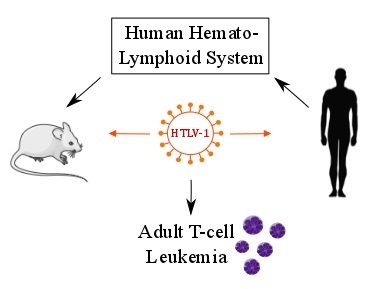
From Immunodeficiency to Humanization: The Contribution of Mouse Models to Explore HTLV-1 Leukemogenesis
Abstract
:1. Introduction
2. The Leukemogenic Activity of HTLV-1
3. The Leukemogenic Potential of Tax and HBZ


4. Mouse Models
From Immunodeficiency-

-to Humanization
5. The Mouse Modeling of HTLV-1-Induced Leukemogenesis
5.1. Xenogeneic Transplantation Assays
5.2. HTLV-1 Infection of Humanized Mice

6. HHLS Mouse Models and HTLV-1 Pathogenesis: The Future Is Now
- (i)
- To infect humanized mice with molecularly cloned HTLV-1 (unpublished data, Pérès et al.) opening a new way not only for understanding in detail the HTLV-1-pathogenesis, but also for delineating the importance of various viral genes on CD4+ T cell transformation and leukemogenesis. Inducible viral gene expression systems could also improve our knowledge [88].
- (ii)
- To mimic the way HTLV-1 is delivered (breast-feeding) and disseminated (through dendritic cells) in the body [89]. One can hypothesize that the gastrointestinal tract can serve as a secondary site of infection in which infected T cells present in the milk would be able to infect dendritic cells in the intestine. Clearly, new humanized mouse models engrafted with appropriate target tissues will be suitable for evaluation of HTLV-1 natural infection.
- (iii)
- To enhance the specific immune response, by using mouse strains transgenic for human HLAs. For example, in NSG-HLA-A2/HDD mice that possess the human HLA-A2 gene, T cell education is performed in a human HLA context. In these mice, a functional HLA-restricted cytotoxic response has been observed after EBV infection [90]. Likewise, in transgenic NOG/HLA-DR4 mice, T cell homeostasis was differentially regulated in HLA-matched humanized NOG mice compared with HLA-mismatched control mice. Furthermore, antibody class switching was induced after immunization of HLA-DR matched mice with exogenous antigens, underlining that this novel mouse strain will contribute to future studies of human humoral immune responses [91].
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baltimore, D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature 1970, 226, 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Temin, H.M.; Mizutani, S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature 1970, 226, 1211–1213. [Google Scholar] [CrossRef] [PubMed]
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419. [Google Scholar] [CrossRef] [PubMed]
- Barre-Sinoussi, F.; Chermann, J.C.; Rey, F.; Nugeyre, M.T.; Chamaret, S.; Gruest, J.; Dauguet, C.; Axler-Blin, C.; Vezinet-Brun, F.; Rouzioux, C.; et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 1983, 220, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Takahashi, T.; Katano, I.; Ito, M. Current advances in humanized mouse models. Cell. Mol. Immunol. 2012, 9, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Rongvaux, A.; Takizawa, H.; Strowig, T.; Willinger, T.; Eynon, E.E.; Flavell, R.A.; Manz, M.G. Human hemato-lymphoid system mice: Current use and future potential for medicine. Annu. Rev. Immunol. 2013, 31, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Brehm, M.A.; Garcia-Martinez, J.V.; Greiner, D.L. Humanized mice for immune system investigation: Progress, promise and challenges. Nat. Rev. Immunol. 2012, 12, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Akkina, R. New generation humanized mice for virus research: Comparative aspects and future prospects. Virology 2013, 435, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Duc Dodon, M.; Villaudy, J.; Gazzolo, L.; Haines, R.; Lairmore, M. What we are learning on HTLV-1 pathogenesis from animal models. