-
 Efficacy and Safety of Anti-Respiratory Syncytial Virus Monoclonal Antibody Nirsevimab in Neonates: A Real-World Monocentric Study
Efficacy and Safety of Anti-Respiratory Syncytial Virus Monoclonal Antibody Nirsevimab in Neonates: A Real-World Monocentric Study -
 Herpesviruses and Alzheimer’s Disease: A Call for Vaccination and Targeted Treatment
Herpesviruses and Alzheimer’s Disease: A Call for Vaccination and Targeted Treatment -
 mRNA-Nanoparticle Vaccine Triggers Protective Immunity to IBV
mRNA-Nanoparticle Vaccine Triggers Protective Immunity to IBV -
 Insect Cell Lines in Biotherapeutics and Vaccine Production
Insect Cell Lines in Biotherapeutics and Vaccine Production -
 Peptide-Based Strep A Vaccines in Infected Mice
Peptide-Based Strep A Vaccines in Infected Mice
Journal Description
Vaccines
Vaccines
is an international, peer-reviewed, open access journal published monthly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, PMC, Embase, CAPlus / SciFinder, and other databases.
- Journal Rank: JCR - Q2 (Medicine, Research and Experimental) / CiteScore - Q1 (Pharmacology (medical))
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 19.6 days after submission; acceptance to publication is undertaken in 2.8 days (median values for papers published in this journal in the first half of 2025).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
Impact Factor:
3.4 (2024);
5-Year Impact Factor:
3.7 (2024)
Latest Articles
Long-Term Follow-Up of T Cell Immunity Against Orthopoxviruses in People Living with HIV After Vaccination and Natural Monkeypox Virus Infection
Vaccines 2025, 13(9), 975; https://doi.org/10.3390/vaccines13090975 (registering DOI) - 13 Sep 2025
Abstract
Background/Objectives: After the 2022 mpox outbreak also outside Africa, risk groups including people living with HIV (PLWH) were vaccinated with the Modified Vaccinia Ankara–Bavarian Nordic vaccine (MVA-BN). Previous data on PLWH showed that two vaccinations induced specific T cell responses in 64% of
[...] Read more.
Background/Objectives: After the 2022 mpox outbreak also outside Africa, risk groups including people living with HIV (PLWH) were vaccinated with the Modified Vaccinia Ankara–Bavarian Nordic vaccine (MVA-BN). Previous data on PLWH showed that two vaccinations induced specific T cell responses in 64% of the patients and natural monkeypox virus (MPXV) infection in 100%. The initial T cell response assay took place at a median of approximately 100 days post-vaccination and 300 days post-infection. Methods: This study investigates the durability of T cell immunity in PLWH by retesting patients approximately two years after initial assessment. We were able to retest 27 of 33 vaccinated patients and 7 of 10 patients after MPXV infection. T cells were stimulated with the same orthopoxvirus-derived peptide pools as in the initial study, and interferon (IFN)-γ and interleukin (IL)-2 ELISpot assays were performed. Results: The ELISpot assays showed specific T cell responses in 59% and 86% of twice vaccinated and previously infected patients, respectively. Paired analysis revealed no significant differences between previous and current data (short- and long-term follow-up), with IL-2 ELISpot results showing positive correlations at both time points (r = 0.67, p = 0.0001). Long-term IFN-γ responses after MPXV infection were 4.3 times higher (p < 0.01), and IL-2 responses were 2.9 times higher (p = 0.05) than after vaccination. Conclusions: Our data indicates that T cell responses to Orthopoxviruses remain overall stable for 2–3 years in PLWH, with long-term immunity being stronger after natural MPXV infection than after two vaccinations.
Full article
(This article belongs to the Special Issue Strategies for the Monitoring, Treatment, and Prevention of Mpox and Other Poxvirus Infections)
►
Show Figures
Open AccessReview
Need for Invasive Meningococcal Disease Prevention Through Vaccination for Young Children in the Americas
by
Gaurav Mathur, Joseph B. Domachowske, Maria Gabriela Graña, Reena Ladak, Joanne M. Langley, Oluwatosin Olaiya, Alysa Pompeo, Laura Taddei and Rodolfo Villena
Vaccines 2025, 13(9), 974; https://doi.org/10.3390/vaccines13090974 (registering DOI) - 13 Sep 2025
Abstract
Background: Invasive meningococcal disease (IMD) is an uncommon but potentially life-threatening condition, resulting in life-long sequelae or death in up to 20% of cases. Most IMD cases are caused by Neisseria meningitidis serogroups (Men) A, B, C, W, X, and Y. The highest
[...] Read more.
Background: Invasive meningococcal disease (IMD) is an uncommon but potentially life-threatening condition, resulting in life-long sequelae or death in up to 20% of cases. Most IMD cases are caused by Neisseria meningitidis serogroups (Men) A, B, C, W, X, and Y. The highest IMD incidence is among children < 5 years of age (YOA). We reviewed IMD epidemiology data and existing national immunization programs (NIP) in the Americas and identify unmet needs to decrease IMD burden in young children. Methods: Using national surveillance data and published literature from 2006 to 2024, we evaluated the IMD burden and national vaccination strategies for children < 5 YOA in the Americas, focusing on Canada, the United States, Brazil, Chile, Argentina. Results: The highest IMD incidence was among infants, followed by children 1–4 YOA, with MenB infections predominating in both age groups. Chile has both MenACWY (2014) and MenB (2023) infant vaccination in its NIP. Argentina and Brazil’s NIPs include MenACWY (2017) and MenC (2010) vaccinations for infants, respectively. In Canada, MenC (2002) vaccination is recommended at 1 YOA (replaced by MenACWY in 2024 in Manitoba); MenB vaccination is selectively recommended. In each country, the incidence of IMD caused by vaccine-preventable serogroups decreased following the introduction of the respective meningococcal vaccination in the NIP. Conclusions: Comprehensive meningococcal vaccination programs in the Americas have the potential to reduce the IMD burden in children < 5 YOA. National recommendations and NIPs could reduce IMD burden by offering equitable access to protection against IMD, aligning with the WHO roadmap to defeat meningitis by 2030.
Full article
(This article belongs to the Section Epidemiology and Vaccination)
►▼
Show Figures

