, M.I. Extracellular Vesicles: A Promising Therapy against SARS-CoV-2 Infection. Mol. Ther. 2023, 31, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Buzas, E.I. The Roles of Extracellular Vesicles in the Immune System. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, W.; Wang, Y.; Guo, Q.; Zhao, F.; Zhu, Z.; Xing, Y.; Zhang, H.; Aljofan, M.; Jarrahi, A.M.; et al. The Potential Role of Extracellular Vesicles in COVID-19 Treatment: Opportunity and Challenge. Front. Mol. Biosci. 2021, 8, 699929. [Google Scholar] [CrossRef]
- Tahyra, A.S.C.; Calado, R.T.; Almeida, F. The Role of Extracellular Vesicles in COVID-19 Pathology. Cells 2022, 11, 2496. [Google Scholar] [CrossRef]
- Wang, L.; Hong, W.; Zhu, H.; He, Q.; Yang, B.; Wang, J.; Weng, Q. Macrophage Senescence in Health and Diseases. Acta Pharm. Sin. B 2024, 14, 1508–1524. [Google Scholar] [CrossRef]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L.; et al. A Proteomic Atlas of Senescence-Associated Secretomes for Aging Biomarker Development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, C.; Han, Y.; Gu, Z.; Sun, C. Immunosenescence, Aging and Successful Aging. Front. Immunol. 2022, 13, 942796. [Google Scholar] [CrossRef]
- Lund, M.E.; To, J.; O’Brien, B.A.; Donnelly, S. The Choice of Phorbol 12-Myristate 13-Acetate Differentiation Protocol Influences the Response of THP-1 Macrophages to a pro-Inflammatory Stimulus. J. Immunol. Methods 2016, 430, 64–70. [Google Scholar] [CrossRef]
- Daigneault, M.; Preston, J.A.; Marriott, H.M.; Whyte, M.K.B.; Dockrell, D.H. The Identification of Markers of Macrophage Differentiation in PMA-Stimulated THP-1 Cells and Monocyte-Derived Macrophages. PLoS ONE 2010, 5, e8668. [Google Scholar] [CrossRef] [PubMed]
- Ngoungoure, F.P.; Owona, B.A. Withaferin A Modulates AIM2 Inflammasome and Caspase-1 Expression in THP-1 Polarized Macrophages. Exp. Cell Res. 2019, 383, 111564. [Google Scholar] [CrossRef]
- Mohd Yasin, Z.N.; Mohd Idrus, F.N.; Hoe, C.H.; Yvonne-Tee, G.B. Macrophage Polarization in THP-1 Cell Line and Primary Monocytes: A Systematic Review. Differentiation 2022, 128, 67–82. [Google Scholar] [CrossRef]
- Genin, M.; Clement, F.; Fattaccioli, A.; Raes, M.; Michiels, C. M1 and M2 Macrophages Derived from THP-1 Cells Differentially Modulate the Response of Cancer Cells to Etoposide. BMC Cancer 2015, 15, 577. [Google Scholar] [CrossRef]
- Kornilov, R.; Puhka, M.; Mannerström, B.; Hiidenmaa, H.; Peltoniemi, H.; Siljander, P.; Seppänen-Kaijansinkko, R.; Kaur, S. Efficient Ultrafiltration-based Protocol to Deplete Extracellular Vesicles from Fetal Bovine Serum. J. Extracell. Vesicles 2018, 7, 1422674. [Google Scholar] [CrossRef]
- Drevets, D.A.; Canono, B.P.; Campbell, P.A. Measurement of Bacterial Ingestion and Killing by Macrophages. Curr. Protoc. Immunol. 2015, 109, 14.6.1–14.6.17. [Google Scholar] [CrossRef]
- Sukumaran, S.K.; Shimada, H.; Prasadarao, N.V. Entry and Intracellular Replication of Escherichia Coli K1 in Macrophages Require Expression of Outer Membrane Protein A. Infect. Immun. 2003, 71, 5951–5961. [Google Scholar] [CrossRef]
- Zheng, M.; Karki, R.; Williams, E.P.; Yang, D.; Fitzpatrick, E.; Vogel, P.; Jonsson, C.B.; Kanneganti, T.D. TLR2 Senses the SARS-CoV-2 Envelope Protein to Produce Inflammatory Cytokines. Nat. Immunol. 2021, 22, 829–838. [Google Scholar] [CrossRef]
- Bi, Z.; Hong, W.; Que, H.; He, C.; Ren, W.; Yang, J.; Lu, T.; Chen, L.; Lu, S.; Peng, X.; et al. Inactivated SARS-CoV-2 Induces Acute Respiratory Distress Syndrome in Human ACE2-Transgenic Mice. Signal Transduct. Target. Ther. 2021, 6, 439. [Google Scholar] [CrossRef]