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, B.; Niewiesk, S.; Lairmore, M.D. Mouse models of human T lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma. Vet. Pathol. 2010, 47, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Panfil, A.R.; Al-Saleem, J.J.; Green, P.L. Animal models utilized in HTLV-1 research. Virol. Res. Treat. 2013, 4, 49–59. [Google Scholar]
- Takatsuki, K. Discovery of adult T-cell leukemia. Retrovirology 2005, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proietti, F.A.; Carneiro-Proietti, A.B.; Catalan-Soares, B.C.; Murphy, E.L. Global epidemiology of HTLV-I infection and associated diseases. Oncogene 2005, 24, 6058–6068. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M. Discovery of HTLV-1, the first human retrovirus, its unique regulatory mechanisms, and insights into pathogenesis. Oncogene 2005, 24, 5931–5937. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Yamada, Y.; Akamatsu, N.; Kamihira, S.; Imaizumi, Y.; Tomonaga, M.; Matsuyama, T. Possible origin of adult T-cell leukemia/lymphoma cells from human T lymphotropic virus type-1-infected regulatory T cells. Cancer Sci. 2005, 96, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Toulza, F.; Heaps, A.; Tanaka, Y.; Taylor, G.P.; Bangham, C.R. High frequency of CD4+FoxP3+ cells in HTLV-1 infection: Inverse correlation with HTLV-1-specific CTL response. Blood 2008, 111, 5047–5053. [Google Scholar] [CrossRef] [PubMed]
- Rowan, A.G.; Bangham, C.R. Is there a role for HTLV-1-specific CTL in adult T-cell leukemia/lymphoma? Leuk. Res. Treat. 2012. [Google Scholar] [CrossRef] [PubMed]
- Uozumi, K. Treatment of adult T-cell leukemia. J. Clin. Exp. Hematopathol. 2010, 50, 9–25. [Google Scholar] [CrossRef]
- Utsunomiya, A.; Choi, I.; Chihara, D.; Seto, M. Recent advances in the treatment of adult T-cell leukemia-lymphomas. Cancer Sci. 2015, 106, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Jeang, K.T. Human T-cell leukemia virus type 1 (HTLV-1) and leukemic transformation: Viral infectivity, Tax, HBZ and therapy. Oncogene 2011, 30, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Gaudray, G.; Gachon, F.; Basbous, J.; Biard-Piechaczyk, M.; Devaux, C.; Mesnard, J.M. The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J. Virol. 2002, 76, 12813–12822. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, B.; Mesnard, J.M. Does chronic infection in retroviruses have a sense? Trends Microbiol. 2015, 23, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Lodewick, J.; Lamsoul, I.; Bex, F. Move or die: The fate of the Tax oncoprotein of HTLV-1. Viruses 2011, 3, 829–857. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Jeang, K.T. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer 2007, 7, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Coscoy, L.; Gonzalez-Dunia, D.; Tangy, F.; Syan, S.; Brahic, M.; Ozden, S. Molecular mechanism of tumorigenesis in mice transgenic for the human T cell leukemia virus Tax gene. Virology 1998, 248, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Grossman, W.J.; Kimata, J.T.; Wong, F.H.; Zutter, M.; Ley, T.J.; Ratner, L. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 1995, 92, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Sawa, H.; Lewis, M.J.; Orba, Y.; Sheehy, N.; Yamamoto, Y.; Ichinohe, T.; Katano, H.; Tsunetsugu-Yokota, Y.; Takahashi, H.; et al. Thymus-derived leukemia-lymphoma in mice transgenic for the Tax gene of human T-lymphotropic virus type I. Nat. Med. 2006, 12, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, J.; Mizukami, T.; Takizawa, K.; Kuramitsu, M.; Momose, H.; Masumi, A.; Ami, Y.; Hasegawa, H.; Hall, W.W.; Tsujimoto, H.; et al. Identification of cancer stem cells in a Tax-transgenic (Tax-Tg) mouse model of adult T-cell leukemia/lymphoma. Blood 2009, 114, 2709–2720. [Google Scholar] [CrossRef] [PubMed]
