Mitigation of Cellular and Bacterial Adhesion on Laser Modified Poly (2-Methacryloyloxyethyl Phosphorylcholine)/Polydimethylsiloxane Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymerisation of 2-Methacryloyloxyethyl Phosphorylcholine (MPC)
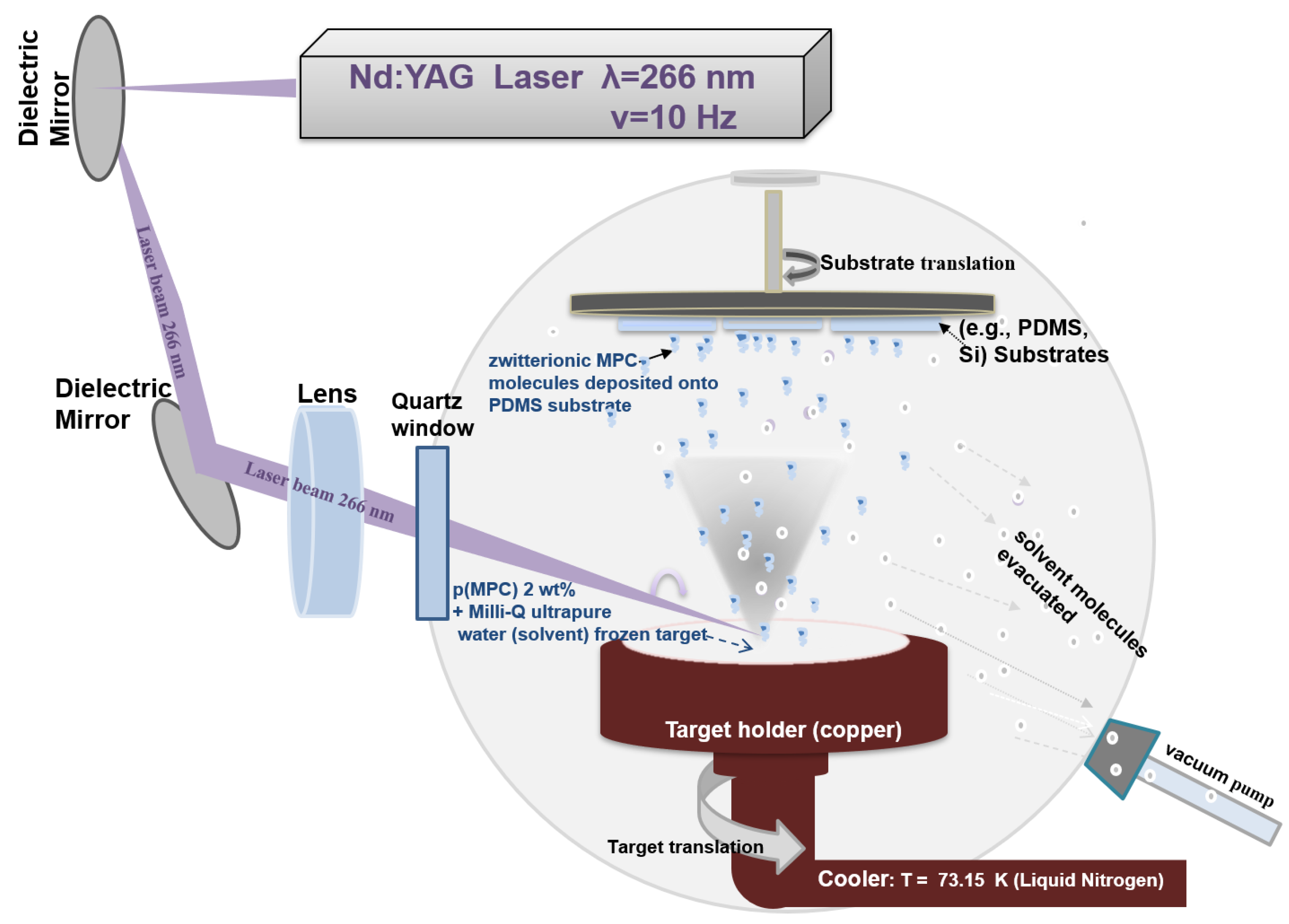
2.2. pMPC Coatings Obtained by MAPLE Method
2.3. PDMS Flat Substrates Preparation and UV–Ozone Treatment
2.4. pMPC Morphological Analysis
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Atomic Force Microscopy (AFM)
2.5. Wettability Characterisation by Contact Angle (CA) and Surface Energy Measurements
2.6. Chemical Profile: Analysis of pMPC
2.6.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.6.2. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.7. Biological Investigations
2.7.1. Antimicrobial Assay
2.7.2. Antibiofilm Effect
2.8. Cell Culture Models
2.8.1. Assessment of Cell Metabolic Activity
2.8.2. Evaluation of Cell Adhesion and Morphology
2.9. Statistical Analysis
3. Results
3.1. Morphological Characterisation of pMPC Coatings Obtained by MAPLE on Si and PDMS
3.2. FTIR and XPS of pMPC Coatings Evaluation
3.3. Wettability
3.4. In Vitro Biological Behaviour
3.4.1. Microbial-Material Surface Interaction
3.4.2. Cell-Material Surface Interplay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Smith, J.R.; Lamprou, D.A. Polymer Coatings for Biomedical Applications: A Review. Trans. IMF 2014, 92, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Adlhart, C.; Verran, J.; Azevedo, N.F.; Olmez, H.; Keinänen-Toivola, M.M.; Gouveia, I.; Melo, L.F.; Crijns, F. Surface Modifications for Antimicrobial Effects in the Healthcare Setting: A Critical Overview. J. Hosp. Infect. 2018, 99, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamdy Makhlouf, A.S.; Perez, A.; Guerrero, E. Chapter 13—Recent Trends in Smart Polymeric Coatings in Biomedicine and Drug Delivery Applications. In Advances in Smart Coatings and Thin Films for Future Industrial and Biomedical Engineering Applications; Makhlouf, A.S.H., Abu-Thabit, N.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 359–381. ISBN 978-0-12-849870-5. [Google Scholar]
- Li, C.; Xia, Y.; Liu, C.; Huang, R.; Qi, W.; He, Z.; Su, R. Lubricin-Inspired Loop Zwitterionic Peptide for Fabrication of Superior Antifouling Surfaces. ACS Appl. Mater. Interfaces 2021, 13, 41978–41986. [Google Scholar] [CrossRef] [PubMed]
- Sibarani, J.; Takai, M.; Ishihara, K. Surface Modification on Microfluidic Devices with 2-Methacryloyloxyethyl Phosphorylcholine Polymers for Reducing Unfavorable Protein Adsorption. Colloids Surfaces B Biointerfaces 2007, 54, 88–93. [Google Scholar] [CrossRef]
