Development and Validation of an LC-MS/MS Method for the Quantitative Determination of Contezolid in Human Plasma and Cerebrospinal Fluid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Development
2.1.1. Chromatography and MS Conditions
2.1.2. Sample Preparation
2.2. Method Validation
2.2.1. Calibration Curve
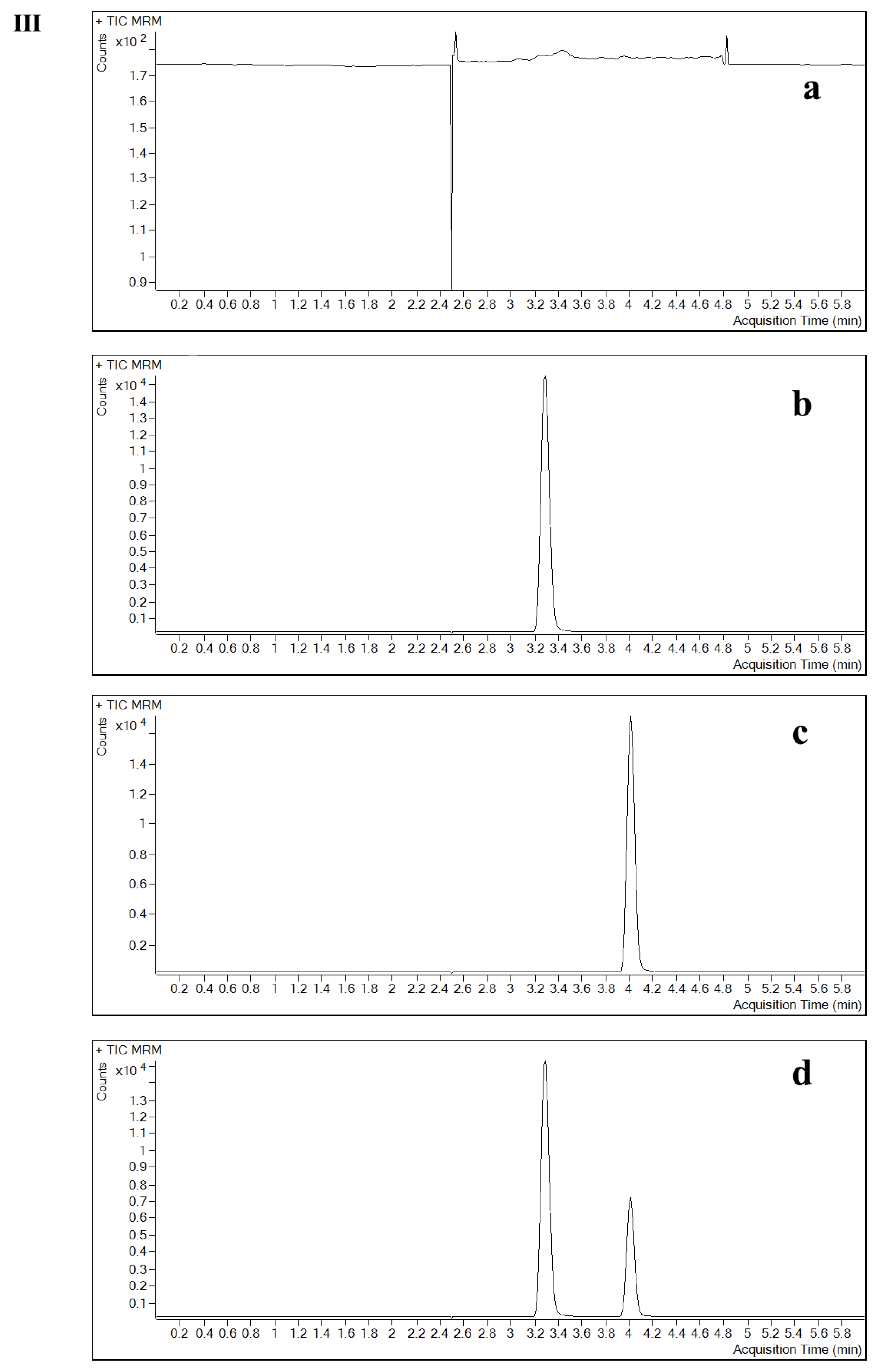
2.2.2. Specificity and Selectivity
2.2.3. Sensitivity
2.2.4. Precision and Accuracy
2.2.5. Recovery
2.2.6. Dilution Integrity
2.2.7. Stability
2.2.8. ME
3. Materials and Methods
3.1. Reference Materials
3.2. Reagents
3.3. Biological Matrix
3.4. LC-MS/MS System
3.5. Preparation of Calibration Standard Curves, Quality Control (QC) and IS Samples
3.6. Sample Preparation
3.7. Method Validation and Acceptance Criteria
3.7.1. Specificity and Selectivity
3.7.2. ME
3.7.3. Calibration Curve and Range
3.7.4. Sensitivity
3.7.5. Precision and Accuracy
3.7.6. Carry-Over
3.7.7. Dilution Integrity
3.7.8. Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| CE | collision energy |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| CV | coefficient of variation |
| DMSO | dimethyl sulfoxide |
| ESI | electrospray ionization |
| FA | formic acid |
| HPLC | high-performance liquid chromatography |
| HQC | high quality control |
| IS | internal standard |
| LC-MS/MS | liquid chromatography-tandem mass spectrometry |
| LLOQ | lower limit of quantification |
| LOQ | low quality control |
| ME | matrix effect |
| MF | matrix factors |
| MQC | medium quality control |
| MRM | multiple reaction monitoring |
| MRSA | methicillin-resistant Staphylococcus aureus |
| PRSP | penicillin-resistant Streptococcus pneumoniae |
| QC | quality control |
| RE | relevant error |
| SNR | signal-to-noise ratio |
| ULOQ | upper limit of quantification |
| VRE | vancomycin-resistant enterococci |
References
- Gao, W.; Chen, Y.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-based local antimicrobial drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, G.; Li, W.; Shen, X.; Perez-Aguilar, J.M.; Chong, Y.; Gao, X.; Chai, Z.; Chen, C.; Ge, C.; Zhou, R. Differential Pd-nanocrystal facets demonstrate distinct antibacterial activity against Gram-positive and Gram-negative bacteria. Nat. Commun. 2018, 9, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozarich, J.W. The biochemistry of disease: Desperately seeking syzygy. Annu. Rev. Biochem. 2009, 78, 55–63. [Google Scholar] [CrossRef]
