Evaluation of Whole-Cell and Acellular Pertussis Vaccines in the Context of Long-Term Herd Immunity
Abstract
:1. Introduction
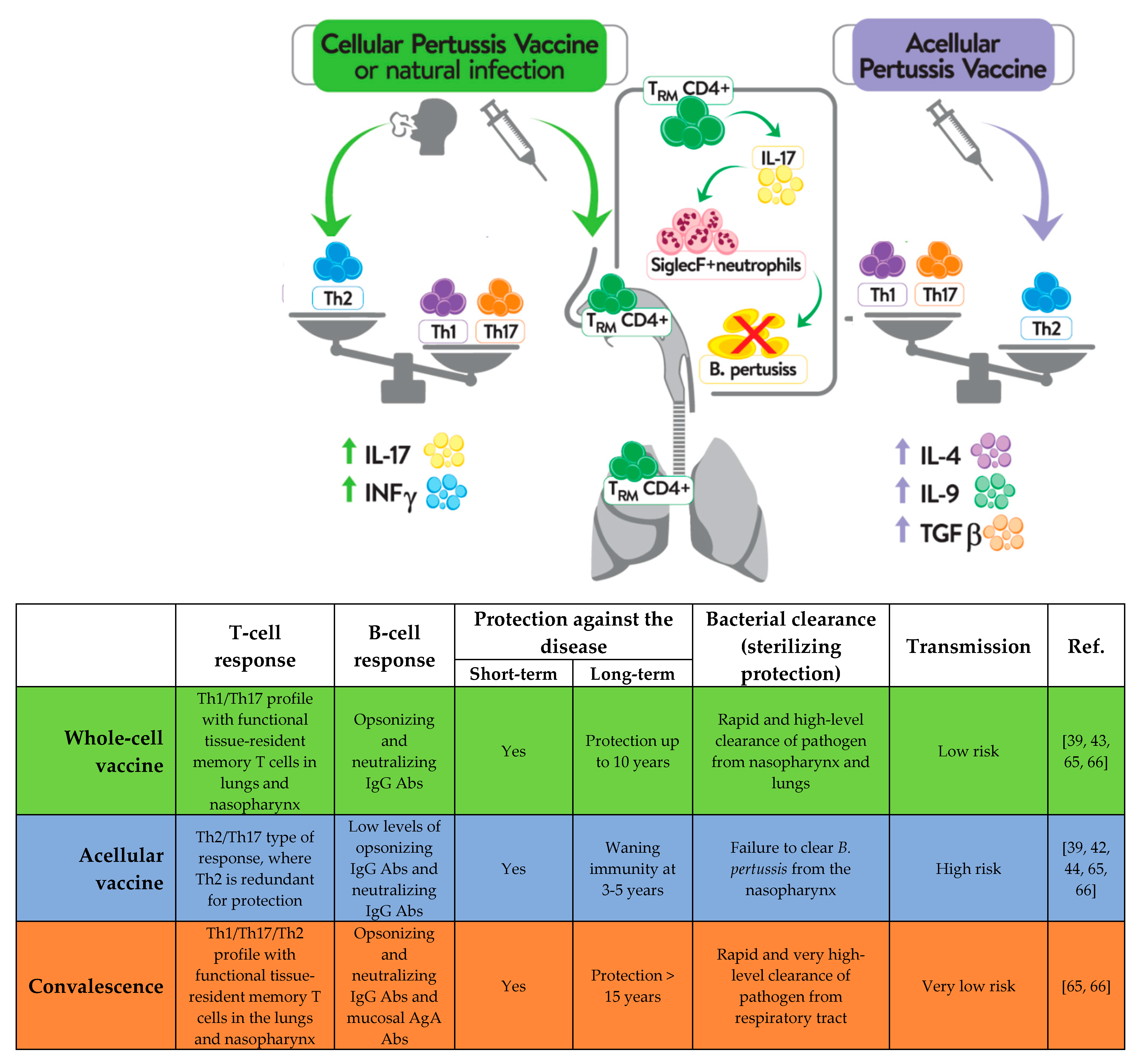
2. Acellular and Whole-Cell Pertussis Vaccine Induce Different Immune Responses
3. The Type of Pertussis Vaccine Affects Both the Response in Later Life and the Loss-of-Immunity Rate
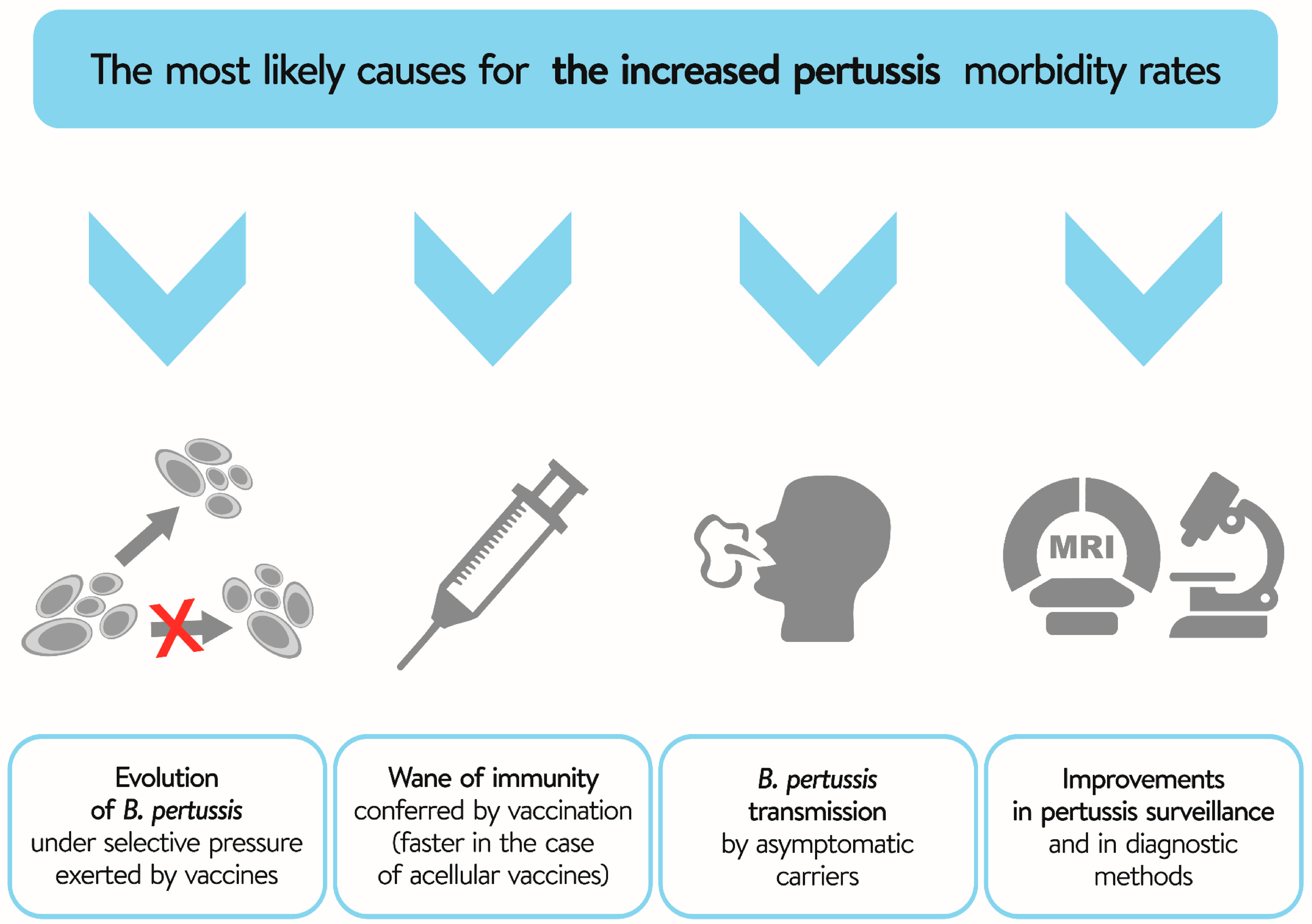
4. Evolution of B. pertussis—The Role of Selection Pressure Exerted by Vaccines
5. Boosters and the Relevance of Immunization during Pregnancy
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yih, W.K.; Lett, S.M.; des Vignes, F.N.; Garrison, K.M.; Sipe, P.L.; Marchant, C.D. The increasing incidence of pertussis in Massachusetts adolescents and adults, 1989–1998. J. Infect. Dis. 2000, 182, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Capili, C.R.; Hettinger, A.; Rigelman-Hedberg, N.; Fink, L.; Boyce, T.; Lahr, B.; Juhn, Y.J. Increased risk of pertussis in patients with asthma. J. Allergy Clin. Immunol. 2012, 129, 957–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.C.; McIntyre, P.; Kaldor, J.M.; Quinn, H.E.; Ridda, I.; Banks, E. Pertussis in older adults: Prospective study of risk factors and morbidity. Clin. Infect. Dis. 2012, 55, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Mbayei, S.A.; Faulkner, A.; Miner, C.; Edge, K.; Cruz, V.; Peña, S.A.; Kudish, K.; Coleman, J.; Pradhan, E.; Thomas, S.; et al. Severe Pertussis Infections in the United States, 2011–2015. Clin. Infect. Dis. 2019, 69, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Caro, V.; Njamkepo, E.; Van Amersfoorth, S.C.; Mooi, F.R.; Advani, A.; Hallander, H.O.; He, Q.; Mertsola, J.; Riffelmann, M.; Vahrenholz, C.; et al. Pulsed-field gel electrophoresis analysis of Bordetella pertussis populations in various European countries with different vaccine policies. Microbes Infect. 2005, 7, 976–982. [Google Scholar] [CrossRef]
- Heininger, U.; André, P.; Chlibek, R.; Kristufkova, Z.; Kutsar, K.; Mangarov, A.; Mészner, Z.; Nitsch-Osuch, A.; Petrović, V.; Prymula, R.; et al. Comparative Epidemiologic Characteristics of Pertussis in 10 Central and Eastern European Countries, 2000–2013. PLoS ONE 2016, 11, e0155949. [Google Scholar] [CrossRef] [Green Version]
- Wong, A.; Opinel, A.; Combes, S.J.; Toubiana, J.; Brisse, S. Determining Factors for Pertussis Vaccination Policy: A Study in Five EU Countries. Vaccines 2020, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Fanget, N. Pertussis: A tale of two vaccines. Nat. Milest. Vaccines 2020. Available online: https://media.nature.com/original/magazine-assets/d42859-020-00013-8/d42859-020-00013-8.pdf (accessed on 25 October 2022).
- European Centre for Disease Prevention and Control. Available online: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=3&SelectedCountryIdByDisease=-1 (accessed on 25 October 2022).
- Kendrick, P.L. Use of Alum-Treated Pertussis Vaccine, and of Alum-Precipitated Combined Pertussis Vaccine and Diphtheria Toxoid, for Active Immunization. Am. J. Public Health Nations Health 1942, 32, 615–626. [Google Scholar] [CrossRef]
