Evaluation of Humoral and Cell-Mediated Immunity Induced by Quadrivalent Influenza Vaccine in Pre-COVID-19 Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants and Vaccines
2.2. Antigens
2.3. HAI Antibody Measurement
2.4. IFN-γ Assay
2.5. Evaluation of Changes in Antibody
2.6. Statistical Analyses
3. Results
3.1. Changes in Antibody Titers Post-Vaccination
3.1.1. Criterion 1: Proportion of Participants with HAI Titers ≥ 1:40
3.1.2. Criterion 2: Seroconversion or ≥4-Fold Increase in HAI Titers
3.2. Changes in CMI (IFN-γ)
3.3. Relationship Between HAI and CMI Before and After Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IIV4 | Quadrivalent inactivated influenza vaccine |
| HAI | Hemagglutination inhibition |
| CMI | Cell-mediated immunity |
| IFN-γ | Interferon-γ |
| ELISA | Enzyme-linked immunosorbent assay |
| GMTR | Geometric mean titer ratio |
| GMT | Geometric mean titer |
| HA | Hemagglutinin |
| GMC | Geometric mean concentration |
| GMCR | Geometric mean concentration ratio |
References
- IASR. Influenza, 2019/2020 Season; National Institute of Infectious Diseases: Shinjuku, Japan, 2020. Available online: https://www.niid.go.jp/niid/en/865-iasr/10759-489te.html (accessed on 1 November 2020).
- National Institute of Infectious Diseases. Weekly Reports of Influenza Virus Isolation/Detection, 2009/2010–2019/2020. Available online: https://www.niid.go.jp/niid/images/iasr/rapid/inf3/2019_36w/in1j_2021331.gif (accessed on 25 March 2025).
- Centers for Disease Control and Prevention. Estimated Flu Disease Burden 2019–2020 Flu Season. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/flu-burden/php/data-vis/2019-2020.html (accessed on 25 March 2025).
- Centers for Disease Control and Prevention. CDC Seasonal Flu Vaccine Effectiveness Studies. Available online: https://www.cdc.gov/flu-vaccines-work/php/effectiveness-studies/index.html (accessed on 25 March 2025).
- Tenforde, M.W.; Kondor, R.J.G.; Chung, J.R.; Zimmerman, R.K.; Nowalk, M.P.; Jackson, M.L.; Jackson, L.A.; Monto, A.S.; Martin, E.T.; Belongia, E.A.; et al. Effect of antigenic drift on influenza vaccine effectiveness in the United States-2019–2020. Clin. Infect. Dis. 2021, 73, e4244–e4250. [Google Scholar] [CrossRef] [PubMed]
- Dawood, F.S.; Chung, J.R.; Kim, S.S.; Zimmerman, R.K.; Nowalk, M.P.; Jackson, M.L.; Jackson, L.A.; Monto, A.S.; Martin, E.T.; Belongia, E.A.; et al. Interim estimates of 2019–20 seasonal influenza vaccine effectiveness—United States, February 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.; Kissling, E.; Emborg, H.D.; Larrauri, A.; McMenamin, J.; Pozo, F.; Trebbien, R.; Mazagatos, C.; Whitaker, H.; Valenciano, M.; et al. Interim 2019/20 influenza vaccine effectiveness: Six European studies, September 2019 to January 2020. Euro Surveill. 2020, 25, 2000153. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S.; Ishikane, M.; Matsunaga, N.; Morioka, S.; Yu, J.; Inagaki, T.; Yamamoto, M.; Ohmagari, N. Interim 2019/2020 Influenza Vaccine Effectiveness in Japan from October 2019 to January 2020. Jpn. J. Infect. Dis. 2021, 74, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; Grohskopf, L.A.; Sambhara, S.; Belser, J.A.; Katz, J.M.; Fry, A.M. Inactivated and recombinant influenza vaccines. In Plotkin’s Vaccines, 8th ed.; Elsevier: Philadelphia, PA, USA, 2023; pp. 514–551.e31. [Google Scholar] [CrossRef]