Figure 1
Open AccessArticle
Immunogenicity and Safety of a Live Attenuated Varicella Vaccine in Healthy Children Aged 12 to 15 Months: A Phase III, Randomized, Double-Blind, Active-Controlled Clinical Trial
by
Nancy Nazaire-Bermal, Ningning Jia, Maria Angela C. Maronilla, Josemaria F. Lopez, Gang Zeng, Wenbin Wu, Adrielle Bernice C. Nimo, Chunfang Luan and Qianqian Xin
Vaccines 2025, 13(9), 973; https://doi.org/10.3390/vaccines13090973 (registering DOI) - 13 Sep 2025
Abstract
Objectives: The varicella vaccine (VarV) produced by Sinovac (Dalian) obtained World Health Organization (WHO) prequalification in November 2022. However, no direct comparative studies have been conducted between VarV and other WHO-prequalified varicella vaccines. The study aimed to assess the immunogenicity and safety
[...] Read more.
Objectives: The varicella vaccine (VarV) produced by Sinovac (Dalian) obtained World Health Organization (WHO) prequalification in November 2022. However, no direct comparative studies have been conducted between VarV and other WHO-prequalified varicella vaccines. The study aimed to assess the immunogenicity and safety of Sinovac’s VarV compared with Merck Sharp & Dohme’s (MSD) VARIVAX® (Moorgate, London, UK) following a single dose administration. Methods: This Phase III, randomized, double-blind, active-controlled, non-inferiority trial was conducted in the Philippines. Healthy children aged 12 to 15 months were enrolled. Eligible participants were randomly assigned (1:1) to receive a single dose of varicella vaccine either manufactured by Sinovac (Test group) or MSD (Active control group). Immunogenicity was evaluated 6 weeks after vaccination by enzyme-linked immunosorbent assay (ELISA). The primary immunogenicity endpoint was seroresponse rate 6 weeks after vaccination. Seroresponse rate was defined as varicella-zoster virus (VZV) antibody concentration ≥ 10 mIU/mL in participants who were seronegative (antibody concentration < 10 mIU/mL) at baseline. The secondary endpoint was the corresponding geometric mean concentration (GMC). Adverse events (AEs) and serious adverse events (SAEs) were monitored for 6 weeks after vaccination. Results: Among the 484 participants analyzed, the seroresponse rates 6 weeks after vaccination were 98.85% and 98.88% in the Test group and Active control group, respectively, with a difference of −0.03% (95% CI: −3.10%, 2.99%), which exceeded the predefined non-inferiority margin of −10%. The corresponding GMCs were 35.73 mIU/mL and 37.34 mIU/mL, respectively, with the ratio of 0.96 (95% CI: 0.86, 1.06), also exceeding the predefined non-inferiority margin of 0.67. Furthermore, the incidence of adverse reactions (ARs) in the Test group was lower than that in the Active control group (38.08% vs. 55.51%). Conclusions: Sinovac’s VarV demonstrated non-inferior immunogenicity to WHO-prequalified comparator vaccine (VARIVAX®) and favorable safety profile. These findings indicated that VarV (Sinovac, Beijing, China) met WHO standards for varicella vaccine evaluation, supporting its global use consideration.
Full article
(This article belongs to the Section Vaccine Advancement, Efficacy and Safety)
►▼
Show Figures

Figure 1
Open AccessArticle
Safety and Immunogenicity of Single-Dose of Adsorbed Tetanus Vaccine in Adults Aged 18–44 Years: Randomized, Double-Blind, Positive-Controlled Phase I/III Clinical Trial
by
Zhiqiang Xie, Liyong Yuan, Yaping Qiao, Wangyang You, Yurong Li, Taotao Zhu, Wei Zhang, Lili Huang, Jiebing Tan, Xiaocan Jia, Zhe Li, Feng Xue, Xiaojuan Lian and Yanxia Wang
Vaccines 2025, 13(9), 972; https://doi.org/10.3390/vaccines13090972 (registering DOI) - 13 Sep 2025
Abstract
Background: The persistence of non-neonatal tetanus (non-NT) highlights the necessity of adult booster vaccines and post-traumatic prophylaxis. This Phase I/III clinical trial aimed to evaluate the safety and immunogenicity of a new adsorbed tetanus vaccine. Methods: A randomized, double-blind, positive-controlled clinical
[...] Read more.
Background: The persistence of non-neonatal tetanus (non-NT) highlights the necessity of adult booster vaccines and post-traumatic prophylaxis. This Phase I/III clinical trial aimed to evaluate the safety and immunogenicity of a new adsorbed tetanus vaccine. Methods: A randomized, double-blind, positive-controlled clinical trial was conducted in Henan Province, China. A total of 1258 healthy participants aged 18–44 years (60 in Phase I and 1198 in Phase III) were enrolled, with no history of tetanus infection, or tetanus toxoid-containing vaccines (TTCVs) vaccination within the past 10 years. The participants were randomly assigned at a 1:1 ratio to receive a single dose of either the investigational vaccine or the licensed control vaccine. The Phase III clinical trial was initiated subsequent to the 7-day safety observation period following vaccination in the Phase I trial. The objective of the Phase III clinical trial was to assess the non-inferiority of the seroconversion rate of tetanus antibodies at 30 days post-vaccination with the investigational vaccine compared to the control vaccine. Serum samples were collected prior to and at 30 days post-vaccination. Adverse events were monitored for 30 days, with serious adverse events (SAEs) followed up for 6 months post-vaccination. Results: The investigational group achieved a seroconversion rate of 99.48%, which was non-inferior to that of the control group (99.66%), with a negligible rate difference of −0.17% (95% confidence interval [CI]: −1.20%, 0.78%). The investigational group exhibited a significantly higher geometric mean concentration (GMC) of antibodies (4.721 IU/mL vs. 3.627 IU/mL, p < 0.0001). Among the susceptible participants, the seroconversion rates were 99.78% in the investigational group and 99.79% in the control group, respectively, with a non-inferior rate difference of −0.01% (95%CI: −1.06%, 0.97%). Furthermore, the investigational group showed a low incidence of adverse reactions (ARs) within 30 days post-vaccination (12.26%), which was comparable to that of the control group (13.65%). All the reported ARs were mild or moderate, and no SAEs were associated with the vaccination. Conclusions: The new adsorbed tetanus vaccine demonstrated favorable safety and comparable immunogenicity to the marketed control vaccine, with a significantly higher antibody GMC, supporting its clinical application in tetanus prevention.
Full article
(This article belongs to the Special Issue Advancements in Vaccine Research: Epidemiology, Immunogenicity, Effectiveness and Safety)
►▼
Show Figures