- Goda, T.; Konno, T.; Takai, M.; Moro, T.; Ishihara, K. Biomimetic Phosphorylcholine Polymer Grafting from Polydimethylsiloxane Surface Using Photo-Induced Polymerization. Biomaterials 2006, 27, 5151–5160. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Kakinoki, S.; Iwasaki, Y. Long-Lasting Hydrophilic Surface Generated on Poly(Dimethyl Siloxane) with Photoreactive Zwitterionic Polymers. Colloids Surfaces B Biointerfaces 2021, 205, 111900. [Google Scholar] [CrossRef] [PubMed]
- Seetasang, S.; Xu, Y. Recent Progress and Perspectives in Applications of 2-Methacryloyloxyethyl Phosphorylcholine Polymers in Biodevices at Small Scales. J. Mater. Chem. B 2022, 10, 2323–2337. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D.P.; Chen, R.; Kalaiselvan, P.; Yasir, M.; Rasul, R.; Kumar, N.; Dutta, D. The Development of an Antimicrobial Contact Lens—From the Laboratory to the Clinic. CPPS 2020, 21, 357–368. [Google Scholar] [CrossRef]
- Hirota, K.; Murakami, K.; Nemoto, K.; Miyake, Y. Coating of a Surface with 2-Methacryloyloxyethyl Phosphorylcholine (MPC) Co-Polymer Significantly Reduces Retention of Human Pathogenic Microorganisms. FEMS Microbiol. Lett. 2005, 248, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Sin, M.-C.; Chen, S.-H.; Chang, Y. Hemocompatibility of Zwitterionic Interfaces and Membranes. Polym. J. 2014, 46, 436–443. [Google Scholar] [CrossRef]
- Ishihara, K. Revolutionary Advances in 2-methacryloyloxyethyl Phosphorylcholine Polymers as Biomaterials. J. Biomed. Mater. Res. 2019, 107, 933–943. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Zwitterionic Materials for Antifouling Membrane Surface Construction. Acta Biomater. 2016, 40, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Ishihara, K. Cell Membrane-Inspired Phospholipid Polymers for Developing Medical Devices with Excellent Biointerfaces. Sci. Technol. Adv. Mater. 2012, 13, 064101. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T.; Sugihara, K.; Ishihara, K.; Iwasaki, Y.; Nakabayashi, N. The Vascular Prosthesis without Pseudointima Prepared by Anti Thrombogenic Phospholipid Polymer. Biomaterials 2002, 23, 1455–1459. [Google Scholar] [CrossRef]
- Seo, J.-H.; Shibayama, T.; Takai, M.; Ishihara, K. Quick and Simple Modification of a Poly(Dimethylsiloxane) Surface by Optimized Molecular Design of the Anti-Biofouling Phospholipid Copolymer. Soft Matter 2011, 7, 2968–2976. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Parasuraman, V.R.; Mekuria, S.L.; Peng, S.; Tsai, H.-C.; Hsiue, G.-H. Plasma Initiated Graft Polymerization of 2-Methacryloyloxyethyl Phosphorylcholine on Silicone Elastomer Surfaces to Enhance Bio(Hemo)Compatibility. Surf. Coat. Technol. 2017, 315, 342–349. [Google Scholar] [CrossRef]
- Xie, R.; Tian, Y.; Peng, S.; Zhang, L.; Men, Y.; Yang, W. Poly(2-Methacryloyloxyethyl Phosphorylcholine)-Based Biodegradable Nanogels for Controlled Drug Release. Polym. Chem. 2018, 9, 4556–4565. [Google Scholar] [CrossRef]