- Satou, Y.; Yasunaga, J.; Yoshida, M.; Matsuoka, M. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc. Natl. Acad. Sci. USA 2006, 103, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Hagiya, K.; Yasunaga, J.; Satou, Y.; Ohshima, K.; Matsuoka, M. ATF3, an HTLV-1 bZip factor binding protein, promotes proliferation of adult T-cell leukemia cells. Retrovirology 2011, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemasson, I.; Lewis, M.R.; Polakowski, N.; Hivin, P.; Cavanagh, M.H.; Thebault, S.; Barbeau, B.; Nyborg, J.K.; Mesnard, J.M. Human T-cell leukemia virus type 1 (HTLV-1) bZIP protein interacts with the cellular transcription factor CREB to inhibit HTLV-1 transcription. J. Virol. 2007, 81, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Mitobe, Y.; Yasunaga, J.I.; Furuta, R.; Matsuoka, M. HTLV-1 bZIP factor RNA and protein impart distinct functions on T-cell proliferation and survival. Cancer Res. 2015, 75, 4143–4152. [Google Scholar] [CrossRef] [PubMed]
- Borowiak, M.; Kuhlmann, A.S.; Girard, S.; Gazzolo, L.; Mesnard, J.M.; Jalinot, P.; Duc Dodon, M. HTLV-1 bZIP factor impedes the menin tumor suppressor and upregulates JunD-mediated transcription of the hTERT gene. Carcinogenesis 2013, 34, 2664–2672. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, A.S.; Villaudy, J.; Gazzolo, L.; Castellazzi, M.; Mesnard, J.M.; Duc Dodon, M. HTLV-1 HBZ cooperates with JunD to enhance transcription of the human telomerase reverse transcriptase gene (hTERT). Retrovirology 2007, 4. [Google Scholar] [CrossRef] [PubMed]
- Satou, Y.; Yasunaga, J.; Zhao, T.; Yoshida, M.; Miyazato, P.; Takai, K.; Shimizu, K.; Ohshima, K.; Green, P.L.; Ohkura, N.; et al. HTLV-1 bZIP factor induces T-cell lymphoma and systemic inflammation in vivo. PLoS Pathog. 2011, 7, e1001274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, T.; Yasunaga, J.; Satou, Y.; Nakao, M.; Takahashi, M.; Fujii, M.; Matsuoka, M. Human T-cell leukemia virus type 1 bZIP factor selectively suppresses the classical pathway of NF-κB. Blood 2009, 113, 2755–2764. [Google Scholar] [CrossRef] [PubMed]
- Tanaka-Nakanishi, A.; Yasunaga, J.; Takai, K.; Matsuoka, M. HTLV-1 bZIP factor suppresses apoptosis by attenuating the function of FoxO3a and altering its localization. Cancer Res. 2014, 74, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.; Zimmerman, B.; Li, M.; Lairmore, M.D.; Green, P.L. Human T-cell leukemia virus type-1 antisense-encoded gene, Hbz, promotes T-lymphocyte proliferation. Blood 2008, 112, 3788–3797. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Taguchi, N.; Satou, Y.; Miyazato, P.; Ohshima, K.; Nakagawa, M.; Katagiri, K.; Kinashi, T.; Matsuoka, M. HTLV-1 bZIP factor induces inflammation through labile Foxp3 expression. PLoS Pathog. 2013, 9, e1003630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitagami, Y.; Yasunaga, J.I.; Kinosada, H.; Ohshima, K.; Matsuoka, M. Interferon-gamma promotes inflammation and development of T-cell lymphoma in HTLV-1 bZIP factor transgenic mice. PLoS Pathog. 