- Jean, S.S.; Tsai, M.H.; Chen, W.C.; Ho, S.J.; Wu, M.H.; Hsueh, P.R. Fatal community-acquired pneumonia caused by Legionella pneumophila and fluoroquinolone-resistant Salmonella enterica serotype Choleraesuis bacteraemia. Int. J. Antimicrob. Agents 2009, 33, 189–190. [Google Scholar] [CrossRef]
- Shinabarger, D. Mechanism of action of the oxazolidinone antibacterial agents. Expert Opin. Investig. Drugs 1999, 8, 1195–1202. [Google Scholar] [CrossRef]
- Jiang, J.; Hou, Y.; Duan, M.; Wang, B.; Wu, Y.; Ding, X.; Zhao, Y. Design, synthesis and antibacterial evaluation of novel oxazolidinone derivatives nitrogen-containing fused heterocyclic moiety. Bioorg. Med. Chem. Lett. 2021, 32, 127660. [Google Scholar] [CrossRef]
- Scaiola, A.; Leibundgut, M.; Boehringer, D.; Caspers, P.; Bur, D.; Locher, H.H.; Rueedi, G.; Ritz, D. Structural basis of translation inhibition by cadazolid, a novel quinoxolidinone antibiotic. Sci. Rep. 2019, 9, 5634. [Google Scholar] [CrossRef] [Green Version]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals from the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef]
- Grau, S.; Rubio-Terrés, C. Pharmacoeconomics of linezolid. Expert Opin. Pharmacother. 2008, 9, 987–1000. [Google Scholar] [CrossRef]
- Rabon, A.D.; Fisher, J.P.; MacVane, S.H. Incidence and Risk Factors for Development of Thrombocytopenia in Patients Treated with Linezolid for 7 Days or Greater. Ann. Pharmacother. 2018, 52, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Gorchynski, J.; Rose, J.K. Complications of MRSA Treatment: Linezolid-induced Myelosuppression Presenting with Pancytopenia. West. J. Emerg. Med. 2008, 9, 177–178. [Google Scholar] [PubMed]
- Gordeev, M.F.; Yuan, Z.Y. New Potent Antibacterial Oxazolidinone (MRX-I) with an Improved Class Safety Profile. J. Med. Chem. 2014, 57, 4487–4497. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-R.; Zhai, Q.-Q.; Wang, X.-K.; Hu, X.-X.; Li, G.-Q.; Zhang, W.-X.; Pang, J.; Lu, X.; Yuan, H.; Gordeev Mikhail, F.; et al. In Vivo Antibacterial Activity of MRX-I, a New Oxazolidinone. Antimicrob. Agents Chemother. 2014, 58, 2418–2421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Cai, C.; Shen, Y.; Sun, C.; Shi, Q.; Wu, N.; Zheng, S.; Qian, J.; Zhang, R.; Zhou, H. In vitro Activity of Contezolid Against Methicillin-Resistant Staphylococcus aureus, Vancomycin-Resistant Enterococcus, and Strains with Linezolid Resistance Genes From China. Front. Microbiol. 2021, 12, 729900. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, J.; Huang, J.; Yu, J.; Zhang, J.; Guo, B. Development and validation of a rapid and robust LC-MS/MS method for quantification of a new oxazolidinone antibiotic MRX-I in human plasma and urine. Clin. J. Infect. Chemother. 