Figure 1
Open AccessArticle
Immunogenicity and Antigenicity of the Recombinant Ectodomain of Rabies Virus Glycoprotein Containing the Human Collagen XVIII Trimerization Domain
by
Izat Smekenov, Gulshat Bayandy, Sanzhar Alybayev, Nuraiym Baltakhozha, Zhanat Batanova, Nurlan Akhmetsadykov and Amangeldy Bissenbaev
Vaccines 2025, 13(9), 971; https://doi.org/10.3390/vaccines13090971 (registering DOI) - 12 Sep 2025
Abstract
Background: Rabies remains a fatal zoonotic disease, necessitating effective and affordable vaccines. While current vaccines are effective, they require multiple doses and may not induce long-lasting immunity in all settings. The rabies virus glycoprotein (RABV-G) is the principal antigen responsible for eliciting
[...] Read more.
Background: Rabies remains a fatal zoonotic disease, necessitating effective and affordable vaccines. While current vaccines are effective, they require multiple doses and may not induce long-lasting immunity in all settings. The rabies virus glycoprotein (RABV-G) is the principal antigen responsible for eliciting virus-neutralizing antibodies, but its recombinant monomeric forms often suffer from poor immunogenicity due to misfolding and aggregation. Methods: A recombinant trimeric RABV-G ectodomain (rRABV-G-XVIII) was engineered by fusing it to a human collagen XVIII-derived trimerization domain. The protein was expressed in E. coli, purified under denaturing conditions, and refolded. Trimer formation was verified using size-exclusion chromatography. Mice were immunized with rRABV-G-XVIII, with or without adjuvant, and compared to a monomeric form (rRABV-GE). Antigen-specific antibody responses were measured by ELISA, neutralizing activity was assessed, and protective efficacy was evaluated via intracerebral challenge with the CVS-27 rabies strain. Results: rRABV-G-XVIII formed stable trimers and induced strong humoral immune responses, with high ELISA titers and virus-neutralizing activity comparable to an inactivated rabies vaccine. Mice immunized with rRABV-GE showed lower antibody responses and partial protection, which improved with adjuvant. All rRABV-G-XVIII-immunized mice were fully protected against rabies challenge, independent of adjuvant use. Conclusions: Stabilization of RABV-G in its native trimeric conformation markedly improves immunogenicity and protective efficacy. This approach offers a promising strategy for the development of rabies subunit vac-cines with simplified formulations and potential for cost-effective production in bacterial systems.
Full article
(This article belongs to the Section Vaccine Design, Development, and Delivery)
►▼
Show Figures

Figure 1
Open AccessReview
Engineering Universal Cancer Immunity: Non-Tumor-Specific mRNA Vaccines Trigger Epitope Spreading in Cold Tumors
by
Matthias Magoola and Sarfaraz K. Niazi
Vaccines 2025, 13(9), 970; https://doi.org/10.3390/vaccines13090970 - 12 Sep 2025
Abstract
The landscape of cancer immunotherapy must shift from personalized neoantigen vaccines toward universal platforms that leverage innate immune activation. This review examines a novel mRNA vaccine strategy that encodes non-tumor-specific antigens, carefully selected pathogen-derived or synthetic sequences designed to transform immunologically “cold” tumors
[...] Read more.
The landscape of cancer immunotherapy must shift from personalized neoantigen vaccines toward universal platforms that leverage innate immune activation. This review examines a novel mRNA vaccine strategy that encodes non-tumor-specific antigens, carefully selected pathogen-derived or synthetic sequences designed to transform immunologically “cold” tumors into inflamed therapy-responsive microenvironments. Unlike conventional approaches requiring patient-specific tumor sequencing and 8–12-week manufacturing timelines, this platform utilizes pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) to trigger broad innate immune activation through multiple pattern recognition receptors (PRRs). The key therapeutic mechanism is epitope spreading, where vaccine-induced inflammation reveals previously hidden tumor antigens, enabling the immune system to mount responses against cancer-specific targets without prior knowledge of these antigens. Delivered via optimized lipid nanoparticles (LNPs) or alternative polymer-based systems, these vaccines induce epitope spreading, enhance checkpoint inhibitor responsiveness, and establish durable antitumor memory. This approach offers several potential advantages, including immediate treatment availability, a cost reduction of up to 100-fold compared to personalized vaccines, scalability for global deployment, and efficacy across diverse tumor types. However, risks such as cytokine release syndrome (CRS), potential for off-target autoimmunity, and challenges with pre-existing immunity must be addressed. By eliminating barriers of time, cost, and infrastructure, this universal platform could help democratize access to advanced cancer treatment, potentially benefiting the 70% of cancer patients in low- and middle-income countries (LMICs) who currently lack immunotherapy options.
Full article
(This article belongs to the Section Vaccination Against Cancer and Chronic Diseases)
►▼
Show Figures

Figure 1
Open AccessArticle
COVID-19 Vaccination Reduces Lower Limb Amputation Rates and Mortality Rate in Patients with Pre-Existing Peripheral Vascular Disease Based on TriNetX Database
by
Shiuan-Tzuen Su, Yu-Hsuan Huang, Jing-Yang Huang and James C.-C. Wei
Vaccines 2025, 13(9), 969; https://doi.org/10.3390/vaccines13090969 - 12 Sep 2025
Abstract
Background: Unvaccinated individuals with peripheral arterial occlusive disease (PAOD) are more likely to develop acute limb ischemia (ALI) following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. We assessed the protective effect of the COVID-19 vaccine in preventing ALI in PAOD patients with
[...] Read more.
Background: Unvaccinated individuals with peripheral arterial occlusive disease (PAOD) are more likely to develop acute limb ischemia (ALI) following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. We assessed the protective effect of the COVID-19 vaccine in preventing ALI in PAOD patients with SARS-CoV-2 infection. Methods: This retrospective cohort study was conducted using the United States TriNetX (Cambridge, MA, USA), using patients with PAOD who were diagnosed with SARS-CoV-2 infection between 1 November 2020 and 31 December 2023. Propensity score matching was performed to adjust for demographic variables, lifestyle factors, medical utilization, and comorbidities. Cox proportional hazards models were used to compare the two matched cohorts. Kaplan–Meier analysis estimated the 3-year cumulative probability of lower limb amputation incidence. We selected 12,948 PAOD patients who received the COVID-19 vaccine and 44,064 PAOD patients who were unvaccinated against COVID-19. Results: A total of 11,822 pairs of COVID-19 vaccinated PAOD patients and unvaccinated individuals were compared. The mean (SD) age was 66.5 (14.1) years; there were 4849 male patients (41%) and 6569 female (55.6%) compared to unvaccinated PAOD patients, and those who received the COVID-19 vaccine had a significantly lower risk of 3-year all-cause mortality (log-rank test, p < 0.001; hazard ratio (HR) was 0.857; 95% CI, 0.796–0.922) and lower limb amputation (log-rank test, p = 0.001, HR = 0.716; 95% CI, 0.587–0.873), though there was no significant difference in ischemic stroke (log-rank test, p = 0.174; HR = 0.958; 95% CI, 0.902–1.019). Conclusions: This study found that patients who received the COVID-19 vaccine had a significantly lower risk of 3-year all-cause mortality and lower limb amputation, though there was no significant difference in ischemic stroke.
Full article
(This article belongs to the Special Issue Vaccines and Immunotherapy for Inflammatory Disease)
►▼
Show Figures