- McElhaney, J.E.; Xie, D.; Hager, W.D.; Barry, M.B.; Wang, Y.; Kleppinger, A.; Ewen, C.; Kane, K.P.; Bleackley, R.C. T cell responses are better correlates of vaccine protection in the elderly. J. Immunol. 2006, 176, 6333–6339. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.M.; Li, C.K.F.; Chui, C.S.C.; Huang, A.K.Y.; Perkins, M.; Liebner, J.C.; Lambkin-Williams, R.; Gilbert, A.; Oxford, J.; Nicholas, B.; et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 2012, 18, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Begom, S.; Bermingham, A.; Hoschler, K.; Adamson, W.; Carman, W.; Bean, T.; Barclay, W.; Deeks, J.J.; Lalvani, A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 2013, 19, 1305–1312. [Google Scholar] [CrossRef]
- Otani, N.; Shima, M.; Ueda, T.; Ichiki, K.; Nakajima, K.; Takesue, Y.; Okuno, T. Evaluation of influenza vaccine-immunogenicity in cell-mediated immunity. Cell. Immunol. 2016, 310, 165–169. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC); World Health Organization Regional Office for Europe. Flu News Europe, 2019. Summary Week; p. 3/2019. Available online: https://www.ema.europa.eu/en/influenza-vaccines-non-clinical-clinical-module-scientific-guideline (accessed on 25 March 2025).
- Otani, N.; Nakajima, K.; Yamada, K.; Ishikawa, K.; Ichiki, K.; Ueda, T.; Takesue, Y.; Yamamoto, T.; Higasa, S.; Tanimura, S.; et al. Timing of assessment of humoral and cell-mediated immunity after influenza vaccination. Vaccines 2024, 12, 584. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Infectious Diseases. Influenza Antibody Status 2019. Available online: https://www.niid.go.jp/niid/ja/je-m/2075-idsc/yosoku/sokuhou/9363-flu-yosoku-rapid2019-2.html (accessed on 25 March 2025).
- Benoit, A.; Beran, J.; Devaster, J.M.; Esen, M.; Launay, O.; Leroux-Roels, G.; McElhaney, J.E.; Oostvogels, L.; van Essen, G.A.; Gaglani, M.; et al. Hemagglutination Inhibition Antibody Titers as a Correlate of Protection Against Seasonal A/H3N2 Influenza Disease. Open Forum Infect. Dis. 2015, 2, ofv067. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Recommended Composition of Influenza Virus Vaccines for Use in the 2023–2024 Northern Hemisphere Influenza Season. Available online: https://www.who.int/teams/global-influenza-programme/vaccines/who-recommendations/candidate-vaccine-viruses (accessed on 24 April 2025).
- Gaglani, M.; Kim, S.S.; Naleway, A.L.; Levine, M.Z.; Edwards, L.; Murthy, K.; Dunnigan, K.; Zunie, T.; Groom, H.; Ball, S.; et al. Effect of repeat vaccination on immunogenicity of quadrivalent cell-culture and recombinant influenza vaccines among healthcare personnel aged 18–64 years: A randomized, open-label trial. Clin. Infect. Dis. 2023, 76, e1168–e1176. [Google Scholar] [CrossRef] [PubMed]
- Tayar, E.; Abdeen, S.; Abed Alah, M.; Chemaitelly, H.; Bougmiza, I.; Ayoub, H.H.; Kaleeckal, A.H.; Latif, A.N.; Shaik, R.M.; Al-Romaihi, H.E.; et al. Effectiveness of influenza vaccination against SARS-CoV-2 infection among healthcare workers in Qatar. J. Infect. Public Health 2023, 16, 250–256. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Infectious Diseases. Influenza Cases Reported Per Sentinel Weekly. Available online: https://www.niid.go.jp/niid/ja/10/2096-weeklygraph/1644-01flu.html (accessed on 25 March 2025).
