Potential for Introduction of Bat-Borne Zoonotic Viruses into the EU: A Review
Abstract
:1. Introduction
2. Virus Distribution and Dynamics
2.1. Infection in Humans and Associated Risk Factors
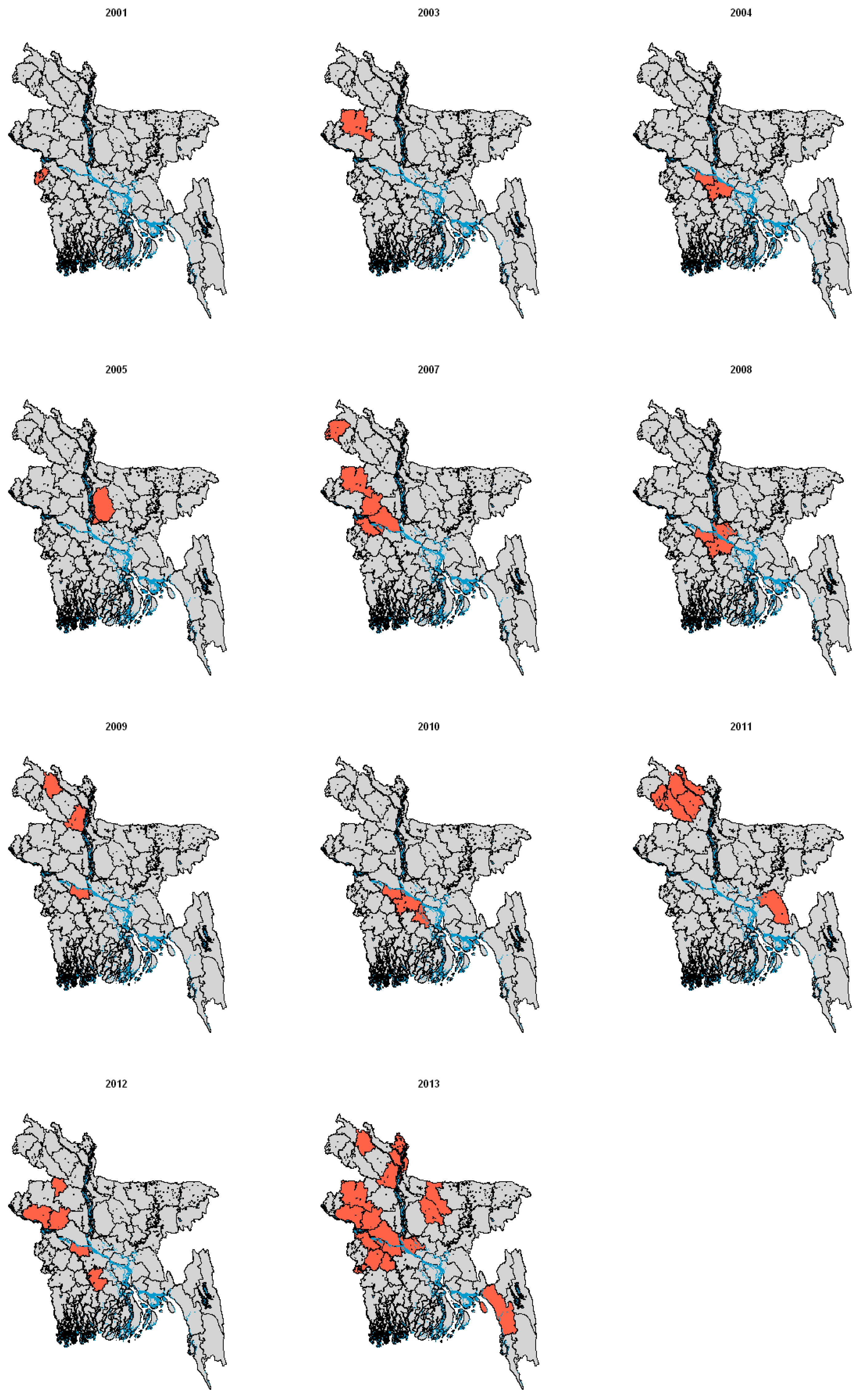
2.2. Infection in Bats
| Location | Human outbreaks | Results of testing for NiV in bats | Bat species tested | |
|---|---|---|---|---|
| Bangladesh | [41,42,43,82] | Antibodies | [42,49] | Pteropus giganteus |
| India | [46,83] | Antibodies | [84] | P. giganteus, |
| Malaysia | [33] | Antibodies, Virus isolation | [62] | P. vampyrus |
| Singapore | [86] | - | - | |
| Indonesia | - | RNA, antibodies | [64] | P. vampyrus |
| Thailand | - | Antibodies | [87] | P. hypomelanus, P. vampyrus, P. lylei, H. larvatus, |
| Viet Nam | - | Antibodies | [88] | R. leschenaultia, Cynopterus sphinx |
| Cambodia | - | Antibodies, virus isolation | [63] | P. lylei |
| China | - | Antibodies | [67] | R. leschenaultia, H. armiger, H. Pomona, Miniopterus spp., M. daubentonii, M. ricketti, R. affinis, R. sinicus |
| Papua New Guinea | - | Antibodies | [89,90] | D. magna, P. alecto, P. conspicillatus |
| East Timor | RNA | [58] | P. vampyrus, R. amplexicaudatus | |
| Madagascar | - | Antibodies | [70] | E. dupreanum, P. rufus, |
| Ghana | Antibodies | [91] | E. helvum, Epomophorus gambianus, Hypsignathus monstrosus | |
| Africa | - | Antibodies | [60,71] | E. helvum |
| Location | Human outbreaks | Results of testing for MARV in bats | Bat species tested | |
|---|---|---|---|---|
| Angola | [92,93] | - | - | |
| DRC Congo | [94] | RNA, antibodies | [13] | Rousettus aegyptiacus, Rhinolophus eloquens, Miniopterus inflatus |
| Uganda | [54] | RNA RNA, antibody, virus isolation, immunohistochemical | [73]
[74] | R. aegyptiacus R. aegyptiacus, Hipposideros spp. |
| Zimbabwe * | [55] | - | - | |
| Kenya | [95] | RNA | [77] | R. aegyptiacus |
| Gabon | - | RNA RNA & antibodies Antibodies | [76]
[72] [75] | R. aegyptiacus R. aegyptiacus R. aegyptiacus, Hypsignathus monstrosus, Micropteropus pusillus, Epomops franqueti |
2.3. Estimation of NiV and MARV “Import Risk” Areas

2.4. Evidence of Viral Load in Bats
2.5. Seasonality
2.6. Survival of Virus/Duration of Infection
3. Routes of Introduction to the EU
3.1. Human Travel

3.2. EU Trade

3.3. Bushmeat
3.4. Bat Migration
3.5. Other Factors
3.6. The Effect of a Changing World
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Luby, S.P. The pandemic potential of Nipah virus. Antivir. Res. 2013, 100, 38–43. [Google Scholar] [CrossRef]
- Clayton, B.A.; Wang, L.F.; Marsh, G.A. Henipaviruses: An Updated Review Focusing on the Pteropid Reservoir and Features of Transmission. Zoonoses Public Health 2013, 60, 69–83. [Google Scholar] [CrossRef]
- Martina, B.E.E.; Osterhaus, A. “Filoviruses”: A real pandemic threat? Embo Mol. Med. 2009, 1, 10–18. [Google Scholar] [CrossRef]
- Memish, Z.A.; Mishra, N.; Olival, K.J.; Fagbo, S.F.; Kapoor, V.; Epstein, J.H.; Alhakeem, R.; Durosinloun, A.; Al Asmari, M.; Islam, A.; et al. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg. Infect. Dis. [Internet]. 2013, 19, 1819–1823. [Google Scholar]
- Ithete, N.L.; Stoffberg, S.; Corman, V.M.; Cottontail, V.M.; Richards, L.R.; Schoeman, M.C.; Drosten, C.; Drexler, J.F.; Preiser, W. Close Relative of Human Middle East Respiratory Syndrome Coronavirus in Bat, South Africa. Emerg. Infect. Dis. 2013, 19, 1697–1699. [Google Scholar] [CrossRef]
- Luby, J.P.; Sanders, C.V. Green Monkey Disease (“Marburg Virus” Disease): A New Zoonosis. Ann. Intern. Med. 1969, 71, 657–660. [Google Scholar] [CrossRef]
- Parashar, U.D.; Sunn, L.M.; Ong, F.; Mounts, A.W.; Arif, M.T.; Ksiazek, T.G.; Kamaluddin, M.A.; Mustafa, A.N.; Kaur, H.; Ding, L.M.; et al. Case-control study of risk factors for human infection with a new zoonotic paramyxovirus, Nipah virus, during a 1998–1999 outbreak of severe encephalitis in Malaysia. J. Infect. Dis. 2000, 181, 1755–1759. [Google Scholar]
- Albariño, C.G.; Foltzer, M.; Towner, J.S.; Rowe, L.A.; Campbell, S.; Jaramillo, C.M.; Bird, B.H.; Reeder, D.M.; Vodzak, M.E.; Rota, P.; et al. Novel Paramyxovirus Associated with Severe Acute Febrile Disease, South Sudan and Uganda, 2012. Emerg. Infect. Dis. [Internet] 2014, 20, 211–216. [Google Scholar] [CrossRef]