- Romanus, V.; Jonsell, R.; Bergquist, S.O. Pertussis in Sweden after the cessation of general immunization in 1979. Pediatr. Infect. Dis. J. 1987, 6, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Donna, H. TV Report on Vaccine Stirs Bitter Controversy; The Washington Post: Washington, DC, USA, 1982. [Google Scholar]
- Gangarosa, E.J.; Galazka, A.M.; Wolfe, C.R.; Phillips, L.M.; Gangarosa, R.E.; Miller, E.; Chen, R.T. Impact of anti-vaccine movements on pertussis control: The untold story. Lancet 1998, 351, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Kuno-Sakai, H. Pertussis vaccines in Japan—A clue toward understanding of Japanese attitude to vaccines. J. Trop. Pediatr. 1991, 37, 45–47. [Google Scholar] [CrossRef]
- Sato, Y.; Kimura, M.; Fukumi, H. Development of a pertussis component vaccine in Japan. Lancet 1984, 1, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Nagai, M. Acellular pertussis vaccines in Japan: Past, present and future. Expert Rev. Vaccines 2005, 4, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Noble, G.R.; Bernier, R.H.; Esber, E.C.; Hardegree, M.C.; Hinman, A.R.; Klein, D.; Saah, A.J. Acellular and whole-cell pertussis vaccines in Japan. Report of a visit by US scientists. JAMA 1987, 257, 1351–1356. [Google Scholar] [CrossRef]
- Ad Hoc Group for the Study of Pertussis Vaccines. Placebo-controlled trial of two acellular pertussis vaccines in Sweden--protective efficacy and adverse events. Lancet 1988, 1, 955–960. [Google Scholar]
- Zhang, L.; Prietsch, S.O.; Axelsson, I.; Halperin, S.A. Acellular vaccines for preventing whooping cough in children. Cochrane Database Syst. Rev. 2014, 2014, CD001478. [Google Scholar] [CrossRef]
- Dewan, K.K.; Linz, B.; DeRocco, S.E.; Harvill, E.T. Acellular Pertussis Vaccine Components: Today and Tomorrow. Vaccines 2020, 8, 217. [Google Scholar] [CrossRef]
- Thierry-Carstensen, B.; Dalby, T.; Stevner, M.A.; Robbins, J.B.; Schneerson, R.; Trollfors, B. Experience with monocomponent acellular pertussis combination vaccines for infants, children, adolescents and adults--a review of safety, immunogenicity, efficacy and effectiveness studies and 15 years of field experience. Vaccine 2013, 31, 5178–5191. [Google Scholar] [CrossRef]
- Gale, J.L.; Thapa, P.B.; Wassilak, S.G.; Bobo, J.K.; Mendelman, P.M.; Foy, H.M. Risk of serious acute neurological illness after immunization with diphtheria-tetanus-pertussis vaccine. A population-based case-control study. JAMA 1994, 271, 37–41. [Google Scholar] [CrossRef]
- Ray, P.; Hayward, J.; Michelson, D.; Lewis, E.; Schwalbe, J.; Black, S.; Shinefield, H.; Marcy, M.; Huff, K.; Ward, J.; et al. Encephalopathy after whole-cell pertussis or measles vaccination: Lack of evidence for a causal association in a retrospective case-control study. Pediatr. Infect. Dis. J. 2006, 25, 768–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Pertussis Vaccines: WHO Position Paper—August 2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/242413/WER9035_433-458.PDF?sequence=1 (accessed on 28 November 2022).