Figure 1
Open AccessArticle
Adjuvant Mucosal Strategies Confer Safe and Effective Immunity Against Mycoplasma pneumoniae and Overcome Vaccine-Associated Enhanced Lung Pathology
by
Zhentao Lei, Dandan Gao, Xiaolong Zhang, Han Cao, Jingping Hu, Yifan Zhou, Ning Luan and Cunbao Liu
Vaccines 2025, 13(9), 968; https://doi.org/10.3390/vaccines13090968 - 12 Sep 2025
Abstract
Background/Objectives: The global spread of Mycoplasma pneumoniae (MP) poses a significant threat to public health; however, no licensed vaccine for human use is currently available. The development of a safe and effective vaccine is a critical priority. This study systematically evaluated the protective
[...] Read more.
Background/Objectives: The global spread of Mycoplasma pneumoniae (MP) poses a significant threat to public health; however, no licensed vaccine for human use is currently available. The development of a safe and effective vaccine is a critical priority. This study systematically evaluated the protective efficacy and safety of an inactivated MP vaccine using different adjuvants and immunization routes. Methods: Mice were immunized with inactivated vaccines via either intramuscular (IM) injection with aluminum hydroxide (alum) or a combination of CpG+QS21 (CQ) or via intranasal (IN) administration of Flagellin from Salmonella Typhimurium (FLA-ST), a potent Toll-like receptor 5 (TLR5) agonist, as a mucosal adjuvant. Vaccine-induced immunogenicity, protective efficacy against MP challenge, and associated lung pathology were assessed. Results: Both IM-vaccinated groups (alum and CQ) exhibited robust systemic immune responses. However, upon subsequent MP challenge, these groups exhibited significant inflammatory pathology in the lung tissues. Notably, the CQ-adjuvanted group displayed severe pulmonary inflammatory infiltration. In stark contrast, compared with the IM-vaccinated group, the IN-immunized group with the FLA-ST mucosal adjuvant achieved significant clearance of MP from the lungs and showed markedly milder histopathological lung damage. Conclusions: Our findings suggest that IM immunization with CQ-adjuvanted inactivated vaccines may represent a suboptimal strategy for MP, given the risk of exacerbating lung immunopathology. Conversely, a mucosal immunization approach using the FLA-ST adjuvant demonstrates considerable promise, offering an effective balance between bacterial clearance and an improved safety profile, highlighting its potential for future MP vaccine development.
Full article
(This article belongs to the Section Vaccine Advancement, Efficacy and Safety)
►▼
Show Figures

Figure 1
Open AccessArticle
Impact of COVID-19 on Mucosal Immunity and Antibody Responses in COVID Vaccinees
by
Priya Kannian, Muruganantham Lillimary Eniya, Pasuvaraj Mahanathi, Arul Gracemary, Nagalingeswaran Kumarasamy and Stephen J. Challacombe
Vaccines 2025, 13(9), 967; https://doi.org/10.3390/vaccines13090967 - 12 Sep 2025
Abstract
Background and Objectives: SARS-CoV-2 infection initiates at mucosal surfaces, and mucosal immunity may influence the nature and severity of infection. Little is known about the induction of mucosal immunity by vaccination in COVID-19 convalescents. Methods: Sera from 205 healthcare workers were
[...] Read more.
Background and Objectives: SARS-CoV-2 infection initiates at mucosal surfaces, and mucosal immunity may influence the nature and severity of infection. Little is known about the induction of mucosal immunity by vaccination in COVID-19 convalescents. Methods: Sera from 205 healthcare workers were collected one month after the first Covishield vaccination and 1/3/6 months after the second vaccination, while paired sera and stimulated whole-mouth fluid (SWMF) was collected 1/3/6 months after the third vaccination (N = 10) and at 0/30/90 days after a COVID-19 episode (N = 8). Anti-SARS-CoV-2 spike antibody detection by ECLIA/ELISA and cytokine detection by ELISA/CBA were performed. Results: One month post-second vaccination, serum antibodies had increased significantly (6-fold) in the COVID-19-naïve group (CNG) but declined (1.5-fold) in the previously COVID-19-exposed group (CEG), who already had high antibody titres. The serum regulatory cytokine IL-10 levels were higher after three antigen exposures (p = 0.0002). New infections (breakthrough infections—BTIs) or reinfections (RIs) with asymptomatic/mild disease occurred in 44% of the CNG and 27% of the CEG (p < 0.01). The mucosal cytokine IL-17 levels were significantly higher in the CEG. Salivary IgG/IgA and secretory IgA antibodies were detectable both after vaccination and COVID-19. Innate cytokines (MIG, MCP-1, IL-8, IL-1β) were higher and sustained in SWMF in contrast to serum. Conclusions: Two vaccinations in the CNG resulted in an antibody boost, but the second vaccination in the CEG induced antibody anergy. Serum/mucosal antibodies declined by six months after vaccination, but the rapid increase at subsequent exposures were indicative of a good T cell/B cell memory response to SARS-CoV-2. A higher percentage of BTI among the CNG than RI among the CEG may indicate better protection due to higher antibody responses in the latter group.
Full article
(This article belongs to the Special Issue A One-Health Perspective on Immunization Against Infectious Diseases)
►▼
Show Figures

Figure 1
Open AccessArticle
Differences in Glycoproteins and the Potential for Early Protection Using LAIV Based on Drift Variants of the A/H1N1pdm09 Influenza Virus
by
Yulia Desheva, Irina Mayorova, Andrey Rekstin, Daniil Sokolovsky, Polina Kudar, Nina Kopylova, Danila Guzenkov, Darya Petrachkova, Andrey Mamontov, Andrey Trullioff and Irina Kiseleva
Vaccines 2025, 13(9), 966; https://doi.org/10.3390/vaccines13090966 - 11 Sep 2025
Abstract
Background/Objectives: Antigenic drift of influenza A(H1N1pdm09) viruses has led to periodic replacement of vaccine strains. Understanding how structural differences in glycoproteins influence immune protection is crucial for improving vaccine effectiveness. Methods: We conducted a structural analysis of the hemagglutinin (HA) and neuraminidase (NA)
[...] Read more.
Background/Objectives: Antigenic drift of influenza A(H1N1pdm09) viruses has led to periodic replacement of vaccine strains. Understanding how structural differences in glycoproteins influence immune protection is crucial for improving vaccine effectiveness. Methods: We conducted a structural analysis of the hemagglutinin (HA) and neuraminidase (NA) glycoproteins from drifted A(H1N1)pdm09 strains: A/South Africa/3626/2008 and A/Guangdong–Maonan/SWL1/2020, as well as their cold-adapted live attenuated vaccine (LAIV) reassortant strains (A/17/South Africa/2013/01(H1N1)pdm09 and A/17/Guangdong–Maonan/2019/211(H1N1)pdm09). We compared their replication in chicken embryo and mammalian cell culture, assessed type I interferon induction, and evaluated post-vaccine protection in mice after homologous and heterogeneous viral challenges. Results: The two vaccine strains had distinct glycosylation patterns for HA and NA. However, they had similar replication capacity in embryonated egg and mammalian cells. In the mouse respiratory tract, both strains replicated similarly. A/17/South Africa/2013/01(H1N1)pdm09 induced significantly higher levels of IFN-α and Mx1 in vitro, and it elicited earlier IgM and IgG response after vaccination in mice. At day 6 after immunization, it provided 70% protection from homologous challenge. A/17/Guangdong–Maonan/2019/211(H1N1)pdm09 did not prevent death, but it reduced viral titer in the lungs. Interestingly, A/17/South Africa/2013/01(H1N1)pdm09 provided full protection from heterologous H5N1 challenge, while A/17/Guangdong–Maonan/2019/211(H1N1)pdm09) only provided partial protection. Conclusions: Differences in HA and NA glycans among A(H1N1)pdm09 strains may influence innate and adaptive immunity, as well as cross-protection. These findings emphasize the importance of glycoprotein structure when selecting vaccine candidates for optimal homologous and cross-protection against influenza.
Full article
(This article belongs to the Section Influenza Virus Vaccines)
►▼
Show Figures