- Luis, A.D.; Hayman, D.T.S.; O’Shea, T.J.; Cryan, P.M.; Gilbert, A.T.; Pulliam, J.R.C.; Mills, J.N.; Timonin, M.E.; Willis, C.K.R.; Cunningham, A.A.; et al. A comparison of bats and rodents as reservoirs of zoonotic viruses: Are bats special? Proc. R. Soc. BBiol. Sci. 2013, 280, 20122753. [Google Scholar] [CrossRef]
- Kurth, A.; Kohl, C.; Brinkmann, A.; Ebinger, A.; Harper, J.A.; Wang, L.F.; Muhldorfer, K.; Wibbelt, G. Novel Paramyxoviruses in Free-Ranging European Bats. PLoS One 2012, 7, e38688. [Google Scholar] [CrossRef]
- Drexler, J.F.; Corman, V.M.; Muller, M.A.; Maganga, G.D.; Vallo, P.; Binger, T.; Gloza-Rausch, F.; Rasche, A.; Yordanov, S.; Seebens, A.; et al. Bats host major mammalian paramyxoviruses. Nat. Commun. 2012, 3, 12. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2013.1. Available online: http://www.iucnredlist.org (accessed on 13 May 2014).
- Swanepoel, R.; Smit, S.B.; Rollin, P.E.; Formenty, P.; Leman, P.A.; Kemp, A.; Burt, F.J.; Grobbelaar, A.A.; Croft, J.; Bausch, D.G.; et al. Studies of reservoir hosts for Marburg virus. Emerg. Infect. Dis. 2007, 13, 1847–1851. [Google Scholar] [CrossRef]
- Epstein, J.H.; Olival, K.J.; Pulliam, J.R.C.; Smith, C.; Westrum, J.; Hughes, T.; Dobson, A.P.; Akbar, Z.; Sohayati Abdul, R.; Misliah Mohamad, B.; et al. Pteropus vampyrus, a hunted migratory species with a multinational home-range and a need for regional management. J. Appl. Ecol. 2009, 46, 991–1002. [Google Scholar] [CrossRef]
- Breed, A.C.; Field, H.E.; Smith, C.S.; Edmonston, J.; Meers, J. Bats Without Borders: Long-Distance Movements and Implications for Disease Risk Management. Ecohealth 2010, 7, 204–212. [Google Scholar] [CrossRef]
- Bausch, D.G.; Borchert, M.; Grein, T.; Roth, C.; Swanepoel, R.; Libande, M.L.; Talarmin, A.; Bertherat, E.; Muyembe-Tamfum, J.J.; Tugume, B.; et al. Risk factors for Marburg hemorrhagic fever, Democratic Republic of the Congo. Emerg. Infect. Dis. 2003, 9, 1531–1537. [Google Scholar] [CrossRef]
- WHO. Case of Marburg Haemorrhagic Fever imported into the Netherlands from Uganda. Available online: http://www.who.int/csr/don/2008_07_10/en/ (accessed on 13 May 2014).
- Timen, A.; Koopmans, M.P.G.; Vossen, A.; van Doornum, G.J.J.; Gunther, S.; van den Berkmortel, F.; Verduin, K.M.; Dittrich, S.; Emmerich, P.; Osterhaus, A.; et al. Response to Imported Case of Marburg Hemorrhagic Fever, the Netherlands. Emerg. Infect. Dis. 2009, 15, 1171–1175. [Google Scholar] [CrossRef]
- Chua, K.B.; Chua, B.H.; Wang, C.W. Anthropogenic deforestation, El Nino and the emergence of Nipah virus in Malaysia. Malays. J. Pathol. 2002, 24, 15–21. [Google Scholar]
- Divljan, A.; Parry-Jones, K.; Eby, P. Death and inuries to Grey-headed Flying-foxes Pteropus poliocephalus shot at an orchard near Sydney, New South Wales. Zoologist 2011, 35, 698–710. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Srinivasulu, B. Greater short-nosed fruit bat (Cynopterus sphinx) foraging and damage in vineyards in India. Acta Chiropterologica 2002, 4, 167–171. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hossain, M.J.; Sultana, S.; Homaira, N.; Khan, S.U.; Rahman, M.; Gurley, E.S.; Rollin, P.E.; Lo, M.K.; Comer, J.A.; et al. Date Palm Sap Linked to Nipah Virus Outbreak in Bangladesh, 2008. Vector Borne Zoonotic Dis. 2012, 12, 65–72. [Google Scholar] [CrossRef]
- Kamins, A.O.; Restif, O.; Ntiamoa-Baidu, Y.; Suu-Ire, R.; Hayman, D.T.S.; Cunningham, A.A.; Wood, J.L.N.; Rowcliffe, J.M. Uncovering the fruit bat bushmeat commodity chain and the true extent of fruit bat hunting in Ghana, West Africa. Biol. Conserv. 2011, 144, 3000–3008. [Google Scholar] [CrossRef]
- Lee, R.J.; Gorog, A.J.; Dwiyahreni, A.; Siwu, S.; Riley, J.; Alexander, H.; Paoli, G.D.; Ramono, W. Wildlife trade and implications for law enforcement in Indonesia: A case study from North Sulawesi. Biol. Conserv. 2005, 123, 477–488. [Google Scholar] [CrossRef]
- Struebig, M.J.; Harrison, M.E.; Cheyne, S.M.; Limin, S.H. Intensive hunting of large flying foxes Pteropus vampyrus natunae in Central Kalimantan, Indonesian Borneo. Oryx 2007, 41, 390–393. [Google Scholar]
- Martini, G.A. Marburg virus disease. Postgrad. Med. J. 1973, 49, 542–546. [Google Scholar] [CrossRef]
- ECDC. Updated rapid risk assessment: Severe respiratory disease associated with Middle East respiratory syndrome coronavirus (MERS-CoV). Eighth update. 6 November 2013. Available online: http://ecdc.europa.eu/en/publications/Publications/mers-cov-risk-assessment-6-november-2013.pdf (accessed on 13 May 2014).