- de Souza, W.D.F.; Zorzella-Pezavento, S.F.G.; Ayupe, M.C.; Salgado, C.L.; Oliveira, B.d.C.; Moreira, F.; da Silva, G.W.; Muraro, S.P.; de Souza, G.F.; Proença-Módena, J.L.; et al. Lung Inflammation Induced by Inactivated SARS-CoV-2 in C57BL/6 Female Mice Is Controlled by Intranasal Instillation of Vitamin D. Cells 2023, 12, 1092. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major Findings, Mechanisms and Recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Postlethwaite, A.; Raghow, R.; Stricklin, G.; Poppleton, H.; Seyer, J.; Kang, A. Modulation of Fibroblast Functions by Interleukin 1: Increased Steady-State Accumulation of Type I Procollagen Messenger RNAs and Stimulation of Other Functions but Not Chemotaxis by Human Recombinant Interleukin 1 Alpha and Beta. J. Cell Biol. 1988, 106, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Sangtian, S.; Anderson, S.M.; Snyder, R.J.; Marshburn, J.D.; Rice, A.B.; Bonner, J.C.; Garantziotis, S. Inflammasome Activation in Airway Epithelial Cells after Multi-Walled Carbon Nanotube Exposure Mediates a Profibrotic Response in Lung Fibroblasts. Part. Fibre Toxicol. 2014, 11, 28. [Google Scholar] [CrossRef]
- de Sá, K.S.G.; Amaral, L.A.; Rodrigues, T.S.; Caetano, C.C.S.; Becerra, A.; Batah, S.S.; Lopes, F.T.; de Oliveira, I.M.; Lopes, L.S.; Almeida, L.; et al. Pulmonary Inflammation and Viral Replication Define Distinct Clinical Outcomes in Fatal Cases of COVID-19. PLoS Pathog. 2024, 20, e1012222. [Google Scholar] [CrossRef]
- Gheitasi, H.; Sabbaghian, M.; Shekarchi, A.A.; Mirmazhary, A.A.; Poortahmasebi, V. Exosome-Mediated Regulation of Inflammatory Pathway during Respiratory Viral Disease. Virol. J. 2024, 21, 30. [Google Scholar] [CrossRef]
- Dixson, A.C.; Dawson, T.R.; Di Vizio, D.; Weaver, A.M. Context-Specific Regulation of Extracellular Vesicle Biogenesis and Cargo Selection. Nat. Rev. Mol. Cell Biol. 2023, 24, 454–476. [Google Scholar] [CrossRef]
- Reinicke, M.; Shamkeeva, S.; Hell, M.; Isermann, B.; Ceglarek, U.; Heinemann, M.L. Targeted Lipidomics for Characterization of PUFAs and Eicosanoids in Extracellular Vesicles. Nutrients 2022, 14, 1319. [Google Scholar] [CrossRef]
- Yang, M.; Walker, S.A.; de León, J.S.A.D.; Davidovich, I.; Broad, K.; Talmon, Y.; Borges, C.R.; Wolfram, J. Extracellular Vesicle Glucose Transporter-1 and Glycan Features in Monocyte-Endothelial Inflammatory Interactions. Nanomedicine 2022, 42, 102515. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular Vesicles and Nanoparticles: Emerging Complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef]
- Stoorvogel, W.; Kleijmeer, M.J.; Geuze, H.J.; Raposo, G. The Biogenesis and Functions of Exosomes. Traffic 2002, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Bobrie, A.; Colombo, M.; Krumeich, S.; Raposo, G.; Théry, C. Diverse Subpopulations of Vesicles Secreted by Different Intracellular Mechanisms Are Present in Exosome Preparations Obtained by Differential Ultracentrifugation. J. Extracell. Vesicles 2012, 1, 18397. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-P.; Hertzberg, M.; Jiang, Y.; Graves, D.T. Interleukin-1 and Tumor Necrosis Factor Receptor Signaling Is Not Required for Bacteria-Induced Osteoclastogenesis and Bone Loss but Is Essential for Protecting the Host from a Mixed Anaerobic Infection. Am. J. Pathol. 1999, 155, 2145–2152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Paolo, N.C.; Shafiani, S.; Day, T.; Papayannopoulou, T.; Russell, D.W.; Iwakura, Y.; Sherman, D.; Urdahl, K.; Shayakhmetov, D.M. Interdependence between Interleukin-1 and Tumor Necrosis Factor Regulates TNF-Dependent Control of Mycobacterium Tuberculosis Infection. Immunity 2015, 43, 1125–1136. [Google Scholar] [CrossRef]