Figure 1
Open AccessArticle
Antibody Responses to SARS-CoV-2 and Common HCoVs in Hemodialysis Patients and Transplant Recipients: Data from the Dominican Republic
by
Lisette Alcantara Sanchez, Eloy Alvarez Guerra, Dongmei Li, Samantha M. King, Shannon P. Hilchey, Qian Zhou, Stephen Dewhurst, Kevin Fiscella and Martin S. Zand
Vaccines 2025, 13(9), 965; https://doi.org/10.3390/vaccines13090965 - 11 Sep 2025
Abstract
Background: Vaccination against SARS-CoV-2 has been pivotal in controlling the COVID-19 pandemic. However, understanding vaccine-induced immunity in immunocompromised individuals remains critical, particularly how prior exposure to other coronaviruses modulates immune responses. The influence of previous infections with endemic human coronaviruses (HCoVs), such as
[...] Read more.
Background: Vaccination against SARS-CoV-2 has been pivotal in controlling the COVID-19 pandemic. However, understanding vaccine-induced immunity in immunocompromised individuals remains critical, particularly how prior exposure to other coronaviruses modulates immune responses. The influence of previous infections with endemic human coronaviruses (HCoVs), such as OC43, on SARS-CoV-2 immunity is not fully understood. This study evaluates antibody responses to COVID-19 vaccination in hemodialysis patients (HD), transplant recipients (TR), and healthy controls (CO), accounting for prior SARS-CoV-2 infection and baseline human coronavirus (HCoV) reactivity. Methods: We obtained longitudinal antibody measurements from 70 subjects (CO: n = 33; HD: n = 13; TR: n = 24) and assessed antibody kinetics across multiple post-vaccination time points using multivariate linear mixed modeling (MLMM). Results: Limited but measurable cross-reactivity was observed between SARS-CoV-2 and endemic HCoVs, particularly the
(This article belongs to the Section COVID-19 Vaccines and Vaccination)
►▼
Show Figures

Figure 1
Open AccessSystematic Review
Strategies to Increase Vaccinations in Adult Cancer Patients: A Systematic Review
by
Giuseppina Lo Moro, Federica Golzio, Sara Claudia Calabrese, Giacomo Scaioli, Alessandro Basile, Roberta Siliquini and Fabrizio Bert
Vaccines 2025, 13(9), 964; https://doi.org/10.3390/vaccines13090964 - 11 Sep 2025
Abstract
Background/Objectives: Although vaccinations are a priority for patients with cancer, achieving high coverage remains challenging. Evidence on effective strategies in oncology settings is still limited. This systematic review aimed to identify interventions to improve vaccination uptake or reduce hesitancy among cancer patients. Methods:
[...] Read more.
Background/Objectives: Although vaccinations are a priority for patients with cancer, achieving high coverage remains challenging. Evidence on effective strategies in oncology settings is still limited. This systematic review aimed to identify interventions to improve vaccination uptake or reduce hesitancy among cancer patients. Methods: A systematic search was conducted in PubMed, Embase, and Scopus, including studies published up to the end of 2023. The protocol was registered in PROSPERO (CRD42024511008). Results: Out of 10,927 non-duplicate records, 15 studies describing unique interventions were included. All studies were published between 2011 and 2022, primarily conducted in Europe/UK (40%) and in North America (40%). The most common study design was pre-post (60%), and 33.3% included a control group. Most interventions were multi-component (60%) and were classified into three main categories: educational materials/campaigns (46.7%), reminders (40%), and patient counselling (33.3%). Additional components included guideline development in two studies. Some studies also highlighted the importance of specific key figures, such as dedicated professionals, general practitioners, and pharmacists. Interventions mainly targeted patients (40%), with 33.3% addressing both healthcare professionals and patients and 26.7% professionals only. They most frequently concerned vaccinations against influenza and pneumococcal disease (26.7%), pneumococcal disease alone (26.7%), or Coronavirus Disease 2019 (COVID-19) (26.7%). Vaccination uptake was the primary outcome in 86.7% of studies, with 66.7% reporting significant improvements. Conclusions: This review identified a variety of strategies, with education, reminders, and counselling as key components. Multicomponent interventions and those involving both patients and providers were most promising. However, methodological limitations and limited generalizability highlighted the need for more rigorous research.
Full article
(This article belongs to the Special Issue Virus Pandemics and Vaccinations)
►▼
Show Figures

