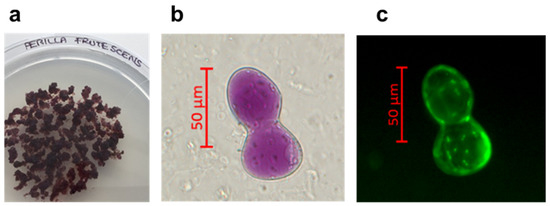
Open AccessArticle
A Novel Perilla frutescens (L.) Britton Cell-Derived Phytocomplex Regulates Keratinocytes Inflammatory Cascade and Barrier Function and Preserves Vaginal Mucosal Integrity In Vivo
by
Giovanna Pressi, Giovanna Rigillo, Paolo Governa, Vittoria Borgonetti, Giulia Baini, Raffaella Rizzi, Chiara Guarnerio, Oriana Bertaiola, Marco Frigo, Matilde Merlin, Stefania Paltrinieri, Roberto Zambonin, Stefano Pandolfo and Marco Biagi
Cited by 6 | Viewed by 2202
Abstract
In the last years, the medicinal plant
Perilla frutescens (L.) Britton has gained scientific interest because leaf extracts, due to the presence of rosmarinic acid and other polyphenols, have shown anti-allergic and skin protective potential in pre-clinical studies. Nevertheless, the lack of standardized
[...] Read more.
In the last years, the medicinal plant
Perilla frutescens (L.) Britton has gained scientific interest because leaf extracts, due to the presence of rosmarinic acid and other polyphenols, have shown anti-allergic and skin protective potential in pre-clinical studies. Nevertheless, the lack of standardized extracts has limited clinical applications to date. In this work, for the first time, a standardized phytocomplex of
P. frutescens, enriched in rosmarinic acid and total polyphenols, was produced through innovative in vitro cell culture biotechnology and tested. The activity of perilla was evaluated in an in vitro inflammatory model of human keratinocytes (HaCaT) by monitoring tight junctions, filaggrin, and loricrin protein levels, the release of pro-inflammatory cytokines and JNK MAPK signaling. In a practical health care application, the perilla biotechnological phytocomplex was tested in a multilayer model of vaginal mucosa, and then, in a preliminary clinical observation to explore its capacity to preserve vaginal mucosal integrity in women in peri-menopause. In keratinocytes cells, perilla phytocomplex demonstrated to exert a marked activity in epidermis barrier maintenance and anti-inflammatory effects, preserving tight junction expression and downregulating cytokines release through targeting JNK activation. Furthermore, perilla showed positive effects in retaining vaginal mucosal integrity in the reconstructed vaginal mucosa model and in vivo tests. Overall, our data suggest that the biotechnological
P. frutescens phytocomplex could represent an innovative ingredient for dermatological applications.
Full article
►▼
Show Figures