- Negredo, A.; Palacios, G.; Vazquez-Moron, S.; Gonzalez, F.; Dopazo, H.; Molero, F.; Juste, J.; Quetglas, J.; Savji, N.; Cruz Martinez, M.; et al. Discovery of an ebolavirus-like filovirus in Europe. PLoS Pathog. 2011, 7, e1002304. [Google Scholar] [CrossRef] [Green Version]
- Warfield, K.L.; Deal, E.M.; Bavari, S. Zoonosis Update Filovirus infections. JavmaJ. Am. Vet. Med. Assoc. 2009, 234, 1130–1139. [Google Scholar] [CrossRef]
- Snary, E.L.; Ramnial, V.; Breed, A.C.; Stephenson, B.; Field, H.E.; Fooks, A.R. Qualitative Release Assessment to Estimate the Likelihood of Henipavirus Entering the United Kingdom. PLoS One 2012, 7, e27918. [Google Scholar]
- Roche, S.E.; Costard, S.; Meers, J.; Field, H.E.; Breed, A.C. Assessing the risk of Nipah virus establishment in Australian flying-foxes. Epidemiol. Infect. 2014, 4, 1–14. [Google Scholar]
- Chang, L.Y.; AbuBakar, S. Nipah virus: Phylogeny and replication. Neurol. Asia 2009, 14, 63–66. [Google Scholar]
- Clayton, B.A.; Middleton, D.; Bergfeld, J.; Haining, J.; Arkinstall, R.; Wang, L.; Marsh, G.A. Transmission routes for nipah virus from Malaysia and Bangladesh. Emerg. Infect. Dis. 2012, 18, 1983–1993. [Google Scholar] [CrossRef]
- DeBuysscher, B.L.; de Wit, E.; Munster, V.J.; Scott, D.; Feldmann, H.; Prescott, J. Comparison of the Pathogenicity of Nipah Virus Isolates from Bangladesh and Malaysia in the Syrian Hamster. PLoS Negl. Trop. Dis. 2013, 7, e2024. [Google Scholar] [CrossRef]
- Chua, K.B.; Koh, C.L.; Hooi, P.S.; Wee, K.F.; Khong, J.H.; Chua, B.H.; Chan, Y.P.; Lim, M.E.; Lam, S.K. Isolation of Nipah virus from Malaysian Island flying-foxes. Microbes Infect. 2002, 4, 145–151. [Google Scholar] [CrossRef]
- Johara, M.Y.; Field, H.; Rashdi, A.M.; Morrissy, C.; van der Heide, B.; Rota, P.; bin Adzhar, A.; White, J.; Daniels, P.; Jamaluddin, A.; et al. Nipah virus infection in bats (order Chiroptera) in peninsular Malaysia. Emerg. Infect. Dis. 2001, 7, 439–441. [Google Scholar] [CrossRef]
- Luby, S.P.; Gurley, E.S.; Hossain, M.J. Transmission of Human Infection with Nipah Virus. Clin. Infect. Dis. 2009, 49, 1743–1748. [Google Scholar] [CrossRef]
- Chua, K.B. Epidemiology, surveillance and control of Nipah virus infections in Malaysia. Malays. J. Pathol. 2010, 32, 69–73. [Google Scholar]
- Tan, C.T.; Goh, K.J.; Wong, K.T.; Sarji, S.A.; Chua, K.B.; Chew, N.K.; Murugasu, P.; Loh, Y.L.; Chong, H.T.; Tan, K.S.; et al. Relapsed and late-onset Nipah encephalitis. Ann. Neurol. 2002, 51, 703–708. [Google Scholar] [CrossRef]
- WHO. Nipah Virus Outbreaks in the WHO South-East Asia Region. Available online: http://searo.who.int/entity/emerging_diseases/links/nipah_virus_outbreaks_sear/en/index.html (accessed on 13 May 2014).
- IEDCR. Institue of Epidemiology, Disease Control and Research. Nipah infection in 2013. Update on 15 May, 2013. Available online: http://www.iedcr.org/index.php?option=com_content&view=article&id=135:23-rd-february-2013-nipah-outbreak&catid=11 (accessed on 13 May 2014).
- Homaira, N.; Rahman, M.; Hossain, M.J.; Epstein, J.H.; Sultana, R.; Khan, M.S.U.; Podder, G.; Nahar, K.; Ahmed, B.; Gurley, E.S.; et al. Nipah virus outbreak with person-to-person transmission in a district of Bangladesh, 2007. Epidemiol. Infect. 2010, 138, 1630–1636. [Google Scholar] [CrossRef]
- Luby, S.P.; Hossain, M.J.; Gurley, E.S.; Ahmed, B.N.; Banu, S.; Khan, S.U.; Homaira, N.; Rota, P.A.; Rollin, P.E.; Comer, J.A.; et al. Recurrent Zoonotic Transmission of Nipah Virus into Humans, Bangladesh, 2001–2007. Emerg. Infect. Dis. 2009, 15, 1229–1235. [Google Scholar] [CrossRef]
- Sazzad, H.M.S.; Hossain, M.J.; Gurley, E.S.; Ameen, K.M.H.; Parveen, S.; Islam, M.S.; Faruque, L.I.; Podder, G.; Banu, S.S.; Lo, M.K.; et al. Nipah virus infection outbreak with nosocomial and corpse-to-human transmission, Bangladesh. Emerg. Infect. Dis. 2013, 19, 210–217. [Google Scholar] [CrossRef]
- Khan, S.U.; Gurley, E.S.; Hossain, M.J.; Nahar, N.; Sharker, M.A.Y.; Luby, S.P. A Randomized Controlled Trial of Interventions to Impede Date Palm Sap Contamination by Bats to Prevent Nipah Virus Transmission in Bangladesh. PLoS One 2012, 7, e42689. [Google Scholar]
- Harit, A.K.; Ichhpujani, R.L.; Gupta, S.; Gill, K.S.; Lal, S.; Ganguly, N.K.; Agarwal, S.P. Nipah/Hendra virus outbreak in Siliguri, West Bengal, India in 2001. Indian J. Med. Res. 2006, 123, 553–560. [Google Scholar]
- icddr, b. Nipah transmission from bats to humans associated with drinking traditional liquor (tari) in northern Bangladesh, 2011. Health and Science Bulletin 2012, 10, 20 (En). 16–20, (Bengali). [Google Scholar]
- Montgomery, J.M.; Hossain, M.J.; Gurley, E.; Carroll, D.S.; Croisier, A.; Bertherat, E.; Asgari, N.; Formenty, P.; Keeler, N.; Comer, J.; et al. Risk factors for Nipah virus encephalitis in Bangladesh. Emerg. Infect. Dis. 2008, 14, 1526–1532. [Google Scholar] [CrossRef]
- Hsu, V.P.; Hossain, M.J.; Parashar, U.D.; Ali, M.M.; Ksiazek, T.G.; Kuzmin, I.; Niezgoda, M.; Rupprecht, C.; Bresee, J.; Breiman, R.F. Nipah virus encephalitis reemergence, Bangladesh. Emerg. Infect. Dis. 2004, 10, 2082–2087. [Google Scholar] [CrossRef]
- Hahn, M.B.; Gurley, E.S.; Epstein, J.H.; Islam, M.S.; Patz, J.A.; Daszak, P.; Luby, S.P. The role of landscape composition and configuration on Pteropus giganteus roosting ecology and Nipah virus spillover risk in Bangladesh. Am. J. Trop. Med. Hyg. 2014, 90, 247–255. [Google Scholar] [CrossRef]