- World Health Organization. Available online: https://cdn.who.int/media/docs/default-source/pvg/global-vaccine-safety/dtp-vaccine-rates-information-sheet.pdf?sfvrsn=511803ec_4&download=true (accessed on 28 November 2022).
- Mooi, F.R.; Van Der Maas, N.A.; De Melker, H.E. Pertussis resurgence: Waning immunity and pathogen adaptation—Two sides of the same coin. Epidemiol. Infect. 2014, 142, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Celentano, L.P.; Massari, M.; Paramatti, D.; Salmaso, S.; Tozzi, A.E.; Group, E.-N. Resurgence of pertussis in Europe. Pediatr. Infect. Dis. J. 2005, 24, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Yi, S.; Cho, S.I. Recent increase in pertussis incidence in Korea: An age-period-cohort analysis. Epidemiol. Health 2021, 43, e2021053. [Google Scholar] [CrossRef] [PubMed]
- Fullen, A.R.; Yount, K.S.; Dubey, P.; Deora, R. Whoop! There it is: The surprising resurgence of pertussis. PLoS Pathog. 2020, 16, e1008625. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.A. Changing pertussis epidemiology: Everything old is new again. J. Infect. Dis. 2014, 209, 978–981. [Google Scholar] [CrossRef]
- Falleiros Arlant, L.H.; de Colsa, A.; Flores, D.; Brea, J.; Avila Aguero, M.L.; Hozbor, D.F. Pertussis in Latin America: Epidemiology and control strategies. Expert Rev. Anti Infect. Ther. 2014, 12, 1265–1275. [Google Scholar] [CrossRef]
- Tan, T.; Dalby, T.; Forsyth, K.; Halperin, S.A.; Heininger, U.; Hozbor, D.; Plotkin, S.; Ulloa-Gutierrez, R.; Wirsing von König, C.H. Pertussis Across the Globe: Recent Epidemiologic Trends From 2000 to 2013. Pediatr. Infect. Dis. J. 2015, 34, e222–e232. [Google Scholar] [CrossRef]
- World Health Organization. The Immunological Basis for Immunization Series: Module 4: Pertussis; World Health Organization: Geneva, Switzerland, 2017. Available online: https://apps.who.int/iris/handle/10665/259388 (accessed on 19 October 2022).
- World Health Organization. Announcing the publication of the WHO immunological basis for immunization series module on pertussis vaccines. Vaccine 2019, 37, 217–218. [Google Scholar] [CrossRef]
- Papagiannis, D.; Thireos, E.; Mariolis, A.; Katsioulis, A.; Gartzonika, K.; Malliaraki, N.; Agnantis, C.; Tsaras, K.; Malli, F.; Rouka, E.C.; et al. Pertussis Prevalence in Adult Population in Greece: A Seroprevalence Nationwide Study. Vaccines 2022, 10, 1511. [Google Scholar] [CrossRef]
- Bahar, E.; Shamarina, D.; Sergerie, Y.; Mukherjee, P. Descriptive Overview of Pertussis Epidemiology among Older Adults in Europe during 2010–2020. Infect. Dis. Ther. 2022, 11, 1821–1838. [Google Scholar] [CrossRef] [PubMed]
- Macina, D.; Evans, K.E. Bordetella pertussis in School-Age Children, Adolescents and Adults: A Systematic Review of Epidemiology and Mortality in Europe. Infect. Dis. Ther. 2021, 10, 2071–2118. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.C.; Wilk, M.M.; Misiak, A.; Borkner, L.; Murphy, D.; Mills, K.H.G. Sustained protective immunity against Bordetella pertussis nasal colonization by intranasal immunization with a vaccine-adjuvant combination that induces IL-17-secreting Trm cells. Mucosal. Immunol. 2018, 11, 1763–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warfel, J.M.; Edwards, K.M. Pertussis vaccines and the challenge of inducing durable immunity. Curr. Opin. Immunol. 2015, 35, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Liko, J.; Robison, S.G.; Cieslak, P.R. Priming with whole-cell versus acellular pertussis vaccine. N. Engl. J. Med. 2013, 368, 581–582. [Google Scholar] [CrossRef]
- Melvin, J.A.; Scheller, E.V.; Miller, J.F.; Cotter, P.A. Bordetella pertussis pathogenesis: Current and future challenges. Nat. Rev. Microbiol. 2014, 12, 274–288. [Google Scholar] [CrossRef] [Green Version]
- Klein, N.P.; Bartlett, J.; Rowhani-Rahbar, A.; Fireman, B.; Baxter, R. Waning protection after fifth dose of acellular pertussis vaccine in children. N. Engl. J. Med. 2012, 367, 1012–1019. [Google Scholar] [CrossRef] [Green Version]
- Klein, N.P.; Bartlett, J.; Fireman, B.; Rowhani-Rahbar, A.; Baxter, R. Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers. Pediatrics 2013, 131, e1716–e1722. [Google Scholar] [CrossRef] [Green Version]
- Klein, N.P.; Bartlett, J.; Fireman, B.; Baxter, R. Waning Tdap Effectiveness in Adolescents. Pediatrics 2016, 137, e20153326. [Google Scholar] [CrossRef] [Green Version]
- Althouse, B.M.; Scarpino, S.V. Asymptomatic transmission and the resurgence of Bordetella pertussis. BMC Med. 2015, 13, 146. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, S.L.; Ware, R.S.; Grimwood, K.; Lambert, S.B. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA 2012, 308, 454–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; He, Q. Immune persistence after pertussis vaccination. Hum. Vaccin Immunother. 2017, 13, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Chasaide, C.N.; Mills, K.H.G. Next-Generation Pertussis Vaccines Based on the Induction of Protective T Cells in the Respiratory Tract. Vaccines 2020, 8, 621. [Google Scholar] [CrossRef] [PubMed]
- Coutte, L.; Alonso, S.; Reveneau, N.; Willery, E.; Quatannens, B.; Locht, C.; Jacob-Dubuisson, F. Role of adhesin release for mucosal colonization by a bacterial pathogen. J. Exp. Med. 2003, 197, 735–742. [Google Scholar] [CrossRef] [Green Version]