Figure 1
Open AccessArticle
Cord Blood RSV-Neutralizing Antibodies and Risk of Hospitalization for RSV-Associated Acute Respiratory Infection in Vietnamese Children: A Case–Cohort Study
by
Michiko Toizumi, Yutaro Yamagata, Hien Anh Thi Nguyen, Hirono Otomaru, Hoang Huy Le, Hiroyuki Moriuchi, Jean-Francois Eleouet, Marie-Anne Rameix-Welti, Makoto Takeda, Hung Thai Do and Lay-Myint Yoshida
Vaccines 2025, 13(9), 963; https://doi.org/10.3390/vaccines13090963 - 11 Sep 2025
Abstract
Background: Respiratory syncytial virus (RSV) is a leading cause of lower respiratory tract infections in children, particularly severe during infancy. Maternal RSV-specific neutralizing antibodies (nAbs), transferred via the placenta, may provide protection in early infancy, but the extent and duration of protection remain
[...] Read more.
Background: Respiratory syncytial virus (RSV) is a leading cause of lower respiratory tract infections in children, particularly severe during infancy. Maternal RSV-specific neutralizing antibodies (nAbs), transferred via the placenta, may provide protection in early infancy, but the extent and duration of protection remain uncertain. Objective: We investigated the association between cord blood RSV-A nAb levels and the risk of hospitalization due to RSV-associated acute respiratory infection (RSV-ARI) by 24 months of age. Methods: We conducted a case–cohort study nested within a birth cohort in Nha Trang, Vietnam. From the full cohort (n = 1977), a random subcohort of 392 infants and all 66 infants hospitalized for RSV-ARI by age 24 months were included for RSV-A nAb testing. RSV-A nAb titers at birth were categorized into three groups in the subcohort (low: lowest quartile; middle; interquartile; high: highest quartile). Weighted Cox proportional hazards regression was used to estimate hazard ratios (HRs) for RSV-ARI hospitalization. Results: The incidence of RSV-ARI hospitalization was 17.92 per 1000 person-years by 24 months, and 25.40 per 1000 person-years among infants aged <12 months. Among infants aged <6 months, those in the low nAb group had a significantly higher risk of hospitalization compared to the middle nAb group (adjusted HR: 4.05; 95% CI: 1.51–10.89). Maternal anemia was consistently associated with increased risk. Conclusions: Lower RSV-nAb titers at birth were associated with an increased risk of RSV-ARI hospitalization during early infancy. These findings support the importance of maternal immunization strategies to enhance infant protection against RSV.
Full article
(This article belongs to the Special Issue Host Immunity and Vaccines for Respiratory Pathogens)
►▼
Show Figures

Figure 1
Open AccessReview
Influenza Vaccines: Current Status, Adjuvant Strategies, and Efficacy
by
Vijay Reddy Mokalla, Shirisha Gundarapu, Radhey S. Kaushik, Mrigendra Rajput and Hemachand Tummala
Vaccines 2025, 13(9), 962; https://doi.org/10.3390/vaccines13090962 - 11 Sep 2025
Abstract
The influenza virus is one of the major global health concerns, causing significant morbidity and mortality in both humans and animals, with substantial impacts on public health. Vaccination remains the primary strategy for managing influenza virus infections; however, the virus undergoes frequent genetic
[...] Read more.
The influenza virus is one of the major global health concerns, causing significant morbidity and mortality in both humans and animals, with substantial impacts on public health. Vaccination remains the primary strategy for managing influenza virus infections; however, the virus undergoes frequent genetic changes through antigenic drift and shift. These mutations lead to new seasonal strains that evade pre-existing immunity. These mutations can potentially result in virulent strains that could trigger future pandemics. Therefore, developing a vaccine capable of providing robust protection despite these genetic changes is essential. Vaccine adjuvants are essential for boosting and directing the immune system’s response, broadening the spectrum of protection, and reducing the amount of antigen required to achieve protection, which is particularly valuable in the face of rapidly evolving strains and during pandemics. Recent advances in adjuvant design and formulation strategies have demonstrated promising improvements in both the overall potency and durability of influenza vaccines, importantly, significant reductions in losses due to influenza infection. This review highlights the current status of different types of influenza virus vaccines, their benefits, and challenges. Further, the review focuses on the role of adjuvants, discussing their advantages, limitations, and methodological approaches, while also considering their potential contribution in developing a universal flu vaccine intended to provide extensive and lasting protection.
Full article
(This article belongs to the Section Vaccine Advancement, Efficacy and Safety)
Open AccessArticle
Vaccination Coverage in Adult Patients with Inflammatory Bowel Disease: Impact of a Tailored Vaccination Pathway Including COVID-19 and Herpes Zoster in a University Hospital Vaccination Center
by
Roberto Venuto, Caterina Elisabetta Rizzo, Daniela Lo Giudice, Walter Fries, Concetta Ceccio, Francesco Fedele, Raffaele Squeri and Cristina Genovese
Vaccines 2025, 13(9), 961; https://doi.org/10.3390/vaccines13090961 - 11 Sep 2025
Abstract
Background/Objectives: Patients with inflammatory bowel disease (IBD) are at increased risk of severe infections, particularly when undergoing immunosuppressive therapy. Vaccination is a key preventive strategy, but coverage in this group is often suboptimal. This study evaluated vaccination coverage among IBD patients at diagnosis/referral
[...] Read more.
Background/Objectives: Patients with inflammatory bowel disease (IBD) are at increased risk of severe infections, particularly when undergoing immunosuppressive therapy. Vaccination is a key preventive strategy, but coverage in this group is often suboptimal. This study evaluated vaccination coverage among IBD patients at diagnosis/referral and after admission to a structured hospital-based vaccination pathway. Methods: We conducted an observational study (February 2022–February 2025) at the Vaccination Center (VC) of the University Hospital “G. Martino” in Messina, Italy. Adult IBD patients referred by gastroenterologists were assessed for vaccination status using hospital and regional registries, and personalized schedules were developed based on Italian National Vaccine Prevention Plan guidelines. Descriptive statistics were applied to assess baseline and post-intervention vaccination coverage. Results: Of 154 participants (mean age 64 years; 51.9% male), 55.4% were on immunosuppressive therapy. Baseline coverage was heterogeneous: influenza, 6.5%; PCV13, 25.5%; PPV23, 26.6%; herpes zoster, 62.3%; and COVID-19 primary cycle, 79.6%. After enrollment, substantial improvements were observed: influenza, 89.2%; PCV13, 74.5%; PPV23, 67.0%; herpes zoster, 75.4%; and COVID-19 primary cycle, 96.8%. Coverage for catch-up vaccines also improved (e.g., HBV went from 1.9% to 44.2%). However, uptake of COVID-19 booster doses during the study period remained low (15.6%). No significant differences emerged by sex or treatment subgroup. Conclusions: A structured, collaborative care pathway between gastroenterologists and public health specialists significantly improved vaccination coverage among IBD patients. Despite gains, gaps persist in COVID-19 booster uptake and catch-up vaccinations. Integration of vaccination services into routine IBD management is essential to enhance protection in this high-risk population.
Full article
(This article belongs to the Special Issue Epidemiology of Diseases Preventable by Vaccination)
►▼
Show Figures