- Chadha, M.S.; Comer, J.A.; Lowe, L.; Rota, P.A.; Rollin, P.E.; Bellini, W.J.; Ksiazek, T.G.; Mishra, A.C. Nipah virus-assodiated encephalitis outbreak, Siliguri, India. Emerg. Infect. Dis. 2006, 12, 235–240. [Google Scholar] [CrossRef]
- Fujita, N.; Miller, A.; Miller, G.; Gershman, K.; Gallagher, N.; Marano, N.; Hale, C.; Jentes, E. Imported Case of Marburg Hemorrhagic Fever-Colorado, 2008 (Reprinted from MMWR, Volume 58, pp. 1377–1381, 2009). Jama J. Am. Med. Assoc. 2010, 203, 413–415. [Google Scholar]
- Leroy, E.M.; Epelboin, A.; Mondonge, V.; Pourrut, X.; Gonzalez, J.P.; Muyembe-Tamfum, J.J.; Formenty, P. Human Ebola Outbreak Resulting from Direct Exposure to Fruit Bats in Luebo, Democratic Republic of Congo, 2007. Vector Borne Zoonotic Dis. 2009, 9, 723–728. [Google Scholar] [CrossRef]
- Adjemian, J.; Farnon, E.C.; Tschioko, F.; Wamala, J.F.; Byaruhanga, E.; Bwire, G.S.; Kansiime, E.; Kagirita, A.; Ahimbisibwe, S.; Katunguka, F.; et al. Outbreak of Marburg Hemorrhagic Fever Among Miners in Kamwenge and Ibanda Districts, Uganda, 2007. J. Infect. Dis. 2011, 204, S796–S799. [Google Scholar] [CrossRef]
- Gear, J.S.S.; Cassel, G.A.; Gear, A.J.; Trappler, B.; Clausen, L.; Meyers, A.M.; Kew, M.C.; Bothwell, T.H.; Sher, R.; Miller, G.B.; et al. Outbreak of Marburg virus disease in Johannesburg. Br. Med. J. 1975, 4, 489–493. [Google Scholar] [CrossRef]
- Paweska, J.T.; van Vuren, P.J.; Masumu, J.; Leman, P.A.; Grobbelaar, A.A.; Birkhead, M.; Clift, S.; Swanepoel, R.; Kemp, A. Virological and serological findings in Rousettus aegyptiacus experimentally inoculated with vero cells-adapted Hogan strain of Marburg virus. PLoS One 2012, 7, e45479. [Google Scholar] [CrossRef]
- Furmankiewicz, J.; Kucharska, M. Migration of bats along a large river valley in southwestern Poland. J. Mammal. 2009, 90, 1310–1317. [Google Scholar] [CrossRef]
- Breed, A.C.; Meers, J.; Sendow, I.; Bossart, K.N.; Barr, J.A.; Smith, I.; Wacharapluesadee, S.; Wang, L.; Field, H.E. The Distribution of Henipaviruses in Southeast Asia and Australasia: Is Wallace’s Line a Barrier to Nipah Virus? PLoS One 2013, 8, e61316. [Google Scholar]
- Gilbert, A.T.; Fooks, A.R.; Hayman, D.T.S.; Horton, D.L.; Mueller, T.; Plowright, R.; Peel, A.J.; Bowen, R.; Wood, J.L.N.; Mills, J.; et al. Deciphering Serology to Understand the Ecology of Infectious Diseases in Wildlife. Ecohealth 2013, 10, 298–313. [Google Scholar] [CrossRef]
- Peel, A.J.; McKinley, T.J.; Baker, K.S.; Barr, J.A.; Crameri, G.; Hayman, D.T.S.; Feng, Y.-R.; Broder, C.C.; Wang, L.-F.; Cunningham, A.A.; et al. Use of cross-reactive serological assays for detecting novel pathogens in wildlife: Assessing an appropriate cutoff for henipavirus assays in African bats. J. Virol. Methods 2013, 193, 295–303. [Google Scholar] [CrossRef]
- Drexler, J.F.; Corman, V.M.; Gloza-Rausch, F.; Seebens, A.; Annan, A.; Ipsen, A.; Kruppa, T.; Muller, M.A.; Kalko, E.K.V.; Adu-Sarkodie, Y.; et al. Henipavirus RNA in African Bats. PLoS One 2009, 4, e6367. [Google Scholar] [CrossRef]
- Rahman, S.A.; Hassan, S.S.; Olival, K.J.; Mohamed, M.; Chang, L.Y.; Hassan, L.; Saad, N.M.; Shohaimi, S.A.; Mamat, Z.C.; Naim, M.S.; et al. Characterization of Nipah virus from naturally infected Pteropus vampyrus bats, Malaysia. Emerg. Infect. Dis. 2010, 16, 1990–1993. [Google Scholar] [CrossRef]
- Reynes, J.M.; Counor, D.; Ong, S.; Faure, C.; Seng, V.; Molia, S.; Walston, J.; Georges-Courbot, M.C.; Deubel, V.; Sarthou, J.L. Nipah virus in lyle's flying foxes, Cambodia. Emerg. Infect. Dis. 2005, 11, 1042–1047. [Google Scholar] [CrossRef]
- Sendow, I.; Ratnawati, A.; Taylor, T.; Adjid, R.M.A.; Saepulloh, M.; Barr, J.; Wong, F.; Daniels, P.; Field, H. Nipah virus in the fruit bat Pteropus vampyrus in Sumatera, Indonesia. PLoS One 2013, 8, e69544. [Google Scholar] [CrossRef]
- Wacharapluesadee, S.; Boongird, K.; Wanghongsa, S.; Ratanasetyuth, N.; Supavonwong, P.; Saengsen, D.; Gongal, G.N.; Hemachudha, T. A longitudinal study of the prevalence of Nipah virus in Pteropus lylei bats in Thailand: Evidence for seasonal preference in disease transmission. Vector Borne and Zoonotic Dis. 2010, 10, 183–190. [Google Scholar] [CrossRef]
- Yadav, P.D.; Raut, C.G.; Shete, A.M.; Mishra, A.C.; Towner, J.S.; Nichol, S.T.; Mourya, D.T. Short report: Detection of Nipah virus RNA in fruit bat (Pteropus giganteus) from India. Am. J. Trop. Med. Hyg. 2012, 87, 576–578. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.M.; Hickey, A.C.; Zhang, Y.Z.; Li, Y.C.; Wu, Y.; Zhang, H.J.; Yuan, J.F.; Han, Z.G.; McEachern, J.; et al. Antibodies to Nipah or Nipah-like Viruses in Bats, China. Emerg. Infect. Dis. 2008, 14, 1974–1976. [Google Scholar] [CrossRef]
- Weiss, S.; Nowak, K.; Fahr, J.; Wibbelt, G.; Mombouli, J.V.; Parra, H.J.; Wolfe, N.D.; Schneider, B.S.; Leendertz, F.H. Henipavirus-related Sequences in Fruit Bat Bushmeat, Republic of Congo. Emerg. Infect. Dis. 2012, 18, 1536–1537. [Google Scholar]
- Baker, K.S.; Todd, S.; Marsh, G.; Fernandez-Loras, A.; Suu-Ire, R.; Wood, J.L.N.; Wang, L.F.; Murcia, P.R.; Cunningham, A.A. Co-circulation of diverse paramyxoviruses in an urban African fruit bat population. J. Gen. Virol. 2012, 93, 850–856. [Google Scholar] [CrossRef]