- Qin, X.-H.; Senturk, B.; Valentin, J.; Malheiro, V.; Fortunato, G.; Ren, Q.; Rottmar, M.; Maniura-Weber, K. Cell-Membrane-Inspired Silicone Interfaces That Mitigate Proinflammatory Macrophage Activation and Bacterial Adhesion. Langmuir 2019, 35, 1882–1894. [Google Scholar] [CrossRef]
- Piqué, A.; Chrisey, D.B.; Spargo, B.J.; Bucaro, M.A.; Vachet, R.W.; Callahan, J.H.; McGill, R.A.; Leonhardt, D.; Mlsna, T.E. Use of Matrix Assisted Pulsed Laser Evaporation (MAPLE) for the Growth of Organic Thin Films. MRS Online Proc. Libr. 1998, 526, 421–426. [Google Scholar] [CrossRef]
- Caricato, A.P.; Luches, A. Applications of the Matrix-Assisted Pulsed Laser Evaporation Method for the Deposition of Organic, Biological and Nanoparticle Thin Films: A Review. Appl. Phys. A 2011, 105, 565–582. [Google Scholar] [CrossRef]
- Dincă, V.; Mocanu, A.; Isopencu, G.; Busuioc, C.; Brajnicov, S.; Vlad, A.; Icriverzi, M.; Roseanu, A.; Dinescu, M.; Stroescu, M.; et al. Biocompatible Pure ZnO Nanoparticles-3D Bacterial Cellulose Biointerfaces with Antibacterial Properties. Arab. J. Chem. 2020, 13, 3521–3533. [Google Scholar] [CrossRef]
- Icriverzi, M.; Bonciu, A.; Rusen, L.; Sima, L.E.; Brajnicov, S.; Cimpean, A.; Evans, R.W.; Dinca, V.; Roseanu, A. Human Mesenchymal Stem Cell Response to Lactoferrin-Based Composite Coatings. Materials 2019, 12, 3414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumitrescu, N.-L.; Icriverzi, M.; Bonciu, A.; Florian, P.; Moldovan, A.; Roseanu, A.; Rusen, L.; Dinca, V.; Grama, F. New Poly(N-Isopropylacrylamide-Butylacrylate) Copolymer Biointerfaces and Their Characteristic Influence on Cell Behavior In Vitro. IJMS 2022, 23, 3988. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, T.; Baassiri, K.; Mahmoudi, Z.; Perumal, A.S.; Rajendran, K.; Rubies, G.M.; Nicolau, D.V. Hydrophobic Recovery of PDMS Surfaces in Contact with Hydrophilic Entities: Relevance to Biomedical Devices. Materials 2022, 15, 2313. [Google Scholar] [CrossRef]
- Ishihara, K.; Ueda, T.; Nakabayashi, N. Preparation of Phospholipid Polyers and Their Properties as Polymer Hydrogel Membranes. Polym. J. 1990, 22, 355–360. [Google Scholar] [CrossRef]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Rabel, W. Einige aspekte der benetzungstheorie und ihre anwendung auf die untersuchung und veränderung der oberflächeneigenschaften von polymeren. Farbe Lack 1971, 77, 997–1005. [Google Scholar]
- Icriverzi, M.; Rusen, L.; Brajnicov, S.; Bonciu, A.; Dinescu, M.; Cimpean, A.; Evans, R.W.; Dinca, V.; Roseanu, A. Macrophage in Vitro Response on Hybrid Coatings Obtained by Matrix Assisted Pulsed Laser Evaporation. Coatings 2019, 9, 236. [Google Scholar] [CrossRef] [Green Version]