2014, 14, 210–215. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Wu, J.; Fan, Y.; Liu, X.; Li, Y.; Hu, J.; Zhang, J.; Guo, B. Development and validation of ultra-performance liquid chromatography-tandem mass spectrometric methods for simultaneous and rapid determination of contezolid and its major metabolite M2 in plasma and urine samples and its application to a study in subjects with moderate liver impairment. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1191, 123129. [Google Scholar] [CrossRef]
- John, C.C.; Carabin, H.; Montano, S.M.; Bangirana, P.; Zunt, J.R.; Peterson, P.K. Global research priorities for infections that affect the nervous system. Nature 2015, 527, S178–S186. [Google Scholar] [CrossRef]
- Kielian, T.; Esen, N.; Bearden, E.D. Toll-like receptor 2 (TLR2) is pivotal for recognition of S. aureus peptidoglycan but not intact bacteria by microglia. Glia 2005, 49, 567–576. [Google Scholar] [CrossRef] [Green Version]
- Lutsar, I.; McCracken, G.H., Jr.; Friedland, I.R. Antibiotic pharmacodynamics in cerebrospinal fluid. Clin. Infect. Dis. 1998, 27, 1117–1129, quiz 1128–1119. [Google Scholar] [CrossRef]
- Dong, L.; Bai, N.; Wang, T.; Cai, Y. Development and validation of a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for the quantification of voriconazole in human cerebrospinal fluid. Anal. Methods 2021, 13, 4585–4593. [Google Scholar] [CrossRef] [PubMed]













| Concentration (ng/mL) | Number | Hemolytic Plasma | Hyperlipidemic Plasma |
|---|---|---|---|
| 150 | 1 | 153.3127 | 155.2132 |
| 2 | 150.9690 | 156.3032 | |
| 3 | 153.2864 | 157.3617 | |
| Mean | 152.5227 | 156.2927 | |
| SD | ±1.3456 | ±1.0743 | |
| %CV | 0.8800 | 0.6900 | |
| Accuracy | 101.6800 | 104.2000 | |
| RE (%) | 1.6800 | 4.2000 | |
| 3750 | 1 | 3767.9127 | 3792.1579 |
| 2 | 3752.9170 | 3775.6060 | |
| 3 | 3770.4290 | 3794.5686 | |
| Mean | 3763.7529 | 3787.4442 | |
| SD | ±9.4681 | ±10.3228 | |
| %CV | 0.2500 | 0.2700 | |
| Accuracy | 100.3700 | 101.0000 | |
| RE (%) | 0.3700 | 1.0000 |
| Concentration (ng/mL) | Batch | Intra-Batch (Mean ± SD) | Intra-Batch Accuracy (%) | Intra-Batch (%CV) | Inter-Batch (Mean ± SD) | Inter-Batch Accuracy (%) | Inter-Batch (%CV) |
|---|---|---|---|---|---|---|---|
| In plasma (n = 6) | |||||||
| 50.0 | 1 | 51.2670 ± 1.5182 | 102.53 | 2.96 | 51.6672 ± 0.5278 | 103.33 | 1.02 |
| 2 | 50.9382 ± 1.7169 | 101.88 | 3.37 | ||||
| 3 | 52.7963 ± 0.7198 | 105.59 | 1.36 | ||||
| 150 | 1 | 142.0762 ± 1.2834 | 94.72 | 0.90 | 146.0054 ± 0.8599 | 97.34 | 0.59 |