- Iehle, C.; Razafitrimo, G.; Razainirina, J.; Andriaholinirina, N.; Goodman, S.M.; Faure, C.; Georges-Courbot, M.-C.; Rousset, D.; Reynes, J.-M. Henipavirus and Tioman virus antibodies in pteropodid bats, Madagascar. Emerg. Infect. Dis. 2007, 13, 159–161. [Google Scholar] [CrossRef]
- Peel, A.J.; Sargan, D.R.; Baker, K.S.; Hayman, D.T.S.; Barr, J.A.; Crameri, G.; Suu-Ire, R.; Broder, C.C.; Lembo, T.; Wang, L.-F.; et al. Continent-wide panmixia of an African fruit bat facilitates transmission of potentially zoonotic viruses. Nat. Commun. 2013, 4, 2770–2770. [Google Scholar]
- Towner, J.S.; Pourrut, X.; Albarino, C.G.; Nkogue, C.N.; Bird, B.H.; Grard, G.; Ksiazek, T.G.; Gonzalez, J.P.; Nichol, S.T.; Leroy, E.M. Marburg Virus Infection Detected in a Common African Bat. PLoS One 2007, 2, e764. [Google Scholar] [CrossRef]
- Towner, J.S.; Amman, B.R.; Sealy, T.K.; Carroll, S.A.R.; Comer, J.A.; Kemp, A.; Swanepoel, R.; Paddock, C.D.; Balinandi, S.; Khristova, M.L.; et al. Isolation of Genetically Diverse Marburg Viruses from Egyptian Fruit Bats. PLoS Pathog. 2009, 5, e1000536. [Google Scholar] [CrossRef]
- Amman, B.R.; Carroll, S.A.; Reed, Z.D.; Sealy, T.K.; Balinandi, S.; Swanepoel, R.; Kemp, A.; Erickson, B.R.; Comer, J.A.; Campbell, S.; et al. Seasonal Pulses of Marburg Virus Circulation in Juvenile Rousettus aegyptiacus Bats Coincide with Periods of Increased Risk of Human Infection. PLoS Pathog. 2012, 8, e1002877. [Google Scholar] [CrossRef]
- Pourrut, X.; Souris, M.; Towner, J.S.; Rollin, P.E.; Nichol, S.T.; Gonzalez, J.P.; Leroy, E. Large serological survey showing cocirculation of Ebola and Marburg viruses in Gabonese bat populations, and a high seroprevalence of both viruses in Rousettus aegyptiacus. BMC Infect. Dis. 2009, 9, 159. [Google Scholar] [CrossRef]
- Maganga, G.D.; Bourgarel, M.; Ella, G.E.; Drexler, J.F.; Gonzalez, J.P.; Drosten, C.; Leroy, E.M. Is Marburg Virus Enzootic in Gabon? J. Infect. Dis. 2011, 204, S800–S803. [Google Scholar] [CrossRef]
- Kuzmin, I.V.; Niezgoda, M.; Franka, R.; Agwanda, B.; Markotter, W.; Breiman, R.F.; Shieh, W.J.; Zaki, S.R.; Rupprecht, C.E. Marburg Virus in Fruit Bat, Kenya. Emerg. Infect. Dis. 2010, 16, 352–354. [Google Scholar] [CrossRef]
- Del Vaglio, M.A.; Nicolaou, H.; Bosso, L.; Russo, D. Feeding habits of the Egyptian fruit bat Rousettus aegyptiacus on Cyrpus island: A first assessment. HystrixItal. J. Mammal. 2011, 22, 281–289. [Google Scholar]
- Albayrak, I.; Asan, N.; Yorulmaz, T. The natural history of the Egyptian fruit bat, Rousettus aegyptiacus, in Turkey (Mammalia: Chiroptera). Turk. J. Zool. 2008, 32, 11–18. [Google Scholar]
- Nogales, M.; Rodriguez-Luengo, J.L.; Marrero, P. Ecological effects and distribution of invasive non-native mammals on the Canary Islands. Mammal Rev. 2006, 36, 49–65. [Google Scholar] [CrossRef]
- Hayman, D.T.S.; McCrea, R.; Restif, O.; Suu-Ire, R.; Fooks, A.R.; Wood, J.L.N.; Cunningham, A.A.; Rowcliffe, J.M. Demography of straw-colored fruit bats in Ghana. J. Mammal. 2012, 93, 1393–1404. [Google Scholar] [CrossRef]
- WHO. Nipah Virus Infection. Available online: http://www.searo.who.int/entity/emerging_diseases/links/CDS_Nipah_Virus.pdf (accessed on 13 May 2014).
- Arankalle, V.A.; Bandyopadhyay, B.T.; Ramdasi, A.Y.; Jadi, R.; Patil, D.R.; Rahman, M.; Majumdar, M.; Banerjee, P.S.; Hati, A.K.; Goswami, R.P.; et al. Genomic Characterization of Nipah Virus, West Bengal, India. Emerg. Infect. Dis. 2011, 17, 907–909. [Google Scholar] [CrossRef]
- Epstein, J.H.; Prakash, V.; Smith, C.S.; Daszak, P.; McLaughlin, A.B.; Meehan, G.; Field, H.E.; Cunningham, A.A. Henipavirus infection in fruit bats (Pteropus giganteus), India. Emerg. Infect. Dis. 2008, 14, 1309–1311. [Google Scholar] [CrossRef]
- Shirai, J.; Sohayati, A.L.; Mohamed Ali, A.L.; Suriani, M.N.; Taniguchi, T.; Sharifah, S.H. Nipah virus survey of flying foxes in Malaysia. Jarq Jpn. Agric. Res. Q. 2007, 41, 69–78. [Google Scholar] [CrossRef]
- Paton, N.I.; Leo, Y.S.; Zaki, S.R.; Auchus, A.P.; Lee, K.E.; Ling, A.E.; Chew, S.K.; Ang, B.; Rollin, P.E.; Umapathi, T.; et al. Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet 1999, 354, 1253–1256. [Google Scholar] [CrossRef]
- Wacharapluesadee, S.; Lumlertdacha, B.; Boongird, K.; Wanghongsa, S.; Chanhome, L.; Rollin, P.; Stockton, P.; Rupprecht, C.E.; Ksiazek, T.G.; Hemachudha, T. Bat Nipah virus, Thailand. Emerg. Infect. Dis. 2005, 11, 1949–1951. [Google Scholar] [CrossRef]
- Hasebe, F.; Nguyen, T.T.T.; Inoue, S.; Yu, F.X.; Kaku, Y.; Watanabe, S.; Akashi, H.; Dat, D.T.; Le, T.Q.M.; Morita, K. Serologic Evidence of Nipah Virus Infection in Bats, Vietnam. Emerg. Infect. Dis. 2012, 18, 536–537. [Google Scholar] [CrossRef]
- Field, H.; de Jong, C.E.; Halpin, K.; Smith, C.S. Henipaviruses and fruit bats, Papua New Guinea. Emerg. Infect. Dis. 2013, 19, 670–671. [Google Scholar] [CrossRef]
- Breed, A.C.; Yu, M.; Barr, J.A.; Crameri, G.; Thalmann, C.M.; Wang, L.-F. Prevalence of Henipavirus and Rubulavirus Antibodies in Pteropid Bats, Papua New Guinea. Emerg. Infect. Dis. 2010, 16, 1997–1999. [Google Scholar] [CrossRef]
- Hayman, D.T.S.; Suu-Ire, R.; Breed, A.C.; McEachern, J.A.; Wang, L.F.; Wood, J.L.N.; Cunningham, A.A. Evidence of Henipavirus Infection in West African Fruit Bats. PLoS One 2008, 3, 4. [Google Scholar]