- Ishibashi, Y.; Nishikawa, A. Bordetella pertussis infection of human respiratory epithelial cells up-regulates intercellular adhesion molecule-1 expression: Role of filamentous hemagglutinin and pertussis toxin. Microb. Pathog. 2002, 33, 115–125. [Google Scholar] [CrossRef]
- Jahnsen, F.L.; Strickland, D.H.; Thomas, J.A.; Tobagus, I.T.; Napoli, S.; Zosky, G.R.; Turner, D.J.; Sly, P.D.; Stumbles, P.A.; Holt, P.G. Accelerated antigen sampling and transport by airway mucosal dendritic cells following inhalation of a bacterial stimulus. J. Immunol. 2006, 177, 5861–5867. [Google Scholar] [CrossRef] [Green Version]
- Klimova, N.; Holubova, J.; Streparola, G.; Tomala, J.; Brazdilova, L.; Stanek, O.; Bumba, L.; Sebo, P. Pertussis toxin suppresses dendritic cell-mediated delivery of B. pertussis into lung-draining lymph nodes. PLoS Pathog. 2022, 18, e1010577. [Google Scholar] [CrossRef]
- Holubova, J.; Stanek, O.; Juhasz, A.; Hamidou Soumana, I.; Makovicky, P.; Sebo, P. The Fim and FhaB adhesins play a crucial role in nasal cavity infection and Bordetella pertussis transmission in a novel mouse catarrhal infection model. PLoS Pathog. 2022, 18, e1010402. [Google Scholar] [CrossRef]
- Esposito, S.; Agliardi, T.; Giammanco, A.; Faldella, G.; Cascio, A.; Bosis, S.; Friscia, O.; Clerici, M.; Principi, N. Long-term pertussis-specific immunity after primary vaccination with a combined diphtheria, tetanus, tricomponent acellular pertussis, and hepatitis B vaccine in comparison with that after natural infection. Infect. Immun. 2001, 69, 4516–4520. [Google Scholar] [CrossRef] [Green Version]
- Esposito, S.; Principi, N. Prevention of pertussis: An unresolved problem. Hum. Vaccin Immunother. 2018, 14, 2452–2459. [Google Scholar] [CrossRef] [Green Version]
- Ross, P.J.; Sutton, C.E.; Higgins, S.; Allen, A.C.; Walsh, K.; Misiak, A.; Lavelle, E.C.; McLoughlin, R.M.; Mills, K.H. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: Towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog. 2013, 9, e1003264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahon, B.P.; Sheahan, B.J.; Griffin, F.; Murphy, G.; Mills, K.H. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-gamma receptor or immunoglobulin mu chain genes. J. Exp. Med. 1997, 186, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; Murphy, G.; Gothefors, L.; Nilsson, L.; Storsaeter, J.; Mills, K.H. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J. Infect. Dis. 1997, 175, 1246–1250. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.; Murphy, G.; Ryan, E.; Nilsson, L.; Shackley, F.; Gothefors, L.; Oymar, K.; Miller, E.; Storsaeter, J.; Mills, K.H. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology 1998, 93, 1–10. [Google Scholar] [CrossRef]
- Ausiello, C.M.; Urbani, F.; la Sala, A.; Lande, R.; Cassone, A. Vaccine- and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infect. Immun. 1997, 65, 2168–2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palazzo, R.; Carollo, M.; Bianco, M.; Fedele, G.; Schiavoni, I.; Pandolfi, E.; Villani, A.; Tozzi, A.E.; Mascart, F.; Ausiello, C.M. Persistence of T-cell immune response induced by two acellular pertussis vaccines in children five years after primary vaccination. New Microbiol. 2016, 39, 35–47. [Google Scholar]
- Wilk, M.M.; Misiak, A.; McManus, R.M.; Allen, A.C.; Lynch, M.A.; Mills, K.H.G. Lung CD4 Tissue-Resident Memory T Cells Mediate Adaptive Immunity Induced by Previous Infection of Mice with Bordetella pertussis. J. Immunol. 2017, 199, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Solans, L.; Debrie, A.S.; Borkner, L.; Aguiló, N.; Thiriard, A.; Coutte, L.; Uranga, S.; Trottein, F.; Martín, C.; Mills, K.H.G.; et al. IL-17-dependent SIgA-mediated protection against nasal Bordetella pertussis infection by live attenuated BPZE1 vaccine. Mucosal. Immunol. 2018, 11, 1753–1762. [Google Scholar] [CrossRef] [Green Version]
- Borkner, L.; Curham, L.M.; Wilk, M.M.; Moran, B.; Mills, K.H.G. IL-17 mediates protective immunity against nasal infection with Bordetella pertussis by mobilizing neutrophils, especially Siglec-F+ neutrophils. Mucosal. Immunol. 2021, 14, 1183–1202. [Google Scholar] [CrossRef]
- Wilk, M.M.; Borkner, L.; Misiak, A.; Curham, L.; Allen, A.C.; Mills, K.H.G. Immunization with whole cell but not acellular pertussis vaccines primes CD4 Trm cells that sustain protective immunity against nasal colonization with Bordetella pertussis. Emerg. Microbes Infect. 2019, 8, 169–185. [Google Scholar] [CrossRef] [Green Version]
- Warfel, J.M.; Zimmerman, L.I.; Merkel, T.J. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc. Natl. Acad. Sci. USA 2014, 111, 787–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redhead, K.; Watkins, J.; Barnard, A.; Mills, K.H. Effective immunization against Bordetella pertussis respiratory infection in mice is dependent on induction of cell-mediated immunity. Infect. Immun. 1993, 61, 3190–3198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Lee, S.; Hendrikx, L.H.; Sanders, E.A.M.; Berbers, G.A.M.; Buisman, A.M. Whole-Cell or Acellular Pertussis Primary Immunizations in Infancy Determines Adolescent Cellular Immune Profiles. Front. Immunol. 2018, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Antunes, R.; Babor, M.; Carpenter, C.; Khalil, N.; Cortese, M.; Mentzer, A.J.; Seumois, G.; Petro, C.D.; Purcell, L.A.; Vijayanand, P.; et al. Th1/Th17 polarization persists following whole-cell pertussis vaccination despite repeated acellular boosters. J. Clin. Investig. 2018, 128, 3853–3865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, K.L.; Blackwood, C.B.; Horspool, A.M.; Pyles, G.M.; Sen-Kilic, E.; Grayson, E.M.; Huckaby, A.B.; Witt, W.T.; DeJong, M.A.; Wolf, M.A.; et al. Long-Term Analysis of Pertussis Vaccine Immunity to Identify Potential Markers of Vaccine-Induced Memory Associated With Whole Cell But Not Acellular Pertussis Immunization in Mice. Front. Immunol. 2022, 13, 838504. [Google Scholar] [CrossRef]