- Dunn, A.C.; Urueña, J.M.; Huo, Y.; Perry, S.S.; Angelini, T.E.; Sawyer, W.G. Lubricity of Surface Hydrogel Layers. Tribol. Lett. 2013, 49, 371–378. [Google Scholar] [CrossRef]
- Nilavarasi, K.; Madhurima, V. Influence of Polar and Dispersive Part of Surface Tension on the Self-Assembly of Droplets on PDMS Surfaces. Mater. Today Proc. 2018, 5, 16424–16432. [Google Scholar] [CrossRef]
- Bowden, N.; Huck, W.; Kateri, P.; Whitesides, G.M. The controlled formation of ordered, sinusoidal structures by plasma oxidation of an elastomeric polymer. Appl. Phys. Lett. 1999, 75, 2557–2559. [Google Scholar] [CrossRef]
- Tan, A.; Pellegrino, L.; Ahmad, Z.; Cabral, J.T. Tunable Structural Color with Gradient and Multiaxial Polydimethylsiloxane Wrinkling. Adv. Opt. Mater. 2022, 10, 2200964. [Google Scholar] [CrossRef]
- Peng, S.; Men, Y.; Xie, R.; Tian, Y.; Yang, W. Biodegradable Phosphorylcholine-Based Zwitterionic Polymer Nanogels with Smart Charge-Conversion Ability for Efficient Inhibition of Tumor Cells. J. Colloid Interface Sci. 2019, 539, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Inoue, Y.; Mahara, A.; Kakinoki, S.; Yamaoka, T.; Ishihara, K. Durable Modification of Segmented Polyurethane for Elastic Blood-Contacting Devices by Graft-Type 2-Methacryloyloxyethyl Phosphorylcholine Copolymer. J. Biomater. Sci. Polym. Ed. 2014, 25, 1514–1529. [Google Scholar] [CrossRef] [PubMed]
- Gökaltun, A.; Kang, Y.B.; Yarmush, M.L.; Usta, O.B.; Asatekin, A. Simple Surface Modification of Poly(dimethylsiloxane) via Surface Segregating Smart Polymers for Biomicrofluidics. Sci. Rep. 2019, 9, 7377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishihara, K.; Ando, B.; Takai, M. Phosphorylcholine Group-immobilized Surface Prepared on Polydimethylsiloxane Membrane by In Situ Reaction for Its Reduced Biofouling. Nanobiotechnology 2007, 3, 83–88. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Wu, C.-H.; Syu, W.-L.; Ho, P.-C.; Tseng, Z.-L.; Yang, M.-C.; Lin, C.-C.; Chen, C.-C.; Chen, C.-C.; Liu, T.-Y. Replica of Bionic Nepenthes Peristome-like and Anti-Fouling Structures for Self-Driving Water and Raman-Enhancing Detection. Polymers 2022, 14, 2465. [Google Scholar] [CrossRef]
- Malecha, K.; Gancarz, I.; Tylus, W. Argon plasma-assisted PDMS–LTCC bonding technique for microsystem applications. J. Micromech. Microeng. 2010, 20, 115006. [Google Scholar] [CrossRef]
- Hillborg, H.; Tomczak, N.; Olàh, A.; Schönherr, H.; Vancso, G.J. Nanoscale Hydrophobic Recovery: A Chemical Force Microscopy Study of UV/Ozone-Treated Cross-Linked Poly(dimethylsiloxane). Langmuir 2004, 20, 785–794. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface Hydration: Principles and Applications toward Low-Fouling/Nonfouling Biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-Review. Res. Rev. J. Eng. Technol. 2017, 6, PMC6133255. [Google Scholar]