- Smetana, J.; Chlibek, R.; Vackova, M. Outbreak of Marburg hemorrhagic fever in Angola. Epidemiol. Mikrobiol. Imunol. 2006, 55, 63–67. [Google Scholar]
- Towner, J.S.; Khristova, M.L.; Sealy, T.K.; Vincent, M.J.; Erickson, B.R.; Bawiec, D.A.; Hartman, A.L.; Comer, J.A.; Zaki, S.R.; Stroher, U.; et al. Marburgvirus Genomics and association with a large hemorrhagic fever outbreak in Angola. J. Virol. 2006, 80, 6497–6516. [Google Scholar] [CrossRef]
- Bausch, D.G.; Nichol, S.T.; Muyembe-Tamfum, J.J.; Borchert, M.; Rollin, P.E.; Sleurs, H.; Campbell, P.; Tshioko, F.K.; Roth, C.; Colebunders, R.; et al. Marburg hemorrhagic fever associated with multiple genetic lineages of virus. N. Engl. J. Med. 2006, 355, 909–919. [Google Scholar] [CrossRef]
- Smith, D.H.; Johnson, B.K.; Isaacson, M.; Swanepoel, R.; Johnson, K.M.; Killey, M.; Bagshawe, A.; Siongok, T.; Keruga, W.K. Marburg-virus disease in Kenya. Lancet 1982, 1, 816–820. [Google Scholar]
- Middleton, D.J.; Westbury, H.A.; Morrissy, C.J.; van der Heide, B.M.; Russell, G.M.; Braun, M.A.; Hyatt, A.D. Experimental Nipah virus infection in pigs and cats. J. Comp. Pathol. 2002, 126, 124–136. [Google Scholar] [CrossRef]
- Hayman, D.T.S.; Bowen, R.A.; Cryan, P.M.; McCracken, G.F.; O’Shea, T.J.; Peel, A.J.; Gilbert, A.; Webb, C.T.; Wood, J.L.N. Ecology of Zoonotic Infectious Diseases in Bats: Current Knowledge and Future Directions. Zoonoses Public Health 2013, 60, 2–21. [Google Scholar] [CrossRef]
- Breed, A.C.; Breed, M.F.; Meers, J.; Field, H.E. Evidence of Endemic Hendra Virus Infection in Flying-Foxes (Pteropus conspicillatus)-Implications for Disease Risk Management. PLoS One 2011, 6, e28816. [Google Scholar]
- Georgea, D.B.; Webb, C.T.; Farnsworth, M.L.; O’Shea, T.J.; Bowen, R.A.; Smith, D.L.; Stanley, T.R.; Ellison, L.E.; Rupprecht, C.E. Host and viral ecology determine bat rabies seasonality and maintenance. Proc. Natl. Acad. Sci. USA 2011, 108, 10208–10213. [Google Scholar] [CrossRef]
- Plowright, R.K.; Field, H.E.; Smith, C.; Divljan, A.; Palmer, C.; Tabor, G.; Daszak, P.; Foley, J.E. Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus). Proc. R. Soc. BBiol. Sci. 2008, 275, 861–869. [Google Scholar] [CrossRef]
- Fogarty, R.; Halpin, K.; Hyatt, A.D.; Daszak, P.; Mungall, B.A. Henipavirus susceptibility to environmental variables. Virus Res. 2008, 132, 140–144. [Google Scholar] [CrossRef]
- Smither, S.J.; Nelson, M.; Eastaugh, L.; Laws, T.R.; Taylor, C.; Smith, S.A.; Salguero, F.J.; Lever, M.S. Experimental respiratory Marburg virus haemorrhagic fever infection in the common marmoset (Callithrix jacchus). Int. J. Exp. Pathol. 2013, 94, 156–168. [Google Scholar] [CrossRef]
- Piercy, T.J.; Smither, S.J.; Steward, J.A.; Eastaugh, L.; Lever, M.S. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J. Appl. Microbiol. 2010, 109, 1531–1539. [Google Scholar]
- FSA. Project: B02014. The Survival and Decontamination of Viruses on Fresh Produce. Available online: http://www.foodbase.org.uk/results.php?f_category_id=&f_report_id=625 (accessed on 13 May 2014).
- Eurostat. Statistics Database. Available online: http://epp.eurostat.ec.europa.eu/portal/page/portal/statistics/search_database (accessed on 13 May 2014).
- ONS. Office for national statistics, United Kingdom census data for 2001 and 2011. Available online: http://www.neighbourhood.statistics.gov.uk/dissemination/ (accessed on 13 May 2014).
- Government., U.D.f.C.a.L. The Bangladeshi Muslim Community in England. Understanding Muslim Ethnic Communities. Available online: http://webarchive.nationalarchives.gov.uk/20120919132719 http://www.communities.gov.uk/documents/communities/pdf/1203189.pdf; (accessed on 13 May 2014).
- Gilsdorf, A.; Morgan, D.; Leitmeyer, K. Guidance for contact tracing of cases of Lassa fever, Ebola or Marburg haemorrhagic fever on an airplane results of a European expert consultation. BMC Public Health 2012, 12. [Google Scholar] [CrossRef]
- Smith, K.M.; Anthony, S.J.; Switzer, W.M.; Epstein, J.H.; Seimon, T.; Jia, H.; Sanchez, M.D.; Huynh, T.T.; Galland, G.G.; Shapiro, S.E.; et al. Zoonotic Viruses Associated with Illegally Imported Wildlife Products. PLoS One 2012, 7, 71–79. [Google Scholar]
- Hodgkison, R.; Balding, S.T.; Zubald, A.; Kunz, T.H. Fruit bats (Chiroptera: Pteropodidae) as seed dispersers and pollinators in a lowland Malaysian rain forest. Biotropica 2003, 35, 491–502. [Google Scholar] [CrossRef]
- Muscarella, R.; Fleming, T.H. The role of frugivorous bats in tropical forest succession. Biol. Rev. 2007, 82, 573–590. [Google Scholar] [CrossRef]
- FaoStat. Trade Data. Available online: http://faostat.fao.org/site/342/default.aspx (accessed on 13 May 2014).
- Anon. Immediate notification report: Report reference: Rage-2013–1 REF OIE 14333, Report Date: 04/11/2013, Country: France. Available online: http://www.oie.int/wahis_2/temp/reports/en_imm_0000014333_20131104_164421.pdf (accessed on 13 May 2014).
- TRACES. Trade Control and Expert System. Available online: https://webgate.ec.europa.eu/sanco/traces/ (accessed on 13 May 2014).
- Defra. Pet Travel: Information for Pet Owners. Available online: https://www.gov.uk/pet-travel-information-for-pet-owners (accessed on 13 May 2014).