- Agius, E.; Lacy, K.E.; Vukmanovic-Stejic, M.; Jagger, A.L.; Papageorgiou, A.-P.; Hall, S.; Reed, J.R.; Curnow, S.J.; Fuentes-Duculan, J.; Buckley, C.D.; et al. Decreased TNF-α Synthesis by Macrophages Restricts Cutaneous Immunosurveillance by Memory CD4+ T Cells during Aging. J. Exp. Med. 2009, 206, 1929–1940. [Google Scholar] [CrossRef]
- Evangelou, K.; Veroutis, D.; Paschalaki, K.; Foukas, P.G.; Lagopati, N.; Dimitriou, M.; Papaspyropoulos, A.; Konda, B.; Hazapis, O.; Polyzou, A.; et al. Pulmonary Infection by SARS-CoV-2 Induces Senescence Accompanied by an Inflammatory Phenotype in Severe COVID-19: Possible Implications for Viral Mutagenesis. Eur. Respir. J. 2022, 60, 2102951. [Google Scholar] [CrossRef] [PubMed]
- Chettimada, S.; Lorenz, D.R.; Misra, V.; Wolinsky, S.M.; Gabuzda, D. Small RNA Sequencing of Extracellular Vesicles Identifies Circulating MiRNAs Related to Inflammation and Oxidative Stress in HIV Patients. BMC Immunol. 2020, 21, 57. [Google Scholar] [CrossRef]
- Takasugi, M. Emerging Roles of Extracellular Vesicles in Cellular Senescence and Aging. Aging Cell 2018, 17, e12734. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Marangio, A.; Biccari, A.; D’Angelo, E.; Sensi, F.; Spolverato, G.; Pucciarelli, S.; Agostini, M. The Study of the Extracellular Matrix in Chronic Inflammation: A Way to Prevent Cancer Initiation? Cancers 2022, 14, 5903. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.Y.F.; Clancy, J.P.; Peng, N.; Li, Y.; Szul, T.J.; Xu, X.; Oster, R.; Sullender, W.; Ambalavanan, N.; Blalock, J.E.; et al. Pulmonary Matrix Metalloproteinase-9 Activity in Mechanically Ventilated Children with Respiratory Syncytial Virus. Eur. Respir. J. 2014, 43, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Taraboletti, G.; D’Ascenzo, S.; Borsotti, P.; Giavazzi, R.; Pavan, A.; Dolo, V. Shedding of the Matrix Metalloproteinases MMP-2, MMP-9, and MT1-MMP as Membrane Vesicle-Associated Components by Endothelial Cells. Am. J. Pathol. 2002, 160, 673–680. [Google Scholar] [CrossRef]
- Shimoda, M.; Khokha, R. Proteolytic Factors in Exosomes. Proteomics 2013, 13, 1624–1636. [Google Scholar] [CrossRef]
- Zucker, S.; Wieman, J.M.; Lysik, R.M.; Wilkie, D.P.; Ramamurthy, N.; Lane, B. Metastatic Mouse Melanoma Cells Release Collagen-Gelatin Degrading Metalloproteinases as Components of Shed Membrane Vesicles. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1987, 924, 225–237. [Google Scholar] [CrossRef]
- Murayama, T.; Kataoka, H.; Koita, H.; Nabeshima, K.; Koono, M. Glycocalyceal Bodies in a Human Rectal Carcinoma Cell Line and Their Interstitial Collagenolytic Activities. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1991, 60, 263–270. [Google Scholar] [CrossRef]
- Yuan, Z.; Syed, M.; Panchal, D.; Joo, M.; Bedi, C.; Lim, S.; Onyuksel, H.; Rubinstein, I.; Colonna, M.; Sadikot, R.T. TREM-1-Accentuated Lung Injury via MiR-155 Is Inhibited by LP17 Nanomedicine. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2016, 310, L426–L438. [Google Scholar] [CrossRef]
- Mashima, R. Physiological Roles of MiR-155. Immunology 2015, 145, 323–333. [Google Scholar] [CrossRef]
- Boucher, J.; Rousseau, A.; Boucher, C.; Subra, C.; Bazié, W.W.; Hubert, A.; Bourgeault, E.; Benmoussa, A.; Goyer, B.; Tessier, P.A.; et al. Immune Cells Release MicroRNA-155 Enriched Extracellular Vesicles That Promote HIV-1 Infection. Cells 2023, 12, 466. [Google Scholar] [CrossRef]