- Valeri, V.; Sochon, A.; Cousu, C.; Chappert, P.; Lecoeuche, D.; Blanc, P.; Weill, J.C.; Reynaud, C.A. The whole-cell pertussis vaccine imposes a broad effector B-cell response in mouse heterologous prime-boost settings. JCI Insight 2022, 8, e157034. [Google Scholar] [CrossRef]
- Diks, A.M.; Versteegen, P.; Teodosio, C.; Groenland, R.J.; de Mooij, B.; Buisman, A.M.; Torres-Valle, A.; Pérez-Andrés, M.; Orfao, A.; Berbers, G.A.M.; et al. Age and Primary Vaccination Background Influence the Plasma Cell Response to Pertussis Booster Vaccination. Vaccines 2022, 10, 136. [Google Scholar] [CrossRef]
- Aalberse, R.C.; Stapel, S.O.; Schuurman, J.; Rispens, T. Immunoglobulin G4: An odd antibody. Clin. Exp. Allergy 2009, 39, 469–477. [Google Scholar] [CrossRef]
- Sallusto, F. Heterogeneity of Human CD4(+) T Cells Against Microbes. Annu. Rev. Immunol. 2016, 34, 317–334. [Google Scholar] [CrossRef]
- Jenkinson, D. Duration of effectiveness of pertussis vaccine: Evidence from a 10 year community study. Br. Med. J. 1988, 296, 612–614. [Google Scholar] [CrossRef] [Green Version]
- Clark, T.A.; Messonnier, N.E.; Hadler, S.C. Pertussis control: Time for something new? Trends Microbiol. 2012, 20, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Burdin, N.; Handy, L.K.; Plotkin, S.A. What Is Wrong with Pertussis Vaccine Immunity? The Problem of Waning Effectiveness of Pertussis Vaccines. Cold Spring Harb Perspect Biol. 2017, 9, a029454. [Google Scholar] [CrossRef] [PubMed]
- McGirr, A.; Fisman, D.N. Duration of pertussis immunity after DTaP immunization: A meta-analysis. Pediatrics 2015, 135, 331–343. [Google Scholar] [CrossRef]
- Salmaso, S.; Mastrantonio, P.; Tozzi, A.E.; Stefanelli, P.; Anemona, A.; Ciofi degli Atti, M.L.; Giammanco, A.; Group, S.I.W. Sustained efficacy during the first 6 years of life of 3-component acellular pertussis vaccines administered in infancy: The Italian experience. Pediatrics 2001, 108, E81. [Google Scholar] [CrossRef] [Green Version]
- Lugauer, S.; Heininger, U.; Cherry, J.D.; Stehr, K. Long-term clinical effectiveness of an acellular pertussis component vaccine and a whole cell pertussis component vaccine. Eur. J. Pediatr. 2002, 161, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, K.; Yam, A.; Simondon, K.; Pinchinat, S.; Simondon, F. Risk factors for acellular and whole-cell pertussis vaccine failure in Senegalese children. Vaccine 2004, 23, 623–628. [Google Scholar] [CrossRef]
- Vickers, D.; Ross, A.G.; Mainar-Jaime, R.C.; Neudorf, C.; Shah, S. Whole-cell and acellular pertussis vaccination programs and rates of pertussis among infants and young children. CMAJ 2006, 175, 1213–1217. [Google Scholar] [CrossRef] [Green Version]
- Tindberg, Y.; Blennow, M.; Granström, M. A ten year follow-up after immunization with a two component acellular pertussis vaccine. Pediatr. Infect. Dis. J. 1999, 18, 361–365. [Google Scholar] [CrossRef]
- Witt, M.A.; Arias, L.; Katz, P.H.; Truong, E.T.; Witt, D.J. Reduced risk of pertussis among persons ever vaccinated with whole cell pertussis vaccine compared to recipients of acellular pertussis vaccines in a large US cohort. Clin. Infect. Dis. 2013, 56, 1248–1254. [Google Scholar] [CrossRef]
- Onorato, I.M.; Wassilak, S.G.; Meade, B. Efficacy of whole-cell pertussis vaccine in preschool children in the United States. JAMA 1992, 267, 2745–2749. [Google Scholar] [CrossRef]
- Chit, A.; Zivaripiran, H.; Shin, T.; Lee, J.K.H.; Tomovici, A.; Macina, D.; Johnson, D.R.; Decker, M.D.; Wu, J. Acellular pertussis vaccines effectiveness over time: A systematic review, meta-analysis and modeling study. PLoS ONE 2018, 13, e0197970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeAngelis, H.; Scarpino, S.V.; Fitzpatrick, M.C.; Galvani, A.P.; Althouse, B.M. Epidemiological and Economic Effects of Priming With the Whole-Cell Bordetella pertussis Vaccine. JAMA Pediatr. 2016, 170, 459–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, T.; Cherry, J.D.; Chang, S.J.; Knoll, M.D.; Lee, M.L.; Barenkamp, S.; Bernstein, D.; Edelman, R.; Edwards, K.M.; Greenberg, D.; et al. Immune responses and antibody decay after immunization of adolescents and adults with an acellular pertussis vaccine: The APERT Study. J. Infect. Dis. 2004, 190, 535–544. [Google Scholar] [CrossRef] [PubMed]
- van Twillert, I.; Bonačić Marinović, A.A.; Kuipers, B.; van Gaans-van den Brink, J.A.; Sanders, E.A.; van Els, C.A. Impact of age and vaccination history on long-term serological responses after symptomatic B. pertussis infection, a high dimensional data analysis. Sci. Rep. 2017, 7, 40328. [Google Scholar] [CrossRef]