- Wacharapluesadee, S.; Sintunawa, C.; Kaewpom, T.; Khongnomnan, K.; Olival, K.J.; Epstein, J.H.; Rodpan, A.; Sangsri, P.; Intarut, N.; Chindamporn, A.; et al. Group C betacoronavirus in bat guano fertilizer, Thailand. Emerg. Infect. Dis. 2013, 19, 1349–1351. [Google Scholar]
- Ronsholt, L.; Sorensen, K.J.; Bruschke, C.J.M.; Wellenberg, G.J.; van Oirschot, J.T.; Johnstone, P.; Whitby, J.E.; Bourhy, H. Clinically silent rabies infection in (zoo) bats. Vet. Rec. 1998, 142, 519–520. [Google Scholar] [CrossRef]
- Chaber, A.-L.; Allebone-Webb, S.; Lignereux, Y.; Cunningham, A.A.; Rowcliffe, J.M. The scale of illegal meat importation from Africa to Europe via Paris. Conserv. Lett. 2010, 3, 317–323. [Google Scholar] [CrossRef]
- Telegraph., T. Frozen porcupines and bats confiscated in Paris exotic food raid. Available online: http://www.telegraph.co.uk/news/worldnews/europe/france/10500982/Frozen-porcupines-and-bats-confiscated-in-Paris-exotic-food-raid.html (accessed on 13 May 2014),.
- Subramanian, M. Zoonotic Disease Risk and the Bushmeat Trade: Assessing Awareness Among Hunters and Traders in Sierra Leone. Ecohealth 2012, 9, 471–482. [Google Scholar] [CrossRef]
- Bair-Brake, H.; Bell, T.; Higgins, A.; Bailey, N.; Duda, M.; Shapiro, S.; Eves, H.E.; Marano, N.; Galland, G. Is that a rodent in your luggage? A mixed method approach to describe bushmeat importation into the United States. Zoonoses Public Health 2013, 61, 97–104. [Google Scholar]
- Mbete, R.A.; Banga-Mboko, H.; Racey, P.; Mfoukou-Ntsakala, A.; Nganga, I.; Vermeulen, C.; Doucet, J.-L.; Hornick, J.-L.; Leroy, P. Household bushmeat consumption in Brazzaville, the Republic of the Congo. Trop. Conserv. Sci. 2011, 4, 187–202. [Google Scholar]
- Mbete, R.A.; Banga-Mboko, H.; Ngokaka, C.; Bouckacka, Q.F., III; Nganga, I.; Hornick, J.-L.; Leroy, P.; Vermeulen, C. Profile of bushmeat sellers and evaluation of biomass commercialized in the municipal markets of Brazzaville, Congo. Trop. Conserv. Sci. 2011, 4, 203–217. [Google Scholar]
- Robinson, J.G.; Bennett, E.L. Hunting for Sustainability in Tropical Forests; Columbia University Press: New York, NY, USA, 2000. [Google Scholar]
- Swensson, J. Bushmeat trade in Techiman, Ghana, West Africa. Available online: http://www.ibg.uu.se/digitalAssets/102/102499_swensson-john.pdf (accessed on 13 May 2014).
- Owusu-Ansah, N. Evaluation of Wildlife Hunting Restrictions on Bushmeat Trade in Five Major Markets around Digya National Park. MA Dissertation, University of Cape Coast: Cape Coast, Ghana, 2010. [Google Scholar]
- Bowen-Jones, E. The African bushmeat trade—A recipe for extinction. In Le Commerce de la Viande de Brousse—Le Meilleur Moyen Pour Aller Droit a L'extinction; Publisher: Cambridge, UK, 1998; pp. 48–51. [Google Scholar]
- Advisory committee on the microbiological safety of food. Review of Possible Microbiological Hazards that may be Associated with the Illegal Importation of Bushmeat. Available online: http://www.food.gov.uk/multimedia/pdfs/acm741.pdf (accessed on 13 May 2014).
- Hoyt, R. Wild Meat Harvest and Trade in Liberia: managing biodiversity, economic and social impacts. Available online: http://www.odi.org.uk/sites/odi.org.uk/files/odi-assets/publications-opinion-files/3300.pdf (accessed on 13 May 2014).
- Defra. Annual review of controls on imports of animal products 2010–2011. Available online: https://www.gov.uk/government/publications/annual-review-of-controls-on-imports-of-animal-products (accessed on 13 May 2014).
- Makosso-Vheiye, G.; Massamba, A.; Mananga, V.; Massamba, J.; Silou, T. Traditional smoking processes of Bushmeat in Congo (Brazzaville). Int. J. Mol. Zool. 2012, 2, 62–69. [Google Scholar]
- WHO. Traditional Medicine Strategy. Available online: http://whqlibdoc.who.int/hq/2002/who_edm_trm_2002.1.pdf (accessed on 13 May 2014),.
- Lelant, V.; Chenaval, N. Note on a meeting with a marabout from Fadial, Senegal, Western Africa who use bats in his medicine. Afr. Bat Conserv. News 2012, 28, 15–16. [Google Scholar]
- Harrison, M.E.; Cheyne, S.M.; Darma, F.; Ribowo, D.A.; Limin, S.H.; Struebig, M.J. Hunting of flying foxes and perception of disease risk in Indonesian Borneo. Biol. Conserv. 2011, 144, 2441–2449. [Google Scholar] [CrossRef]
- Ghosh, A.K. Ethnobiology: Therapeutics and Natural Resources; Daya Publishing House: Delhi, India, 2008. [Google Scholar]
- Zhou, J.; Xie, G.; Yan, X. Encyclopedia of Traditional Chinese Medicines—Molecular Structures, Pharmacological Activities, Natural Sources and Applications: Vol. 4: Isolated Compounds N-S, Springer-Verlag: Berlin, Germany, 2011.