2015, 11, e1005120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, T.; Satou, Y.; Matsuoka, M. Development of T cell lymphoma in HTLV-1 bZIP factor and Tax double transgenic mice. Arch. Virol. 2014, 159, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Ohsugi, T. A transgenic mouse model of human T cell leukemia virus type 1-associated diseases. Front. Microbiol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Nerenberg, M.; Hinrichs, S.H.; Reynolds, R.K.; Khoury, G.; Jay, G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science 1987, 237, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Aizawa, S.; Suda, Y.; Ikawa, Y.; Kishimoto, H.; Asano, Y.; Tada, T.; Hikikoshi, A.; Yoshida, M.; Seiki, M. Thymic atrophy characteristic in transgenic mice that harbor pX genes of human T-cell leukemia virus type I. J. Virol. 1989, 63, 3185–3189. [Google Scholar] [PubMed]
- Ruddle, N.H.; Li, C.B.; Horne, W.C.; Santiago, P.; Troiano, N.; Jay, G.; Horowitz, M.; Baron, R. Mice transgenic for HTLV-I LTR-tax exhibit tax expression in bone, skeletal alterations, and high bone turnover. Virology 1993, 197, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Rauch, D.A.; Ratner, L. Targeting HTLV-1 activation of NFκB in mouse models and ATLL patients. Viruses 2011, 3, 886–900. [Google Scholar] [CrossRef] [PubMed]
- Rauch, D.; Gross, S.; Harding, J.; Niewiesk, S.; Lairmore, M.; Piwnica-Worms, D.; Ratner, L. Imaging spontaneous tumorigenesis: Inflammation precedes development of peripheral NK tumors. Blood 2009, 113, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Thebault, S.; Basbous, J.; Hivin, P.; Devaux, C.; Mesnard, J.M. HBZ interacts with JunD and stimulates its transcriptional activity. FEBS Lett. 2004, 562, 165–170. [Google Scholar] [CrossRef]
- Sugata, K.; Satou, Y.; Yasunaga, J.; Hara, H.; Ohshima, K.; Utsunomiya, A.; Mitsuyama, M.; Matsuoka, M. HTLV-1 bZIP factor impairs cell-mediated immunity by suppressing production of Th1 cytokines. Blood 2012, 119, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Sugata, K.; Yasunaga, J.I.; Mitobe, Y.; Miura, M.; Miyazato, P.; Kohara, M.; Matsuoka, M. Protective effect of cytotoxic T lymphocytes targeting HTLV-1 bZIP factor. Blood 2015, 126, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, S.P. “Nude”, a new hairless gene with pleiotropic effects in the mouse. Genet. Res. 1966, 8, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Bosma, G.C.; Custer, R.P.; Bosma, M.J. A severe combined immunodeficiency mutation in the mouse. Nature 1983, 301, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Schweitzer, P.A.; Christianson, S.W.; Gott, B.; Schweitzer, I.B.; Tennent, B.; McKenna, S.; Mobraaten, L.; Rajan, T.V.; Greiner, D.L.; et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 1995, 154, 180–191. [Google Scholar] [PubMed]
- Traggiai, E.; Chicha, L.; Mazzucchelli, L.; Bronz, L.; Piffaretti, J.C.; Lanzavecchia, A.; Manz, M.G. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 2004, 304, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Parrula, C.; Zimmerman, B.; Nadella, P.; Shu, S.; Rosol, T.; Fernandez, S.; Lairmore, M.; Niewiesk, S. Expression of tumor invasion factors determines systemic engraftment and induction of humoral hypercalcemia in a mouse model of adult T-cell leukemia. Vet. Pathol. 2009, 46, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Kawahara, M.; Hishizawa, M.; Shimazu, Y.; Sugino, N.; Fujii, S.; Kadowaki, N.; Takaori-Kondo, A. T memory stem cells are the hierarchical apex of adult T-cell leukemia. Blood 2015, 125, 3527–3535. [Google Scholar] [CrossRef] [PubMed]