| Influenza Vaccine Antigen | 2018/2019 Season | 2019/2020 Season | HAI ≥ 1:40 (Pre) | HAI ≥ 1:40 (2 w) | HAI ≥ 1:40 (5 m) | ≥4-fold Increase (2 w) | ≥4-fold Increase (5 m) |
|---|---|---|---|---|---|---|---|
| A/H1N1 | A/Singapore/GP1908/2015(IVR-180) | A/Brisbane/02/2018(IVR-190) | 28% (7/25) | 40% (10/25) | 39% (9/23) | 4% (1/25) | 0% (0/23) |
| A/H3N2 | A/Singapore/INFMH-16-0019/2016(IVR-186) | A/Kansas/14/2017(X327) | 32% (8/25) | 60% (15/25) | 57% (13/23) | 20% (5/25) | 17% (4/23) |
| B/Yamagata lineage | B/Phuket/3073/2013 | B/Phuket/3073/2013 | 60% (15/25) | 68% (17/25) | 61% (14/23) | 0% (0/25) | 0% (0/23) |
| B/Victoria lineage | B/Maryland/15/2016(NYMC BX-69A) | B/Maryland/15/2016(NYMC BX-69A) | 44% (11/25) | 48% (12/25) | 52% (12/23) | 4% (1/25) | 0% (0/23) |
| Influenza Vaccine Antigen | HAI | ||
|---|---|---|---|
| GMT Pre- and Post-Vaccination (GMTR) | |||
| Pre-Vaccination | 2 Weeks | 5 Months | |
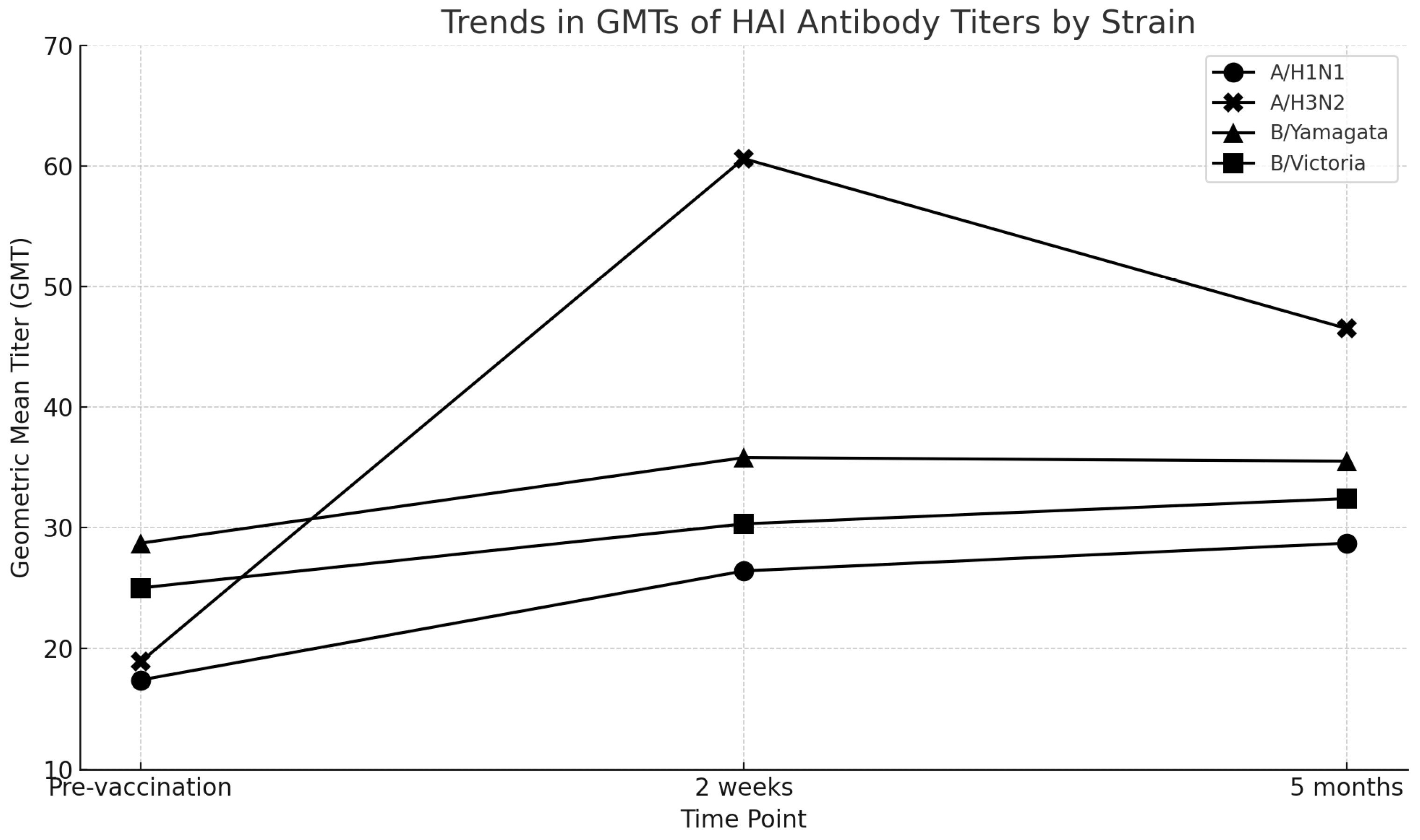
| A/H1N1 | 17.4 | 26.4 (1.5) | 28.7 (1.6) |
| A/H3N2 | 18.9 | 60.6 (3.2) | 46.5 (2.5) |
| B/Yamagata lineage | 28.7 | 35.8 (1.2) | 35.5 (1.2) |
| B/Victoria lineage | 25.0 | 30.3 (1.2) | 32.4 (1.3) |
| Influenza Vaccine Antigen | 5 HA Units | 10 HA Units | ||
|---|---|---|---|---|
| Pre-Vaccination GMC | 2 Weeks GMC (GMCR) | Pre-Vaccination GMC | 2 Weeks GMC (GMCR) | |
| A/H1N1 | 79.4 | 100.0 (1.3) | 134.0 | 130.0 (1.0) |
| A/H3N2 | 71.1 | 89.3 (1.3) | 105.9 | 117.0 (1.1) |
| B/Yamagata lineage | 75.6 | 104.0 (1.4) | 118.8 | 144.9 (1.2) |