- Kaneko, T.; Saito, T.; Shobuike, T.; Miyamoto, H.; Matsuda, J.; Fukazawa, K.; Ishihara, K.; Tanaka, S.; Moro, T. 2-Methacryloyloxyethyl Phosphorylcholine Polymer Coating Inhibits Bacterial Adhesion and Biofilm Formation on a Suture: An In Vitro and In Vivo Study. BioMed Res. Int. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rzhepishevska, O.; Hakobyan, S.; Ruhal, R.; Gautrot, J.; Barberoc, D.; Ramstedt, M. The surface charge of anti-bacterial coatings alters motility and biofilm architecture. Biomater. Sci. 2013, 1, 589–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arima, Y.; Iwata, H. Effect of Wettability and Surface Functional Groups on Protein Adsorption and Cell Adhesion Using Well-Defined Mixed Self-Assembled Monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
- Ross, A.M.; Jiang, Z.; Bastmeyer, M.; Lahann, J. Physical Aspects of Cell Culture Substrates: Topography, Roughness, and Elasticity. Small 2012, 8, 336–355. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xiao, Y.; Liu, C. The Horizon of Materiobiology: A Perspective on Material-Guided Cell Behaviors and Tissue Engineering. Chem. Rev. 2017, 117, 4376–4421. [Google Scholar] [CrossRef]
- Kang, S.H.; Bengtson, B.P.; Heo, C.Y. Various Properties of Silicone Breast Implant Surfaces and Multimodal Techniques for the Functional Surface Modification. Clin. Plast. Surg. 2021, 48, 87–99. [Google Scholar] [CrossRef]
- McWhorter, F.Y.; Davis, C.T.; Liu, W.F. Physical and Mechanical Regulation of Macrophage Phenotype and Function. Cell Mol. Life Sci. 2015, 72, 1303–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Takai, M.; Ishihara, K. Protein Adsorption and Cell Adhesion on Cationic, Neutral, and Anionic 2-Methacryloyloxyethyl Phosphorylcholine Copolymer Surfaces. Biomaterials 2009, 30, 4930–4938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kanetaka, H.; Sano, Y.; Kano, M.; Kudo, T.; Shimizu, Y. MPC Polymer Regulates Fibrous Tissue Formation by Modulating Cell Adhesion to the Biomaterial Surface. Dent. Mater. J. 2010, 29, 518–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, J.D.; Ebert, M.; Ward, R.; Anderson, J.M.S. Epidermidis Biofilm Formation: Effects of Biomaterial Surface Chemistry and Serum Proteins. J. Biomed. Mater. Res. A 2007, 80, 742–751. [Google Scholar] [CrossRef]
- Jiang, S.; Cao, Z. Ultralow-Fouling, Functionalizable, and Hydrolyzable Zwitterionic Materials and Their Derivatives for Biological Applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef]
- Chen, S.-H.; Chang, Y.; Ishihara, K. Reduced Blood Cell Adhesion on Polypropylene Substrates through a Simple Surface Zwitterionization. Langmuir 2017, 33, 611–621. [Google Scholar] [CrossRef]