- Feuer, G.; Fraser, J.K.; Zack, J.A.; Lee, F.; Feuer, R.; Chen, I.S. Human T-cell leukemia virus infection of human hematopoietic progenitor cells: Maintenance of virus infection during differentiation in vitro and in vivo. J. Virol. 1996, 70, 4038–4044. [Google Scholar] [PubMed]
- Villaudy, J.; Wencker, M.; Gadot, N.; Gillet, N.A.; Scoazec, J.Y.; Gazzolo, L.; Manz, M.G.; Bangham, C.R.; Duc Dodon, M. HTLV-1 propels thymic human T cell development in “human immune system” Rag2/ gamma c/ mice. PLoS Pathog. 2011, 7, e1002231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tezuka, K.; Xun, R.; Tei, M.; Ueno, T.; Tanaka, M.; Takenouchi, N.; Fujisawa, J. An animal model of adult T-cell leukemia: Humanized mice with HTLV-1-specific immunity. Blood 2014, 123, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Mosier, D.E.; Gulizia, R.J.; Baird, S.M.; Wilson, D.B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature 1988, 335, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Namikawa, R.; Weilbaecher, K.N.; Kaneshima, H.; Yee, E.J.; McCune, J.M. Long-term human hematopoiesis in the SCID-hu mouse. J. Exp. Med. 1990, 172, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- McCune, J.M.; Namikawa, R.; Shih, C.C.; Rabin, L.; Kaneshima, H. Suppression of HIV infection in AZT-treated SCID-hu mice. Science 1990, 247, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Melkus, M.W.; Estes, J.D.; Padgett-Thomas, A.; Gatlin, J.; Denton, P.W.; Othieno, F.A.; Wege, A.K.; Haase, A.T.; Garcia, J.V. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat. Med. 2006, 12, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, F.; Yasukawa, M.; Lyons, B.; Yoshida, S.; Miyamoto, T.; Yoshimoto, G.; Watanabe, T.; Akashi, K.; Shultz, L.D.; Harada, M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γ chainnull mice. Blood 2005, 106, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Kobayashi, K.; Nakahata, T. NOD/Shi-scid IL2rγnull (NOG) mice more appropriate for humanized mouse models. Curr. Top. Microbiol. Immunol. 2008, 324, 53–76. [Google Scholar] [PubMed]
- Rongvaux, A.; Willinger, T.; Martinek, J.; Strowig, T.; Gearty, S.V.; Teichmann, L.L.; Saito, Y.; Marches, F.; Halene, S.; Palucka, A.K.; et al. Development and function of human innate immune cells in a humanized mouse model. Nat. Biotechnol. 2014, 32, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Shores, E.W.; Hu-Li, J.; Anver, M.R.; Kelsall, B.L.; Russell, S.M.; Drago, J.; Noguchi, M.; Grinberg, A.; Bloom, E.T.; et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity 1995, 2, 223–238. [Google Scholar] [CrossRef]
- Brehm, M.A.; Wiles, M.V.; Greiner, D.L.; Shultz, L.D. Generation of improved humanized mouse models for human infectious diseases. J. Immunol. Methods 2014, 410, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Cachat, A.; Villaudy, J.; Rigal, D.; Gazzolo, L.; Duc Dodon, M. Mice are not Men and yet… how humanized mice inform us about human infectious diseases. Med. Sci. 2012, 28, 63–68. [Google Scholar]
- Legrand, N.; Huntington, N.D.; Nagasawa, M.; Bakker, A.Q.; Schotte, R.; Strick-Marchand, H.; de Geus, S.J.; Pouw, S.M.; Bohne, M.; Voordouw, A.; et al. Functional CD47/signal regulatory protein alpha (SIRPα) interaction is required for optimal human T-and natural killer-(NK) cell homeostasis in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 13224–13229. [Google Scholar] [CrossRef] [PubMed]