| B/Victoria lineage | 74.7 | 95.8 (1.3) | 115.7 | 136.4 (1.2) |
| Influenza Vaccine Antigen | GMCR ≥ 1.5 |
|---|---|
| 2 Weeks Post-Vaccination | |
| H1N1 | 28% (7/25) |
| H3N2 | 28% (7/25) |
| B/Yamagata lineage | 36% (9/25) |
| B/Victoria lineage | 32% (8/25) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otani, N.; Okuno, T.; Yamada, K.; Tsuchida, T.; Ishikawa, K.; Ichiki, K.; Ueda, T.; Takesue, Y.; Higasa, S.; Nakajima, K. Evaluation of Humoral and Cell-Mediated Immunity Induced by Quadrivalent Influenza Vaccine in Pre-COVID-19 Japan. Viruses 2025, 17, 626. https://doi.org/10.3390/v17050626
Otani N, Okuno T, Yamada K, Tsuchida T, Ishikawa K, Ichiki K, Ueda T, Takesue Y, Higasa S, Nakajima K. Evaluation of Humoral and Cell-Mediated Immunity Induced by Quadrivalent Influenza Vaccine in Pre-COVID-19 Japan. Viruses. 2025; 17(5):626. https://doi.org/10.3390/v17050626
Chicago/Turabian StyleOtani, Naruhito, Toshiomi Okuno, Kumiko Yamada, Toshie Tsuchida, Kaori Ishikawa, Kaoru Ichiki, Takashi Ueda, Yoshio Takesue, Satoshi Higasa, and Kazuhiko Nakajima. 2025. "Evaluation of Humoral and Cell-Mediated Immunity Induced by Quadrivalent Influenza Vaccine in Pre-COVID-19 Japan" Viruses 17, no. 5: 626. https://doi.org/10.3390/v17050626
APA StyleOtani, N., Okuno, T., Yamada, K., Tsuchida, T., Ishikawa, K., Ichiki, K., Ueda, T., Takesue, Y., Higasa, S., & Nakajima, K. (2025). Evaluation of Humoral and Cell-Mediated Immunity Induced by Quadrivalent Influenza Vaccine in Pre-COVID-19 Japan. Viruses, 17(5), 626. https://doi.org/10.3390/v17050626