- Bazié, W.W.; Boucher, J.; Goyer, B.; Traoré, I.T.; Kania, D.; Somé, D.Y.; Alary, M.; Gilbert, C. Plasma Vesicular MiR-155 as a Biomarker of Immune Activation in Antiretroviral Treated People Living with HIV. Front. Immunol. 2022, 13, 916599. [Google Scholar] [CrossRef]
- Bazié, W.W.; Boucher, J.; Traoré, I.T.; Kania, D.; Somé, D.Y.; Alary, M.; Gilbert, C. Vesicular MicroRNA as Potential Biomarkers of Viral Rebound. Cells 2022, 11, 859. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yu, Y.; Tan, S. The Role of the MiR-21-5p-mediated Inflammatory Pathway in Ulcerative Colitis. Exp. Ther. Med. 2019, 19, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Behmoaras, J.; Gil, J. Similarities and Interplay between Senescent Cells and Macrophages. J. Cell Biol. 2021, 220, e202010162. [Google Scholar] [CrossRef]
- Iakovou, E.; Kourti, M. A Comprehensive Overview of the Complex Role of Oxidative Stress in Aging, The Contributing Environmental Stressors and Emerging Antioxidant Therapeutic Interventions. Front. Aging Neurosci. 2022, 14, 827900. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Souroullas, G.P.; Diekman, B.O.; Krishnamurthy, J.; Hall, B.M.; Sorrentino, J.A.; Parker, J.S.; Sessions, G.A.; Gudkov, A.V.; Sharpless, N.E. Cells Exhibiting Strong P16INK4a Promoter Activation in Vivo Display Features of Senescence. Proc. Natl. Acad. Sci. USA 2019, 116, 2603–2611. [Google Scholar] [CrossRef]
- Olivieri, F.; Rippo, M.R.; Monsurrò, V.; Salvioli, S.; Capri, M.; Procopio, A.D.; Franceschi, C. MicroRNAs Linking Inflamm-Aging, Cellular Senescence and Cancer. Ageing Res. Rev. 2013, 12, 1056–1068. [Google Scholar] [CrossRef]
- Mensà, E.; Guescini, M.; Giuliani, A.; Bacalini, M.G.; Ramini, D.; Corleone, G.; Ferracin, M.; Fulgenzi, G.; Graciotti, L.; Prattichizzo, F.; et al. Small Extracellular Vesicles Deliver MiR-21 and MiR-217 as Pro-senescence Effectors to Endothelial Cells. J. Extracell. Vesicles 2020, 9, 1725285. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva-Neto, P.V.; de Carvalho, J.C.S.; Toro, D.M.; Oliveira, B.T.M.; Cominal, J.G.; Castro, R.C.; Almeida, M.A.; Prado, C.M.; Arruda, E.; Frantz, F.G.; et al. TREM-1-Linked Inflammatory Cargo in SARS-CoV-2-Stimulated Macrophage Extracellular Vesicles Drives Cellular Senescence and Impairs Antibacterial Defense. Viruses 2025, 17, 610. https://doi.org/10.3390/v17050610
da Silva-Neto PV, de Carvalho JCS, Toro DM, Oliveira BTM, Cominal JG, Castro RC, Almeida MA, Prado CM, Arruda E, Frantz FG, et al. TREM-1-Linked Inflammatory Cargo in SARS-CoV-2-Stimulated Macrophage Extracellular Vesicles Drives Cellular Senescence and Impairs Antibacterial Defense. Viruses. 2025; 17(5):610. https://doi.org/10.3390/v17050610
Chicago/Turabian Styleda Silva-Neto, Pedro V., Jonatan C. S. de Carvalho, Diana M. Toro, Bianca T. M. Oliveira, Juçara G. Cominal, Ricardo C. Castro, Maria A. Almeida, Cibele M. Prado, Eurico Arruda, Fabiani G. Frantz, and et al. 2025. "TREM-1-Linked Inflammatory Cargo in SARS-CoV-2-Stimulated Macrophage Extracellular Vesicles Drives Cellular Senescence and Impairs Antibacterial Defense" Viruses 17, no. 5: 610. https://doi.org/10.3390/v17050610
APA Styleda Silva-Neto, P. V., de Carvalho, J. C. S., Toro, D. M., Oliveira, B. T. M., Cominal, J. G., Castro, R. C., Almeida, M. A., Prado, C. M., Arruda, E., Frantz, F. G., Ramos, A. P., Ciancaglini, P., Martins, R. B., da Silveira, J. C., Almeida, F., Malmegrim, K. C. R., & Sorgi, C. A. (2025). TREM-1-Linked Inflammatory Cargo in SARS-CoV-2-Stimulated Macrophage Extracellular Vesicles Drives Cellular Senescence and Impairs Antibacterial Defense. Viruses, 17(5), 610. https://doi.org/10.3390/v17050610