- Mooi, F.R.; van Loo, I.H.; van Gent, M.; He, Q.; Bart, M.J.; Heuvelman, K.J.; de Greeff, S.C.; Diavatopoulos, D.; Teunis, P.; Nagelkerke, N.; et al. Bordetella pertussis strains with increased toxin production associated with pertussis resurgence. Emerg. Infect. Dis. 2009, 15, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Bart, M.J.; Harris, S.R.; Advani, A.; Arakawa, Y.; Bottero, D.; Bouchez, V.; Cassiday, P.K.; Chiang, C.S.; Dalby, T.; Fry, N.K.; et al. Global population structure and evolution of Bordetella pertussis and their relationship with vaccination. mBio 2014, 5, e01074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallander, H.O.; Advani, A.; Donnelly, D.; Gustafsson, L.; Carlsson, R.M. Shifts of Bordetella pertussis variants in Sweden from 1970 to 2003, during three periods marked by different vaccination programs. J. Clin. Microbiol. 2005, 43, 2856–2865. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.W.; Pawloski, L.; Williams, M.; Weening, K.; DeBolt, C.; Qin, X.; Reynolds, L.; Kenyon, C.; Giambrone, G.; Kudish, K.; et al. Pertactin-negative Bordetella pertussis strains: Evidence for a possible selective advantage. Clin. Infect. Dis. 2015, 60, 223–227. [Google Scholar] [CrossRef] [Green Version]
- Safarchi, A.; Octavia, S.; Luu, L.D.; Tay, C.Y.; Sintchenko, V.; Wood, N.; Marshall, H.; McIntyre, P.; Lan, R. Pertactin negative Bordetella pertussis demonstrates higher fitness under vaccine selection pressure in a mixed infection model. Vaccine 2015, 33, 6277–6281. [Google Scholar] [CrossRef]
- Esposito, S.; Stefanelli, P.; Fry, N.K.; Fedele, G.; He, Q.; Paterson, P.; Tan, T.; Knuf, M.; Rodrigo, C.; Weil Olivier, C.; et al. Pertussis Prevention: Reasons for Resurgence, and Differences in the Current Acellular Pertussis Vaccines. Front. Immunol. 2019, 10, 1344. [Google Scholar] [CrossRef] [Green Version]
- Otsuka, N.; Han, H.J.; Toyoizumi-Ajisaka, H.; Nakamura, Y.; Arakawa, Y.; Shibayama, K.; Kamachi, K. Prevalence and genetic characterization of pertactin-deficient Bordetella pertussis in Japan. PLoS ONE 2012, 7, e31985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeddeman, A.; van Gent, M.; Heuvelman, C.J.; van der Heide, H.G.; Bart, M.J.; Advani, A.; Hallander, H.O.; Wirsing von Konig, C.H.; Riffelman, M.; Storsaeter, J.; et al. Investigations into the emergence of pertactin-deficient Bordetella pertussis isolates in six European countries, 1996 to 2012. Eurosurveill 2014, 19, 20881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carriquiriborde, F.; Regidor, V.; Aispuro, P.M.; Magali, G.; Bartel, E.; Bottero, D.; Hozbor, D. Rare Detection of Bordetella pertussis Pertactin-Deficient Strains in Argentina. Emerg. Infect. Dis. 2019, 25, 2048–2054. [Google Scholar] [CrossRef] [PubMed]
- Mir-Cros, A.; Moreno-Mingorance, A.; Martín-Gómez, M.T.; Abad, R.; Bloise, I.; Campins, M.; González-Praetorius, A.; Gutiérrez, M.N.; Martín-González, H.; Muñoz-Almagro, C.; et al. Pertactin-Deficient Bordetella pertussis with Unusual Mechanism of Pertactin Disruption, Spain, 1986–2018. Emerg. Infect. Dis. 2022, 28, 967–976. [Google Scholar] [CrossRef]
- Ma, L.; Caulfield, A.; Dewan, K.K.; Harvill, E.T. Pertactin-Deficient Bordetella pertussis, Vaccine-Driven Evolution, and Reemergence of Pertussis. Emerg. Infect. Dis. 2021, 27, 1561–1566. [Google Scholar] [CrossRef]
- Bouchez, V.; Brun, D.; Cantinelli, T.; Dore, G.; Njamkepo, E.; Guiso, N. First report and detailed characterization of B. pertussis isolates not expressing Pertussis Toxin or Pertactin. Vaccine 2009, 27, 6034–6041. [Google Scholar] [CrossRef]
- Bouchez, V.; Hegerle, N.; Strati, F.; Njamkepo, E.; Guiso, N. New Data on Vaccine Antigen Deficient Bordetella pertussis Isolates. Vaccines 2015, 3, 751–770. [Google Scholar] [CrossRef]
- Linz, B.; Ivanov, Y.V.; Preston, A.; Brinkac, L.; Parkhill, J.; Kim, M.; Harris, S.R.; Goodfield, L.L.; Fry, N.K.; Gorringe, A.R.; et al. Acquisition and loss of virulence-associated factors during genome evolution and speciation in three clades of Bordetella species. BMC Genom. 2016, 17, 767. [Google Scholar] [CrossRef] [Green Version]
- Sealey, K.L.; Harris, S.R.; Fry, N.K.; Hurst, L.D.; Gorringe, A.R.; Parkhill, J.; Preston, A. Genomic analysis of isolates from the United Kingdom 2012 pertussis outbreak reveals that vaccine antigen genes are unusually fast evolving. J. Infect. Dis. 2015, 212, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Sealey, K.L.; Belcher, T.; Preston, A. Bordetella pertussis epidemiology and evolution in the light of pertussis resurgence. Infect. Genet. Evol. 2016, 40, 136–143. [Google Scholar] [CrossRef]
- Lefrancq, N.; Bouchez, V.; Fernandes, N.; Barkoff, A.M.; Bosch, T.; Dalby, T.; Åkerlund, T.; Darenberg, J.; Fabianova, K.; Vestrheim, D.F.; et al. Global spatial dynamics and vaccine-induced fitness changes of. Sci. Transl. Med. 2022, 14, eabn3253. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women. Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 131–135. [Google Scholar]
- Vygen-Bonnet, S.; Hellenbrand, W.; Garbe, E.; von Kries, R.; Bogdan, C.; Heininger, U.; Röbl-Mathieu, M.; Harder, T. Safety and effectiveness of acellular pertussis vaccination during pregnancy: A systematic review. BMC Infect. Dis. 2020, 20, 136. [Google Scholar] [CrossRef] [Green Version]
- Donegan, K.; King, B.; Bryan, P. Safety of pertussis vaccination in pregnant women in UK: Observational study. BMJ 2014, 349, g4219. [Google Scholar] [CrossRef]