- Shigeta, M.; Tanaka, T.; Koike, N.; Yamakawa, N.; Usui, M. Suppression of Fibroblast and Bacterial Adhesion by MPC Coating on Acrylic Intraocular Lenses. J. Cataract. Refract. Surg. 2006, 32, 859–866. [Google Scholar] [CrossRef]
- Li, D.; Wei, Q.; Wu, C.; Zhang, X.; Xue, Q.; Zheng, T.; Cao, M. Superhydrophilicity and Strong Salt-Affinity: Zwitterionic Polymer Grafted Surfaces with Significant Potentials Particularly in Biological Systems. Adv. Colloid Interface Sci. 2020, 278, 102141. [Google Scholar] [CrossRef]
- Ishihara, K.; Fukazawa, K.; Sharma, V.; Liang, S.; Shows, A.; Dunbar, D.C.; Zheng, Y.; Ge, J.; Zhang, S.; Hong, Y.; et al. Antifouling Silicone Hydrogel Contact Lenses with a Bioinspired 2-Methacryloyloxyethyl Phosphorylcholine Polymer Surface. ACS Omega 2021, 6, 7058–7067. [Google Scholar] [CrossRef]
- Cheng, Q.; Asha, A.B.; Liu, Y.; Peng, Y.-Y.; Diaz-Dussan, D.; Shi, Z.; Cui, Z.; Narain, R. Antifouling and Antibacterial Polymer-Coated Surfaces Based on the Combined Effect of Zwitterions and the Natural Borneol. ACS Appl. Mater. Interfaces 2021, 13, 9006–9014. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nistorescu, S.; Icriverzi, M.; Florian, P.; Bonciu, A.; Marascu, V.; Dumitrescu, N.; Pircalabioru, G.G.; Rusen, L.; Mocanu, A.; Roseanu, A.; et al. Mitigation of Cellular and Bacterial Adhesion on Laser Modified Poly (2-Methacryloyloxyethyl Phosphorylcholine)/Polydimethylsiloxane Surface. Nanomaterials 2023, 13, 64. https://doi.org/10.3390/nano13010064
Nistorescu S, Icriverzi M, Florian P, Bonciu A, Marascu V, Dumitrescu N, Pircalabioru GG, Rusen L, Mocanu A, Roseanu A, et al. Mitigation of Cellular and Bacterial Adhesion on Laser Modified Poly (2-Methacryloyloxyethyl Phosphorylcholine)/Polydimethylsiloxane Surface. Nanomaterials. 2023; 13(1):64. https://doi.org/10.3390/nano13010064
Chicago/Turabian StyleNistorescu, Simona, Madalina Icriverzi, Paula Florian, Anca Bonciu, Valentina Marascu, Nicoleta Dumitrescu, Gratiela Gradisteanu Pircalabioru, Laurentiu Rusen, Alexandra Mocanu, Anca Roseanu, and et al. 2023. "Mitigation of Cellular and Bacterial Adhesion on Laser Modified Poly (2-Methacryloyloxyethyl Phosphorylcholine)/Polydimethylsiloxane Surface" Nanomaterials 13, no. 1: 64. https://doi.org/10.3390/nano13010064
APA StyleNistorescu, S., Icriverzi, M., Florian, P., Bonciu, A., Marascu, V., Dumitrescu, N., Pircalabioru, G. G., Rusen, L., Mocanu, A., Roseanu, A., Cimpean, A., Grama, F., Dinca, V., & Cristian, D. A. (2023). Mitigation of Cellular and Bacterial Adhesion on Laser Modified Poly (2-Methacryloyloxyethyl Phosphorylcholine)/Polydimethylsiloxane Surface. Nanomaterials, 13(1), 64. https://doi.org/10.3390/nano13010064