- Walker, S. Some informal correspondance on local people’s medicinal uses of fruit bats. Bat Net Newsl. Newsl. Chiropt. Conserv. Inf. Netw. South Asia 2005, 6, 6. [Google Scholar]
- Padmanabhan, P.S.; Sujana, K.A. Animal products in traditional medicine from Attappady hills of Western Ghats. Indian J. Tradit. Knowl. 2008, 7, 326–329. [Google Scholar]
- Fujita, M.S.T.; Tuttle, M.D. Flying foxes: Threatened animals of key ecological and economic importance. Conserv. Biol. 1991, 5, 455–463. [Google Scholar] [CrossRef]
- Lev, E. Traditional healing with animals (zootherapy): Medieval to present-day Levantine practice. J. Ethnopharmacol. 2003, 85, 107–118. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Nguyen, T. An Overview of the Use of Plants and Animals in Traditional Medicine Systems in Vietnam; TRAFFIC Southeast Asia, Greater Mekong Programme: Ha Noi, Vietnam, 2008; p. 92. [Google Scholar]
- Ng, T.P.; Wong, M.L.; Hong, C.Y.; Koh, K.T.; Goh, L.G. The use of complementary and alternative medicine by asthma patients. QJM 2003, 96, 747–754. [Google Scholar] [CrossRef]
- Sinha, R.K.S.; Sinha, S. Ethnobiology (Role of Indigenous and Ethnic Societies in Biodiversity Conservation, Human Health Protection and Sustainable Development. Surabhi Publication: Jaipur, India, 2005. [Google Scholar]
- Fleming, T.H.; Eby, P. Ecology of bat migration. Bat Ecol. 2003, 4, 156–208. [Google Scholar]
- Hutterer, R.; Ivanova, T.; Meyer-Cord, C.; Rodrigues, L. Bat Migrations in Europe: A Review of Banding Data and Literature. Naturschutz und Biologische Vielfalt. 2005, 28, 1–172. [Google Scholar]
- Kanuch, P. On the occurrence of Pipistrellus nathusii in central Slovakia. Vespertilio 2012, 16, 165–166. [Google Scholar]
- Tsoar, A.; Nathan, R.; Bartan, Y.; Vyssotski, A.; Dell’Omo, G.; Ulanovsky, N. Large-scale navigational map in a mammal. Proc. Natl. Acad. Sci. USA 2011, 108, E718–E724. [Google Scholar]
- Benda, P.; Vallo, P.; Hulva, P.; Horacek, I. The Egyptian fruit bat Rousettus aegyptiacus (Chiroptera: Pteropodidae) in the Palaearctic: Geographical variation and taxonomic status. Biologia 2012, 67, 1230–1244. [Google Scholar] [CrossRef]
- Richter, H.V.; Cumming, G.S. First application of satellite telemetry to track African straw-coloured fruit bat migration. J. Zool. 2008, 275, 172–176. [Google Scholar] [CrossRef]
- Ossa, G.; Kramer-Schadt, S.; Peel, A.J.; Scharf, A.K.; Voigt, C.C. The Movement Ecology of the Straw-Colored Fruit Bat, Eidolon helvum, in Sub-Saharan Africa Assessed by Stable Isotope Ratios. PLoS One 2012, 7, e45729. [Google Scholar]
- Voigt, C.C.; Popa-Lisseanu, A.G.; Niermann, I.; Kramer-Schadt, S. The catchment area of wind farms for European bats: A plea for international regulations. Biol. Conserv. 2012, 153, 80–86. [Google Scholar] [CrossRef]
- Stansfield, G. Parti-coloured bat Vespevtilio muvinus L. from a North Sea drilling rig. J. Zool. 1966, 150, 491–492. [Google Scholar] [CrossRef]
- McFarlane, R.; Becker, N.; Field, H. Investigation of the Climatic and Environmental Context of Hendra Virus Spillover Events 1994–2010. PLoS One 2011, 6, e28374. [Google Scholar] [CrossRef]
- Parsons, J.G.; Blair, D.; Luly, J.; Robson, S.K.A. Bat Strikes in the Australian Aviation Industry. J. Wildl. Manag. 2009, 73, 526–529. [Google Scholar] [CrossRef]
- Cleary, C.C.; Dolbeer, R.A.; Wright, S.E. Wildlife Strikes to Civil Aircraft in the United States1990–2005. Available online: http://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1006&context=birdstrikeother (accessed on 13 May 2014).
- Constantine, D.G. Geographic translocation of bats: Known and potential problems. Emerg. Infect. Dis. 2003, 9, 17–21. [Google Scholar]
- Casanova, L.; Rutala, W.A.; Weber, D.J.; Sobsey, M.D. Survival of surrogate coronaviruses in water. Water Res. 2009, 43, 1893–1898. [Google Scholar] [CrossRef]
- Kazmierczak, J.; Davis, J.P.; Bell, T.R.; Marienau, K.; Cohen, N.J.; Marano, N.; Recuenco, S.; Rupprecht, C.; Buttke, D.; Tack, D.; et al. Rabies risk assessment of exposures to a bat on a commercial airliner—United States, August 2011. Morb. Mortal. Wkly. Rep. 2012, 61, 242–244. [Google Scholar]
- Gale, P.; Adkin, A.; Drew, T.; Wooldridge, M. Predicting the impact of climate change on livestock disease in Great Britain. Vet. Rec. 2008, 162, 214–215. [Google Scholar] [CrossRef]
- Gale, P.; Breed, A.C. Horizon Scanning for Emergence of New Viruses: From Constructing Complex Scenarios to Online Games. Transbound. Emerg. Dis. 2013, 60, 472–474. [Google Scholar] [CrossRef]
- Benda, P.; Hotoviv, J. Hibernation record of Pipistrellus nathusii in southern Moravia (Czech Republic). Vespertilio 2004, 8, 137–139. [Google Scholar]
- Lundy, M.; Montgomery, I.; Russ, J. Climate change-linked range expansion of Nathusius’ pipistrelle bat, Pipistrellus nathusii (Keyserling & Blasius, 1839). J. Biogeogr. 2010, 37, 2232–2242. [Google Scholar] [CrossRef]
- Daszak, P.; Zambrana-Torrelio, C.; Bogich, T.L.; Fernandez, M.; Epstein, J.H.; Murray, K.A.; Hamilton, H. Interdisciplinary approaches to understanding disease emergence: The past, present, and future drivers of Nipah virus emergence. Proc. Natl. Acad. Sci. USA 2013, 110, 3681–3688. [Google Scholar] [CrossRef]
- Izhaki, I.; Korine, C.; Arad, Z. The effect of bat (Rousettus aegyptiacus) dispersal on seed-germination in eastern mediterranean habitats. Oecologia 1995, 101, 335–342. [Google Scholar] [CrossRef]
- Breed, A.; Field, H.; Perkins, N.; Eby, P.; Cunningham, A.; Prowse, S. Re: Flying foxes carrying Hendra virus in Queensland pose a potential problem for other states. Aust. Vet. J. 2010, 88, N24–N24. [Google Scholar] [CrossRef]
- Streicker, D.G.; Recuenco, S.; Valderrama, W.; Benavides, J.G.; Vargas, I.; Pacheco, V.; Condori, E.C.; Montgomery, J.; Rupprecht, C.E.; Rohani, P.; et al. Ecological and anthropogenic drivers of rabies exposure in vampire bats: Implications for transmission and control. Proc. R. Soc. BBiol. Sci. 2012, 279, 3384–3392. [Google Scholar] [CrossRef]
- Nazmun, N.; Mondal, U.K.; Sultana, R.; Hossain, M.J.; Khan, M.S.U.; Gurley, E.S.; Oliveras, E.; Luby, S.P. Piloting the use of indigenous methods to prevent Nipah virus infection by interrupting bats’ access to date palm sap in Bangladesh. Health Promot. Int. 2013, 28, 378–386. [Google Scholar] [CrossRef]
- Department of Agriculture Fisheries and Forestry, Australia. Guidelines for Veterinarians Handling Potential Hendra Virus Infection in Horses. Available online: http://www.nationalarchives.gov.uk/doc/open-government-licence/open-government-licence.htm. (accessed on 13 May 2014).
© 2014 Crown Copyright. This article is published under the terms of the free Open Government Licence, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited. See http://www.nationalarchives.gov.uk/doc/open-government-licence/open-government-licence.htm.
Share and Cite
Simons, R.R.L.; Gale, P.; Horigan, V.; Snary, E.L.; Breed, A.C. Potential for Introduction of Bat-Borne Zoonotic Viruses into the EU: A Review. Viruses 2014, 6, 2084-2121. https://doi.org/10.3390/v6052084
Simons RRL, Gale P, Horigan V, Snary EL, Breed AC. Potential for Introduction of Bat-Borne Zoonotic Viruses into the EU: A Review. Viruses. 2014; 6(5):2084-2121. https://doi.org/10.3390/v6052084
Chicago/Turabian StyleSimons, Robin R. L., Paul Gale, Verity Horigan, Emma L. Snary, and Andrew C. Breed. 2014. "Potential for Introduction of Bat-Borne Zoonotic Viruses into the EU: A Review" Viruses 6, no. 5: 2084-2121. https://doi.org/10.3390/v6052084
APA StyleSimons, R. R. L., Gale, P., Horigan, V., Snary, E. L., & Breed, A. C. (2014). Potential for Introduction of Bat-Borne Zoonotic Viruses into the EU: A Review. Viruses, 6(5), 2084-2121. https://doi.org/10.3390/v6052084