- Villarreal Pérez, J.Z.; Ramírez Aranda, J.M.; de la O Cavazos, M.; Zamudio Osuna, M.J.; Perales Dávila, J.; Ballesteros Elizondo, M.R.; Gómez Meza, M.V.; García Elizondo, F.J.; Rodríguez González, A.M. Randomized clinical trial of the safety and immunogenicity of the Tdap vaccine in pregnant Mexican women. Hum. Vaccin Immunother. 2017, 13, 128–135. [Google Scholar] [CrossRef] [Green Version]
- D’Heilly, C.; Switzer, C.; Macina, D. Safety of Maternal Immunization Against Pertussis: A Systematic Review. Infect. Dis. Ther. 2019, 8, 543–568. [Google Scholar] [CrossRef] [Green Version]
- Munoz, F.M.; Bond, N.H.; Maccato, M.; Pinell, P.; Hammill, H.A.; Swamy, G.K.; Walter, E.B.; Jackson, L.A.; Englund, J.A.; Edwards, M.S.; et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: A randomized clinical trial. JAMA 2014, 311, 1760–1769. [Google Scholar] [CrossRef]
- Kandeil, W.; van den Ende, C.; Bunge, E.M.; Jenkins, V.A.; Ceregido, M.A.; Guignard, A. A systematic review of the burden of pertussis disease in infants and the effectiveness of maternal immunization against pertussis. Expert Rev. Vaccines 2020, 19, 621–638. [Google Scholar] [CrossRef]
- Abu Raya, B.; Srugo, I.; Kessel, A.; Peterman, M.; Vaknin, A.; Bamberger, E. The Decline of Pertussis-Specific Antibodies after Tetanus, Diphtheria, and Acellular Pertussis Immunization in Late Pregnancy. J. Infect. Dis. 2015, 212, 1869–1873. [Google Scholar] [CrossRef] [Green Version]
- Halperin, S.A.; Langley, J.M.; Ye, L.; MacKinnon-Cameron, D.; Elsherif, M.; Allen, V.M.; Smith, B.; Halperin, B.A.; McNeil, S.A.; Vanderkooi, O.G.; et al. A Randomized Controlled Trial of the Safety and Immunogenicity of Tetanus, Diphtheria, and Acellular Pertussis Vaccine Immunization during Pregnancy and Subsequent Infant Immune Response. Clin. Infect. Dis. 2018, 67, 1063–1071. [Google Scholar] [CrossRef]
- Hoang, H.T.; Leuridan, E.; Maertens, K.; Nguyen, T.D.; Hens, N.; Vu, N.H.; Caboré, R.N.; Duong, H.T.; Huygen, K.; Van Damme, P.; et al. Pertussis vaccination during pregnancy in Vietnam: Results of a randomized controlled trial Pertussis vaccination during pregnancy. Vaccine 2016, 34, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Maertens, K.; Hoang, T.T.; Nguyen, T.D.; Caboré, R.N.; Duong, T.H.; Huygen, K.; Hens, N.; Van Damme, P.; Dang, D.A.; Leuridan, E. The Effect of Maternal Pertussis Immunization on Infant Vaccine Responses to a Booster Pertussis-Containing Vaccine in Vietnam. Clin. Infect. Dis. 2016, 63, S197–S204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Raya, B.; Maertens, K.; Munoz, F.M.; Zimmermann, P.; Curtis, N.; Halperin, S.A.; Rots, N.; Barug, D.; Holder, B.; Kampmann, B.; et al. The Effect of Tetanus-Diphtheria-Acellular-Pertussis Immunization during Pregnancy on Infant Antibody Responses: Individual-Participant Data Meta-Analysis. Front. Immunol. 2021, 12, 689394. [Google Scholar] [CrossRef] [PubMed]
- Sapuan, S.; Andrews, N.; Hallis, B.; Hole, L.; Jones, C.E.; Matheson, M.; Miller, E.; Snape, M.D.; Heath, P.T. An observational, cohort, multi-centre, open label phase IV extension study comparing preschool DTAP-IPV booster vaccine responses in children whose mothers were randomised to one of two pertussis-containing vaccines or received no pertussis-containing vaccine in pregnancy in England. Vaccine 2022, 40, 7050–7056. [Google Scholar] [CrossRef] [PubMed]
- Wanlapakorn, N.; Maertens, K.; Vongpunsawad, S.; Puenpa, J.; Tran, T.M.P.; Hens, N.; Van Damme, P.; Thiriard, A.; Raze, D.; Locht, C.; et al. Quantity and Quality of Antibodies After Acellular Versus Whole-cell Pertussis Vaccines in Infants Born to Mothers Who Received Tetanus, Diphtheria, and Acellular Pertussis Vaccine During Pregnancy: A Randomized Trial. Clin. Infect. Dis. 2020, 71, 72–80. [Google Scholar] [CrossRef]
- Ibrahim, R.; Ali, S.A.; Kazi, A.M.; Rizvi, A.; Guterman, L.B.; Bednarczyk, R.A.; Kim, E.; Park, S.; Paulos, S.; Jeyachandran, A.; et al. Impact of maternally derived pertussis antibody titers on infant whole-cell pertussis vaccine response in a low income setting. Vaccine 2018, 36, 7048–7053. [Google Scholar] [CrossRef]
- Fabricius, G.; Martin Aispuro, P.; Bergero, P.; Bottero, D.; Gabrielli, M.; Hozbor, D. Pertussis epidemiology in Argentina: TRENDS after the introduction of maternal immunisation. Epidemiol. Infect. 2018, 146, 858–866. [Google Scholar] [CrossRef] [Green Version]
- Hozbor, D. New Pertussis Vaccines: A Need and a Challenge. Adv. Exp. Med. Biol. 2019, 1183, 115–126. [Google Scholar] [CrossRef]
- Edwards, K.M. Challenges to Pertussis Control. Pediatrics 2019, 144, e20191276. [Google Scholar] [CrossRef]
- Domenech de Cellès, M.; Magpantay, F.M.; King, A.A.; Rohani, P. The pertussis enigma: Reconciling epidemiology, immunology and evolution. Proc. Biol. Sci. 2016, 283, 20152309. [Google Scholar] [CrossRef] [Green Version]
- Pillsbury, A.; Quinn, H.E.; McIntyre, P.B. Australian vaccine preventable disease epidemiological review series: Pertussis, 2006–2012. Commun. Dis. Intell. Q. Rep. 2014, 38, E179–E194. [Google Scholar] [PubMed]
- Aslanabadi, A.; Ghabili, K.; Shad, K.; Khalili, M.; Sajadi, M.M. Emergence of whooping cough: Notes from three early epidemics in Persia. Lancet Infect. Dis. 2015, 15, 1480–1484. [Google Scholar] [CrossRef] [PubMed]