Figure 1
Open AccessCase Report
Case Report: A Multi-Peptide Vaccine Targeting Individual Somatic Mutations Induces Tumor Infiltration of Neoantigen-Specific T Cells in a Patient with Metastatic Colorectal Cancer
by
Armin Rabsteyn, Henning Zelba, Borong Shao, Lisa Oenning, Christina Kyzirakos, Simone Kayser, Tabea Riedlinger, Johannes Harter, Magdalena Feldhahn, Dirk Hadaschik, Florian Battke, Veit Scheble, Alfred Königsrainer and Saskia Biskup
Vaccines 2025, 13(9), 960; https://doi.org/10.3390/vaccines13090960 - 11 Sep 2025
Abstract
Background/Objectives: Fully personalized peptide vaccines targeting tumor-specific mutations are a promising treatment option for patients in an adjuvant but also advanced/metastatic disease situation in addition to non-personalized standard therapies. Here, we report a patient’s case with advanced metastatic colorectal cancer (mCRC) who was
[...] Read more.
Background/Objectives: Fully personalized peptide vaccines targeting tumor-specific mutations are a promising treatment option for patients in an adjuvant but also advanced/metastatic disease situation in addition to non-personalized standard therapies. Here, we report a patient’s case with advanced metastatic colorectal cancer (mCRC) who was treated with a neoantigen-derived multi-peptide vaccine in addition to standard of care. Methods: Tumor-specific mutations were identified by whole exome and transcriptome sequencing. An individualized peptide vaccine was designed using an in-house developed epitope prediction and vaccine design platform. In this case, the vaccine consisted of 20 peptides targeting 18 distinct mutations. The vaccine was administered according to a prime-boost scheme for a total of 12 vaccinations. Vaccine immunogenicity was determined by stimulation of patient T cells with vaccinated peptides and subsequent intracellular cytokine staining (ICS). Tumor-infiltrating lymphocytes (TIL) were analyzed by ICS and T cell receptor beta chain (TCRβ) sequencing. Results: The patient survived for 41 months since initial diagnosis despite continuous disease progression under all therapeutic interventions. The vaccination induced multiple neoantigen-specific T cell responses in the patient without notable side effects. Two liver metastases were resected five months after the start of vaccination, and TIL were extracted and cultured. Analysis of TIL cultures revealed tumor infiltration by vaccine-induced neoantigen-specific T cells in only one of the metastases. TCRβ sequencing of neoantigen-specific T cells and tumor tissues supported this finding. Vaccine-targeted variants were reduced or absent in the metastasis with vaccine-specific T cell infiltration. Conclusions: This case demonstrates immunogenicity of a neoantigen-derived peptide vaccine and highlights tumor-infiltrating capabilities and potential cytotoxicity of vaccine-induced T cells in mCRC.
Full article
(This article belongs to the Special Issue The Development of Peptide-Based Vaccines)
►▼
Show Figures

Figure 1
Open AccessReview
Recent Advances in Clinical Research of Prophylactic Vaccines Against Tuberculosis
by
Buyun Xu, Mengjuan Yuan, Lisa Yang, Lan Huang, Jingxin Li and Zhongming Tan
Vaccines 2025, 13(9), 959; https://doi.org/10.3390/vaccines13090959 - 10 Sep 2025
Abstract
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB), is one of the leading infectious causes of adult mortality worldwide. The Bacillus Calmette–Guérin (BCG) vaccine is currently the only approved vaccine for TB prevention, but its protective efficacy against adult pulmonary TB is limited, and
[...] Read more.
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB), is one of the leading infectious causes of adult mortality worldwide. The Bacillus Calmette–Guérin (BCG) vaccine is currently the only approved vaccine for TB prevention, but its protective efficacy against adult pulmonary TB is limited, and it lacks effective protection against primary or latent TB infection. There is an urgent need to develop more effective preventive TB vaccines. Currently, preventive TB vaccines under clinical investigation globally include live attenuated vaccines, recombinant subunit vaccines, viral vector vaccines, and mRNA vaccines. This article reviews and summarizes recent progress in the clinical development of preventive TB vaccines, analyzing and comparing their safety, immunogenicity, and protective efficacy. It also explores novel strategies for next-generation TB vaccine development, aiming to provide insights and directions for future research.
Full article
(This article belongs to the Special Issue Research Progress of New Tuberculosis Vaccines and Vaccine Design for Intracellular Pathogens)
Open AccessArticle
A Comparative Study on Immune Protection Efficacy: An HSV-1 Trivalent Antigen Subunit Vaccine Formulated with a Cellular Immunity-Inducing Adjuvant Versus an mRNA Vaccine
by
Han Cao, Jingping Hu, Fengyuan Zeng, Ning Luan, Dandan Gao, Zhentao Lei, Jishuai Cheng and Cunbao Liu
Vaccines 2025, 13(9), 958; https://doi.org/10.3390/vaccines13090958 - 10 Sep 2025
Abstract
Background: Herpes simplex virus (HSV) is a neurotropic virus that can be categorized into two serotypes: HSV-1 and HSV-2. HSV-1 causes symptoms such as herpes labialis, herpetic keratitis, genital ulcers, and encephalitis, and primarily establishes latent infection in the trigeminal ganglion. The
[...] Read more.
Background: Herpes simplex virus (HSV) is a neurotropic virus that can be categorized into two serotypes: HSV-1 and HSV-2. HSV-1 causes symptoms such as herpes labialis, herpetic keratitis, genital ulcers, and encephalitis, and primarily establishes latent infection in the trigeminal ganglion. The complexity of membrane fusion mechanisms and potential infection in nerves allow HSV to easily evade recognition and clearance by host immune cells. Therefore, developing a vaccine that can prevent both primary and reactivated HSV-1 infection is critical. Currently, no preventive or therapeutic HSV-1 vaccines have been approved for marketing. Methods: In this study, we utilized the gC, gD, and gE proteins of HSV-1, which are associated with viral fusion and immune escape, to design a trivalent antigen vaccine that is capable of inducing a cellular immune response. Two formulations of the vaccine are available: a subunit vaccine incorporating oligodeoxynucleotides with CpG motifs (CpG ODNs) and QS-21 as adjuvants, as well as an mRNA vaccine. Mice were immunized via intramuscular injection to evaluate and compare the immunological responses and protective efficacy of the two vaccines. Results: After the challenge, the viral load in the tissues of both vaccine groups was significantly lower than that in the positive control group, indicating that both vaccines were able to control viral proliferation in the tissues. Conclusions: The findings indicated that both mRNA and subunit vaccines were capable of eliciting comparable humoral and cellular immune responses.
Full article
(This article belongs to the Section Vaccine Design, Development, and Delivery)
►▼
Show Figures