| 2 | 139.9441 ± 2.4430 | 93.30 | 1.75 | ||||
| 3 | 155.9958 ± 0.7632 | 104.00 | 0.49 | ||||
| 2000 | 1 | 2008.7019 ± 10.5603 | 100.44 | 0.53 | 2004.4452 ± 2.1689 | 100.22 | 0.11 |
| 2 | 1973.4304 ± 7.4792 | 98.67 | 0.38 | ||||
| 3 | 2031.2032 ± 11.6640 | 101.56 | 0.57 | ||||
| 3750 | 1 | 3712.0060 ± 14.6794 | 98.99 | 0.40 | 3721.1817 ± 3.8675 | 99.23 | 0.10 |
| 2 | 3651.3176 ± 10.4087 | 97.37 | 0.29 | ||||
| 3 | 3800.2216 ± 18.1291 | 101.34 | 0.48 | ||||
| In CSF (n = 6) | |||||||
| 20.0 | 1 | 20.7859 ± 0.6668 | 103.93 | 3.21 | 21.1237 ± 0.4009 | 105.62 | 1.90 |
| 2 | 21.0129 ± 0.4486 | 105.06 | 2.13 | ||||
| 3 | 21.5723 ± 1.2258 | 107.86 | 5.68 | ||||
| 60.0 | 1 | 62.7186 ± 1.6451 | 104.53 | 2.62 | 61.3609 ± 1.4473 | 102.27 | 2.36 |
| 2 | 58.7197 ± 0.8075 | 97.87 | 1.38 | ||||
| 3 | 62.6443 ± 3.6259 | 104.41 | 5.79 | ||||
| 350 | 1 | 359.8353 ± 3.2945 | 102.81 | 0.92 | 347.9318 ± 4.6878 | 99.41 | 1.35 |
| 2 | 342.4519 ± 4.3888 | 97.84 | 1.28 | ||||
| 3 | 341.5083 ± 11.9056 | 97.57 | 3.49 | ||||
| 750 | 1 | 738.0302 ± 17.8846 | 98.40 | 2.42 | |||
| 2 | 751.4551 ± 4.7026 | 100.19 | 0.63 | 747.1321 ± 19.1938 | 99.62 | 2.57 | |
| 3 | 751.9110 ± 42.5167 | 100.25 | 5.65 | ||||
| Number | Nominal Concentration (ng/mL) | Actual Concentration (ng/mL) | Accuracy (%) | RE (%) |
|---|---|---|---|---|
| In plasma (n = 6, diluted five-fold) | ||||
| 1 | 20,000 | 20,858.37 | 104.29 | 4.29 |
| 2 | 20,820.85 | 104.10 | 4.10 | |
| 3 | 20,873.87 | 104.37 | 4.37 | |
| 4 | 20,757.35 | 103.79 | 3.79 | |
| 5 | 20,675.17 | 103.38 | 3.38 | |
| 6 | 20,909.58 | 104.55 | 4.55 | |
| Mean | - | 20,815.87 | 104.08 | - |
| SD | - | ±86.25 | - | - |
| %CV | - | 0.41 | - | - |
| RE (%) | - | 4.08 | - | - |
| In CSF (n = 6, diluted five-fold) | ||||
| 1 | 1750 | 1739.23 | 99.38 | −0.62 |
| 2 | 1747.11 | 99.84 | −0.16 | |
| 3 | 1757.69 | 100.44 | 0.44 | |
| 4 | 1654.12 | 94.52 | −5.48 | |
| 5 | 1657.21 | 94.70 | −5.30 | |
| 6 | 1648.88 | 94.22 | −5.78 | |
| Mean | - | 1700.71 | 97.18 | - |
| SD | - | ±52.22 | - | - |
| %CV | - | 3.07 | - | - |
| RE (%) | - | −2.82 | - | - |
| Nominal Concentration (ng/mL) | 150 | 3750 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | %CV | RE (%) | Mean | SD | %CV | RE (%) | ||
| In plasma (n = 3) | |||||||||
| 0 h | Determined concentration (ng/mL) | 154.68 | ±1.51 | 0.98 | 3827.04 | ±13.58 | 0.35 | ||
| Accuracy (%) | 103.12 | 3.12 | 102.05 | 2.05 | |||||
| 3.5 h—at room temperature | Determined concentration (ng/mL) | 154.03 | ±2.92 | 1.90 | 3812.67 | ±32.46 | 0.85 | ||
| Accuracy (%) | 102.68 | 2.68 | 101.67 | 1.67 | |||||