- Berbers, G.; van Gageldonk, P.; Kassteele, J.V.; Wiedermann, U.; Desombere, I.; Dalby, T.; Toubiana, J.; Tsiodras, S.; Ferencz, I.P.; Mullan, K.; et al. Circulation of pertussis and poor protection against diphtheria among middle-aged adults in 18 European countries. Nat. Commun. 2021, 12, 2871. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.H.; Ross, P.J.; Allen, A.C.; Wilk, M.M. Do we need a new vaccine to control the re-emergence of pertussis? Trends Microbiol. 2014, 22, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Wilk, M.M.; Mills, K.H.G. CD4 Trm cells following infection and immunization: Implications for more effective vaccine design. Front. Immunol. 2018, 9, 1860. [Google Scholar] [CrossRef]
- Soumana, I.H.; Linz, B.; Dewan, K.K.; Sarr, D.; Gestal, M.C.; Howard, L.K.; Caulfield, A.D.; Rada, B.; Harvill, E.T. Modeling Immune Evasion and Vaccine Limitations by Targeted Nasopharyngeal Bordetella pertussis Inoculation in Mice. Emerg. Infect. Dis. 2021, 27, 2107–2116. [Google Scholar] [CrossRef]
- Préziosi, M.P.; Halloran, M.E. Effects of pertussis vaccination on transmission: Vaccine efficacy for infectiousness. Vaccine 2003, 21, 1853–1861. [Google Scholar] [CrossRef]
- Wearing, H.J.; Rohani, P. Estimating the duration of pertussis immunity using epidemiological signatures. PLoS Pathog. 2009, 5, e1000647. [Google Scholar] [CrossRef] [Green Version]
- Blackwood, J.C.; Cummings, D.A.; Broutin, H.; Iamsirithaworn, S.; Rohani, P. Deciphering the impacts of vaccination and immunity on pertussis epidemiology in Thailand. Proc. Natl. Acad. Sci. USA 2013, 110, 9595–9600. [Google Scholar] [CrossRef] [Green Version]
- Damron, F.H.; Barbier, M.; Dubey, P.; Edwards, K.M.; Gu, X.X.; Klein, N.P.; Lu, K.; Mills, K.H.G.; Pasetti, M.F.; Read, R.C.; et al. Overcoming Waning Immunity in Pertussis Vaccines: Workshop of the National Institute of Allergy and Infectious Diseases. J. Immunol. 2020, 205, 877–882. [Google Scholar] [CrossRef]
- Bottero, D.; Gaillard, M.E.; Zurita, E.; Moreno, G.; Martinez, D.S.; Bartel, E.; Bravo, S.; Carriquiriborde, F.; Errea, A.; Castuma, C.; et al. Characterization of the immune response induced by pertussis OMVs-based vaccine. Vaccine 2016, 34, 3303–3309. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, M.E.; Bottero, D.; Errea, A.; Ormazábal, M.; Zurita, M.E.; Moreno, G.; Rumbo, M.; Castuma, C.; Bartel, E.; Flores, D.; et al. Acellular pertussis vaccine based on outer membrane vesicles capable of conferring both long-lasting immunity and protection against different strain genotypes. Vaccine 2014, 32, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Asensio, C.J.; Gaillard, M.E.; Moreno, G.; Bottero, D.; Zurita, E.; Rumbo, M.; van der Ley, P.; van der Ark, A.; Hozbor, D. Outer membrane vesicles obtained from Bordetella pertussis Tohama expressing the lipid A deacylase PagL as a novel acellular vaccine candidate. Vaccine 2011, 29, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Raeven, R.H.M.; Rockx-Brouwer, D.; Kanojia, G.; van der Maas, L.; Bindels, T.H.E.; Ten Have, R.; van Riet, E.; Metz, B.; Kersten, G.F.A. Intranasal immunization with outer membrane vesicle pertussis vaccine confers broad protection through mucosal IgA and Th17 responses. Sci. Rep. 2020, 10, 7396. [Google Scholar] [CrossRef]
- DeJong, M.A.; Wolf, M.A.; Bitzer, G.J.; Hall, J.M.; Sen-Kilic, E.; Blake, J.M.; Petty, J.E.; Wong, T.Y.; Barbier, M.; Campbell, J.D.; et al. CpG 1018® adjuvant enhances Tdap immune responses against Bordetella pertussis in mice. Vaccine 2022, 40, 5229–5240. [Google Scholar] [CrossRef]
- Pitisuttithum, P.; Dhitavat, J.; Sirivichayakul, C.; Pitisuthitham, A.; Sabmee, Y.; Chinwangso, P.; Kerdsomboon, C.; Fortuna, L.; Spiegel, J.; Chauhan, M.; et al. Antibody persistence 2 and 3 years after booster vaccination of adolescents with recombinant acellular pertussis monovalent aP. eClinicalMedicine 2021, 37, 100976. [Google Scholar] [CrossRef]
- Jahnmatz, M.; Richert, L.; Al-Tawil, N.; Storsaeter, J.; Colin, C.; Bauduin, C.; Thalen, M.; Solovay, K.; Rubin, K.; Mielcarek, N.; et al. Safety and immunogenicity of the live attenuated intranasal pertussis vaccine BPZE1: A phase 1b, double-blind, randomised, placebo-controlled dose-escalation study. Lancet Infect. Dis. 2020, 20, 1290–1301. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwejser-Zawislak, E.; Wilk, M.M.; Piszczek, P.; Krawczyk, J.; Wilczyńska, D.; Hozbor, D. Evaluation of Whole-Cell and Acellular Pertussis Vaccines in the Context of Long-Term Herd Immunity. Vaccines 2023, 11, 1. https://doi.org/10.3390/vaccines11010001
Szwejser-Zawislak E, Wilk MM, Piszczek P, Krawczyk J, Wilczyńska D, Hozbor D. Evaluation of Whole-Cell and Acellular Pertussis Vaccines in the Context of Long-Term Herd Immunity. Vaccines. 2023; 11(1):1. https://doi.org/10.3390/vaccines11010001
Chicago/Turabian StyleSzwejser-Zawislak, Ewa, Mieszko M. Wilk, Piotr Piszczek, Justyna Krawczyk, Daria Wilczyńska, and Daniela Hozbor. 2023. "Evaluation of Whole-Cell and Acellular Pertussis Vaccines in the Context of Long-Term Herd Immunity" Vaccines 11, no. 1: 1. https://doi.org/10.3390/vaccines11010001
APA StyleSzwejser-Zawislak, E., Wilk, M. M., Piszczek, P., Krawczyk, J., Wilczyńska, D., & Hozbor, D. (2023). Evaluation of Whole-Cell and Acellular Pertussis Vaccines in the Context of Long-Term Herd Immunity. Vaccines, 11(1), 1. https://doi.org/10.3390/vaccines11010001