- Brehm, M.A.; Cuthbert, A.; Yang, C.; Miller, D.M.; Dilorio, P.; Laning, J.; Burzenski, L.; Gott, B.; Foreman, O.; Kavirayani, A.; et al. Parameters for establishing humanized mouse models to study human immunity: Analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rγnull mutation. Clin. Immunol. 2010, 135, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, K.; Prasolava, T.K.; Wang, J.C.; Mortin-Toth, S.M.; Khalouei, S.; Gan, O.I.; Dick, J.E.; Danska, J.S. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat. Immunol. 2007, 8, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dole, K.; Stanley, J.R.; Richard, V.; Rosol, T.J.; Ratner, L.; Lairmore, M.; Feuer, G. Engraftment and tumorigenesis of HTLV-1 transformed T cell lines in SCID/bg and NOD/SCID mice. Leuk. Res. 2002, 26, 561–567. [Google Scholar] [CrossRef]
- Takajo, I.; Umeki, K.; Morishita, K.; Yamamoto, I.; Kubuki, Y.; Hatakeyama, K.; Kataoka, H.; Okayama, A. Engraftment of peripheral blood mononuclear cells from human T-lymphotropic virus type 1 carriers in NOD/SCID/γnull (NOG) mice. Int. J. Cancer 2007, 121, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Kawano, N.; Ishikawa, F.; Shimoda, K.; Yasukawa, M.; Nagafuji, K.; Miyamoto, T.; Baba, E.; Tanaka, T.; Yamasaki, S.; Gondo, H.; et al. Efficient engraftment of primary adult T-cell leukemia cells in newborn NOD/SCID/β2-microglobulinnull mice. Leukemia 2005, 19, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.S.; Lambert, S.; Bouttier, M.; Benit, L.; Ruscetti, F.W.; Hermine, O.; Pique, C. Molecular aspects of HTLV-1 entry: Functional domains of the HTLV-1 surface subunit (SU) and their relationships to the entry receptors. Viruses 2011, 3, 794–810. [Google Scholar] [CrossRef] [PubMed]
- Uota, S.; Zahidunnabi Dewan, M.; Saitoh, Y.; Muto, S.; Itai, A.; Utsunomiya, A.; Watanabe, T.; Yamamoto, N.; Yamaoka, S. An IκB kinase 2 inhibitor IMD-0354 suppresses the survival of adult T-cell leukemia cells. Cancer Sci. 2012, 103, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Satou, Y.; Nosaka, K.; Koya, Y.; Yasunaga, J.I.; Toyokuni, S.; Matsuoka, M. Proteasome inhibitor, bortezomib, potently inhibits the growth of adult T-cell leukemia cells both in vivo and in vitro. Leukemia 2004, 18, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, K.; Kunami, N.; Katsuya, H.; Nogami, R.; Ishikawa, C.; Yotsumoto, F.; Tanji, H.; Mori, N.; Takeshita, M.; Miyamoto, S.; et al. Targeting Bcl-2 family proteins in adult T-cell leukemia/lymphoma: In vitro and in vivo effects of the novel Bcl-2 family inhibitor ABT-737. Cancer Lett. 2012, 317, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Hisatomi, T.; Sueoka-Aragane, N.; Sato, A.; Tomimasu, R.; Ide, M.; Kurimasa, A.; Okamoto, K.; Kimura, S.; Sueoka, E. NK314 potentiates antitumor activity with adult T-cell leukemia-lymphoma cells by inhibition of dual targets on topoisomerase IIα and DNA-dependent protein kinase. Blood 2011, 117, 3575–3584. [Google Scholar] [CrossRef] [PubMed]
- Parrula, C.; Fernandez, S.A.; Zimmerman, B.; Lairmore, M.; Niewiesk, S. Measles virotherapy in a mouse model of adult T-cell leukaemia/lymphoma. J. Gen. Virol. 2011, 92, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Ishida, T.; Utsunomiya, A.; Sato, F.; Mori, F.; Yano, H.; Inagaki, A.; Suzuki, S.; Takino, H.; Ri, M.; et al. Defucosylated anti-CCR4 monoclonal antibody exerts potent ADCC against primary ATLL cells mediated by autologous human immune cells in NOD/Shi-scid, IL-2R γnull mice in vivo. J. Immunol. 2009, 183, 4782–4791. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Muta, H.; Oflazoglu, E.; Yoshikai, Y. Susceptibility of human T-cell leukemia virus type I-infected cells to humanized anti-CD30 monoclonal antibodies in vitro and in vivo. Cancer Sci. 2010, 101, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Bangham, C.R. CTL quality and the control of human retroviral infections. Eur. J. Immunol. 2009, 39, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Crawford, L.; Samuelson, E.; Feuer, G. Hematopoietic stem cells and retroviral infection. Retrovirology 2010, 7. [Google Scholar] [CrossRef] [PubMed]