| 116 h—in the automatic sampler * | Determined concentration (ng/mL) | 161.21 | ±7.12 | 4.42 | 3819.02 | ±12.55 | 0.33 | ||
| Accuracy (%) | 107.47 | 7.47 | 101.84 | 1.84 | |||||
| Repeated freeze-thaw three times from −20 °C to room temperature | Determined concentration (ng/mL) | 153.76 | ±1.53 | 1.00 | 3791.53 | ±21.06 | 0.56 | ||
| Accuracy (%) | 102.50 | 2.50 | 101.11 | 1.11 | |||||
| Repeated freeze-thaw three times from −80 °C to room temperature | Determined concentration (ng/mL) | 152.80 | ±1.61 | 1.05 | 3793.19 | ±9.28 | 0.24 | ||
| Accuracy (%) | 101.86 | 1.86 | 101.15 | 1.15 | |||||
| 28 days—at −20 °C | Determined concentration (ng/mL) | 138.46 | ±2.15 | 1.55 | 3516.93 | ±38.64 | 1.10 | ||
| Accuracy (%) | 92.30 | −7.70 | 93.78 | −6.22 | |||||
| 28 days—at −80 °C | Determined concentration (ng/mL) | 135.19 | ±1.57 | 1.16 | 3524.58 | ±53.28 | 1.51 | ||
| Accuracy (%) | 90.13 | −9.87 | 93.99 | −6.01 | |||||
| 118 days—at −80 °C | Determined concentration (ng/mL) | 152.07 | ±5.64 | 3.71 | 3219.72 | ±46.30 | 1.44 | ||
| Accuracy (%) | 101.38 | 1.38 | 85.86 | −14.14 | |||||
| In CSF (n = 3) | |||||||||
| 0 h | Determined concentration (ng/mL) | 62.77 | ±4.33 | 6.90 | 792.67 | ±49.44 | 6.24 | ||
| Accuracy (%) | 104.61 | 4.61 | 105.69 | 5.69 | |||||
| 6 h–at room temperature | Determined concentration (ng/mL) | 62.57 | ±0.64 | 1.03 | 768.33 | ±2.52 | 0.33 | ||
| Accuracy (%) | 104.28 | 4.28 | 102.44 | 2.44 | |||||
| 23.5 h–in the automatic sampler * | Determined concentration (ng/mL) | 60.45 | ±0.71 | 1.17 | 707.28 | ±5.09 | 0.72 | ||
| Accuracy (%) | 100.75 | 0.75 | 94.30 | −5.70 | |||||
| Repeated freeze-thaw three times from −20 °C to room temperature | Determined concentration (ng/mL) | 62.53 | ±1.07 | 1.71 | 766.00 | ±14.42 | 1.88 | ||
| Accuracy (%) | 104.22 | 4.22 | 102.13 | 2.13 | |||||
| Repeated freeze-thaw three times from −80 °C to room temperature | Determined concentration (ng/mL) | 61.43 | ±1.59 | 2.59 | 781.33 | ±22.68 | 2.90 | ||
| Accuracy (%) | 102.39 | 2.39 | 104.18 | 4.18 | |||||
| 90 days—at −20 °C | Determined concentration (ng/mL) | 68.53 | ±0.62 | 0.62 | 734.52 | ±17.95 | 2.44 | ||
| Accuracy (%) | 114.22 | 14.22 | 97.94 | −2.06 | |||||
| 90 days—at −80 °C | Determined concentration (ng/mL) | 65.62 | ±0.89 | 1.36 | 753.42 | ±6.14 | 0.82 | ||
| Accuracy (%) | 109.37 | 9.37 | 100.46 | 0.46 | |||||
| Number | LQC (150 ng/mL) | MQC (2000 ng/mL) | HQC (3750 ng/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Area Ratio of Analyte | Area Ratio of IS | MF | Area Ratio of Analyte | Area Ratio of IS | MF | Area Ratio of Analyte | Area Ratio of IS | MF | |