Figure 1
Open AccessReview
Emerging Therapeutic Strategies for Lung Cancer: The Role of Immunotherapy and HPV-Targeted Cancer Vaccines
by
Krupa Bhaliya, Muneera Anwer and Ming Q. Wei
Vaccines 2025, 13(9), 957; https://doi.org/10.3390/vaccines13090957 - 8 Sep 2025
Abstract
Background/Objectives: Lung cancer remains the leading cause of cancer-related deaths globally, with non-small-cell lung cancer (NSCLC) accounting for most cases. Although advances in targeted therapies and immunotherapy have improved outcomes, long-term survival remains limited. This review aims to explore current immunotherapeutic strategies,
[...] Read more.
Background/Objectives: Lung cancer remains the leading cause of cancer-related deaths globally, with non-small-cell lung cancer (NSCLC) accounting for most cases. Although advances in targeted therapies and immunotherapy have improved outcomes, long-term survival remains limited. This review aims to explore current immunotherapeutic strategies, the evolving role of therapeutic cancer vaccines, and the emerging potential of human papillomavirus-targeted interventions in lung cancer, particularly among non-smoker populations. Methods: A comprehensive search of the literature was conducted using PubMed, Scopus, and Web of Science databases to identify relevant articles published between 2015 and 2024. Studies focusing on immune checkpoint inhibitors, vaccine platforms, HPV-associated lung cancer, tumor microenvironment modulation, and novel delivery systems such as bacterial ghosts were included. Relevant clinical trials and preclinical studies were critically evaluated and synthesized. Results: Immune checkpoint inhibitors targeting PD-1, PD-L1, and CTLA-4 have demonstrated clinical efficacy in NSCLC, yet their effectiveness is often limited by resistance mechanisms and lack of robust predictive biomarkers. Cancer vaccines, including peptide-based, mRNA, DNA, dendritic cell, and bacterial ghost platforms are emerging as complementary strategies to enhance antitumor immunity. Moreover, accumulating evidence suggests a potential association between high-risk HPV infection and lung cancer development, supporting the rationale for HPV-targeted vaccine strategies. Conclusions: Immunotherapy and therapeutic vaccination hold significant promise in reshaping lung cancer treatment. Advancements in vaccine design, delivery platforms like bacterial ghosts, and better understanding of HPV’s role in lung oncogenesis could support more effective, personalized immunotherapeutic approaches in the future.
Full article
(This article belongs to the Special Issue Innovative Vaccines That Modulate the Immune System to Enhance Cancer Therapy)
►▼
Show Figures

Figure 1
Open AccessArticle
Presence of Vaccine-Induced Antibodies Against Leptospira spp. Complicates the Diagnosis of Leptospirosis by the Microscopic Agglutination Test
by
Katharina Gesa Schmitt, Michèle Bergmann, Hans van der Linden, Ahmed A. Ahmed, Reinhard K. Straubinger, Yury Zablotski and Katrin Hartmann
Vaccines 2025, 13(9), 956; https://doi.org/10.3390/vaccines13090956 - 8 Sep 2025
Abstract
Background: Leptospirosis is a potentially fatal infectious disease. Therefore, annual revaccination of dogs is recommended, but this can lead to diagnostic interference due to vaccine-induced antibodies. This study determined the prevalence of Leptospira spp.-specific antibodies in 97 healthy adult dogs revaccinated with a
[...] Read more.
Background: Leptospirosis is a potentially fatal infectious disease. Therefore, annual revaccination of dogs is recommended, but this can lead to diagnostic interference due to vaccine-induced antibodies. This study determined the prevalence of Leptospira spp.-specific antibodies in 97 healthy adult dogs revaccinated with a 4-serovar vaccine (Nobivac® L4). Methods: Antibodies were measured with a microscopic agglutination test against 12 serovars before (week 0) and 2, 4, 12, 26, and 52 weeks after revaccination. Logistic regression analysis was performed to determine the presence of pre-revaccination antibodies. Mixed-effect logistic regression analyses and chi-squared tests were used to compare differences between antibodies against vaccine serovars and between vaccine and non-vaccine serovars at different time points. Results: Overall, 63/97 dogs (64.9%) had antibodies against vaccine serovars before revaccination. During the study period, antibodies against
(This article belongs to the Special Issue Animal Infectious Diseases and Vaccinology in One Health)
►▼
Show Figures
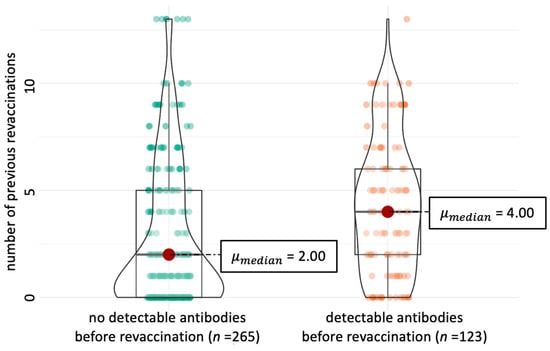
Figure 1

Journal Menu
► ▼ Journal Menu-
- Vaccines Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
26 June 2025
Meet Us at the 12th Congress on Trends in Medical Mycology (TIMM-12), 19–22 September 2025, Bilbao, Spain
Meet Us at the 12th Congress on Trends in Medical Mycology (TIMM-12), 19–22 September 2025, Bilbao, Spain

11 September 2025
Vaccines | Interview with Dr. Stephanie Longet—Session Chair of the 3rd International Online Conference on Vaccines
Vaccines | Interview with Dr. Stephanie Longet—Session Chair of the 3rd International Online Conference on Vaccines

Topics
Topic in
Animals, Arthropoda, Insects, Vaccines, Veterinary Sciences, Pathogens
Ticks and Tick-Borne Pathogens: 2nd Edition
Topic Editors: Alina Rodriguez-Mallon, Alejandro Cabezas-CruzDeadline: 31 March 2026

Conferences
Special Issues
Special Issue in
Vaccines
Inequality in Immunization 2025
Guest Editors: Ahmad Reza Hosseinpoor, Devaki Nambiar, Nicole Bergen, M. Carolina Danovaro, Hope Johnson, Ibrahim DadariDeadline: 15 September 2025
Special Issue in
Vaccines
Vaccination Strategies for Global Public Health
Guest Editors: Mathumalar Fahrni, Antonio Ivan LazzarinoDeadline: 30 September 2025
Special Issue in
Vaccines
The Current Development of Glycoconjugate Vaccines for Infectious Diseases
Guest Editor: Fatme MawasDeadline: 30 September 2025
Special Issue in
Vaccines
Vaccine Against Sexually Transmitted Diseases
Guest Editor: Weiming TangDeadline: 30 September 2025
Topical Collections
Topical Collection in
Vaccines
The Safety and Immunogenicity of the Bivalent Omicron-Containing mRNA-1273.214 Booster Vaccine
Collection Editors: Lalit Batra, Shailendra Kumar Verma
Topical Collection in
Vaccines
Factors Associated with Vaccine Hesitancy
Collection Editor: Brian D. Poole
Topical Collection in
Vaccines
Topic Advisory Panel Members’ Collection Series: Immunization and Vaccines for Infectious Diseases
Collection Editors: Shumaila Hanif, Ravinder Kumar
Topical Collection in
Vaccines
COVID-19 Vaccine Hesitancy: Correlates and Interventions
Collection Editors: Manoj Sharma, Kavita Batra