- Wencker, M.; Gazzolo, L.; Duc Dodon, M. The leukemogenic activity of Tax HTLV-1 during αβ T cell development. Front. Biosci. 2009, 1, 194–204. [Google Scholar] [CrossRef]
- El Hajj, H.; El-Sabban, M.; Hasegawa, H.; Zaatari, G.; Ablain, J.; Saab, S.T.; Janin, A.; Mahfouz, R.; Nasr, R.; Kfoury, Y.; et al. Therapy-induced selective loss of leukemia-initiating activity in murine adult T cell leukemia. J. Exp. Med. 2010, 207, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Centlivre, M.; Legrand, N.; Berkhout, B. A conditionally replicating human immunodeficiency virus in BRG-HIS mice. In Humanized Mice for HIV Research; Polakowski, N., Garcia, J.V., Koyanagi, Y., Manz, M.G., Tager, A.M., Eds.; Springer: New York, NY, USA, 2014; pp. 443–454. [Google Scholar]
- Martin-Latil, S.; Gnadig, N.F.; Mallet, A.; Desdouits, M.; Guivel-Benhassine, F.; Jeannin, P.; Prevost, M.C.; Schwartz, O.; Gessain, A.; Ozden, S.; et al. Transcytosis of HTLV-1 across a tight human epithelial barrier and infection of subepithelial dendritic cells. Blood 2012, 120, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Saito, Y.; Najima, Y.; Tanaka, S.; Ochi, T.; Tomizawa, M.; Doi, T.; Sone, A.; Suzuki, N.; Fujiwara, H.; et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r γnull humanized mice. Proc. Natl. Acad. Sci. USA 2010, 107, 13022–13027. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Takahashi, T.; Katano, I.; Ito, R.; Ito, M.; Harigae, H.; Ishii, N.; Sugamura, K. Induction of human humoral immune responses in a novel HLA-DR-expressing transgenic NOD/Shi-scid/gammacnull mouse. Int. Immunol. 2012, 24, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, H.; Shivalila, C.S.; Cheng, A.W.; Shi, L.; Jaenisch, R. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell 2013, 154, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Grupp, S.A. Advances in T-cell therapy for ALL. Best Pract. Res. Clin. Haematol. 2014, 27, 222–228. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérès, E.; Bagdassarian, E.; This, S.; Villaudy, J.; Rigal, D.; Gazzolo, L.; Duc Dodon, M. From Immunodeficiency to Humanization: The Contribution of Mouse Models to Explore HTLV-1 Leukemogenesis. Viruses 2015, 7, 6371-6386. https://doi.org/10.3390/v7122944
Pérès E, Bagdassarian E, This S, Villaudy J, Rigal D, Gazzolo L, Duc Dodon M. From Immunodeficiency to Humanization: The Contribution of Mouse Models to Explore HTLV-1 Leukemogenesis. Viruses. 2015; 7(12):6371-6386. https://doi.org/10.3390/v7122944
Chicago/Turabian StylePérès, Eléonore, Eugénie Bagdassarian, Sébastien This, Julien Villaudy, Dominique Rigal, Louis Gazzolo, and Madeleine Duc Dodon. 2015. "From Immunodeficiency to Humanization: The Contribution of Mouse Models to Explore HTLV-1 Leukemogenesis" Viruses 7, no. 12: 6371-6386. https://doi.org/10.3390/v7122944
APA StylePérès, E., Bagdassarian, E., This, S., Villaudy, J., Rigal, D., Gazzolo, L., & Duc Dodon, M. (2015). From Immunodeficiency to Humanization: The Contribution of Mouse Models to Explore HTLV-1 Leukemogenesis. Viruses, 7(12), 6371-6386. https://doi.org/10.3390/v7122944