| In plasma | |||||||||
| 1 | 0.8719 | 0.8896 | 0.9801 | 0.9442 | 0.9589 | 0.9847 | 0.9581 | 0.9760 | 0.9817 |
| 2 | 0.8314 | 0.8589 | 0.9680 | 0.9390 | 0.9529 | 0.9854 | 0.8791 | 0.8903 | 0.9874 |
| 3 | 0.8229 | 0.8313 | 0.9899 | 0.9110 | 0.9240 | 0.9859 | 0.8979 | 0.9153 | 0.9810 |
| 4 | 0.9893 | 1.0272 | 0.9631 | 0.9108 | 0.9319 | 0.9774 | 0.9367 | 0.9459 | 0.9903 |
| 5 | 0.7877 | 0.7994 | 0.9854 | 1.2185 | 1.2303 | 0.9904 | 1.2196 | 1.2489 | 0.9765 |
| 6 | 0.7650 | 0.8032 | 0.9524 | 0.9052 | 0.9273 | 0.9762 | 0.9204 | 0.9408 | 0.9783 |
| Mean | - | - | 0.9732 | - | - | 0.9833 | - | - | 0.9825 |
| SD | - | - | ±0.0144 | - | - | ±0.0055 | - | - | ±0.0053 |
| %CV | - | - | 1.48 | - | - | 0.56 | - | - | 0.54 |
| In CSF | |||||||||
| 1 | 0.9520 | 1.1002 | 0.8653 | 0.9000 | 0.9550 | 0.9424 | 0.8648 | 1.0114 | 0.8551 |
| 2 | 0.9370 | 0.9040 | 1.0365 | 0.9190 | 0.9743 | 0.9432 | 0.9371 | 1.0000 | 0.9371 |
| 3 | 0.9700 | 1.0200 | 0.9510 | 0.9429 | 0.9058 | 1.0410 | 0.9837 | 1.0343 | 0.9511 |
| 4 | 0.9970 | 1.0156 | 0.9817 | 0.9381 | 0.8822 | 1.0634 | 0.9907 | 1.0435 | 0.9494 |
| 5 | 0.9970 | 0.9265 | 1.0761 | 0.9333 | 0.9893 | 0.9434 | 0.9510 | 0.8902 | 1.0683 |
| 6 | 0.9760 | 1.0067 | 0.9695 | 0.9190 | 0.9850 | 0.9330 | 0.9604 | 1.0000 | 0.9604 |
| Mean | - | - | 0.9800 | - | - | 0.9777 | - | - | 0.9536 |
| SD | - | - | ±0.0729 | - | - | ±0.0582 | - | - | ±0.0681 |
| %CV | - | - | 7.44 | - | - | 5.95 | - | - | 7.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Zhang, N.; Dong, L.; Bai, N.; Cai, Y. Development and Validation of an LC-MS/MS Method for the Quantitative Determination of Contezolid in Human Plasma and Cerebrospinal Fluid. Pharmaceuticals 2023, 16, 32. https://doi.org/10.3390/ph16010032
Zhang G, Zhang N, Dong L, Bai N, Cai Y. Development and Validation of an LC-MS/MS Method for the Quantitative Determination of Contezolid in Human Plasma and Cerebrospinal Fluid. Pharmaceuticals. 2023; 16(1):32. https://doi.org/10.3390/ph16010032
Chicago/Turabian StyleZhang, Guanxuanzi, Na Zhang, Liuhan Dong, Nan Bai, and Yun Cai. 2023. "Development and Validation of an LC-MS/MS Method for the Quantitative Determination of Contezolid in Human Plasma and Cerebrospinal Fluid" Pharmaceuticals 16, no. 1: 32. https://doi.org/10.3390/ph16010032
APA StyleZhang, G., Zhang, N., Dong, L., Bai, N., & Cai, Y. (2023). Development and Validation of an LC-MS/MS Method for the Quantitative Determination of Contezolid in Human Plasma and Cerebrospinal Fluid. Pharmaceuticals, 16(1), 32. https://doi.org/10.3390/ph16010032







