Biventricular Strain Imaging with Cardiac MRI in Genotyped and Histology Validated Amyloid Cardiomyopathy
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Histopathological Analysis and Genotyping of Endomyocardial Biopsies
2.3. Contrast-Enhanced Cardiac MRI Protocol
2.4. Strain Analysis
2.5. Statistical Methods
3. Results
3.1. Histopathology of EMB Samples
3.2. Echocardiographic Assessment of Left Ventricular (LV) Longitudinal Strains
3.3. Cardiac MRI Assessment of Left Ventricular (LV) Deformation
3.3.1. LV Circumferential, Radial and Longitudinal Strains
3.3.2. LV Strain Rates
3.4. Cardiac MRI Assessment of Right Ventricular (RV) Deformation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wechalekar, A.D.; Gillmore, J.D.; Hawkins, P.N. Systemic amyloidosis. Lancet 2016, 387, 2641–2654. [Google Scholar] [CrossRef]
- Rubin, J.; Maurer, M.S. Cardiac Amyloidosis: Overlooked, Underappreciated, and Treatable. Annu. Rev. Med. 2020, 71, 203–219. [Google Scholar] [CrossRef] [Green Version]
- Bart, N.K.; Thomas, L.; Korczyk, D.; Atherton, J.J.; Stewart, G.J.; Fatkin, D. Amyloid Cardiomyopathy. Heart Lung Circ. 2019, 29, 575–583. [Google Scholar] [CrossRef] [Green Version]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef]
- Kyriakou, P.; Mouselimis, D.; Tsarouchas, A.; Rigopoulos, A.; Bakogiannis, C.; Noutsias, M.; Vassilikos, V. Diagnosis of cardiac amyloidosis: A systematic review on the role of imaging and biomarkers. BMC Cardiovasc. Disord. 2018, 18, 221. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Hahn, V.S.; Yanek, L.R.; Vaishnav, J.; Ying, W.; Vaidya, D.; Lee, Y.Z.J.; Riley, S.J.; Subramanya, V.; Brown, E.E.; Hopkins, C.D.; et al. Endomyocardial Biopsy Characterization of Heart Failure with Preserved Ejection Fraction and Prevalence of Cardiac Amyloidosis. JACC Heart Fail. 2020, 8, 712–724. [Google Scholar] [CrossRef]
- Johnson, C.; Kuyt, K.; Oxborough, D.; Stout, M. Practical tips and tricks in measuring strain, strain rate and twist for the left and right ventricles. Echo Res. Pract. 2019, 6, R87–R98. [Google Scholar] [CrossRef] [Green Version]
- Knight, D.S.; Zumbo, G.; Barcella, W.; Steeden, J.A.; Muthurangu, V.; Martinez-Naharro, A.; Treibel, T.; Abdel-Gadir, A.; Bulluck, H.; Kotecha, T.; et al. Cardiac Structural and Functional Consequences of Amyloid Deposition by Cardiac Magnetic Resonance and Echocardiography and Their Prognostic Roles. JACC Cardiovasc. Imaging 2019, 12, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Tian, Z.; Fang, Q. Diagnostic accuracy of cardiovascular magnetic resonance for patients with suspected cardiac amyloidosis: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2016, 16, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phelan, D.; Collier, P.; Thavendiranathan, P.; Popović, Z.B.; Hanna, M.; Plana, J.C.; Marwick, T.H.; Thomas, J.D. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012, 98, 1442–1448. [Google Scholar] [CrossRef]
- Lopez-Candales, A.; Hernandez-Suarez, D.F. Strain Imaging Echocardiography: What Imaging Cardiologists Should Know. Curr. Cardiol. Rev. 2017, 13, 118–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arvidsson, S.; Henein, M.Y.; Wikström, G.; Suhr, O.B.; Lindqvist, P. Right ventricular involvement in transthyretin amyloidosis. Amyloid 2018, 25, 160–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthikeyan, B.; Sonkawade, S.D.; Pokharel, S.; Preda, M.; Schweser, F.; Zivadinov, R.; Kim, M.; Sharma, U.C. Tagged cine magnetic resonance imaging to quantify regional mechanical changes after acute myocardial infarction. Magn. Reson. Imaging 2020, 66, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Heiberg, E.; Sjögren, J.; Ugander, M.; Carlsson, M.; Engblom, H.; Arheden, H. Design and validation of Segment-freely available software for cardiovascular image analysis. BMC Med. Imaging 2010, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Heyde, B.; Jasaityte, R.; Barbosa, D.; Robesyn, V.; Bouchez, S.; Wouters, P.; Maes, F.; Claus, P.; D’Hooge, J. Elastic image registration versus speckle tracking for 2-D myocardial motion estimation: A direct comparison in vivo. IEEE Trans. Med. Imaging 2013, 32, 449–459. [Google Scholar] [CrossRef]
- Morais, P.; Heyde, B.; Barbosa, D.; Queirós, S.; Claus, P.; D’Hooge, J. Cardiac Motion and Deformation Estimation from Tagged MRI Sequences Using a Temporal Coherent Image Registration Framework. In International Conference on Functional Imaging and Modeling of the Heart; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Morais, P.; Marchi, A.; Bogaert, J.A.; Dresselaers, T.; Heyde, B.; D’Hooge, J. Cardiovascular magnetic resonance myocardial feature tracking using a non-rigid, elastic image registration algorithm: Assessment of variability in a real-life clinical setting. J. Cardiovasc. Magn. Reson. 2017, 19, 24. [Google Scholar] [CrossRef] [Green Version]
- Pedrizzetti, G.; Claus, P.; Kilner, P.J.; Nagel, E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J. Cardiovasc. Magn. Reson. 2016, 18, 51. [Google Scholar] [CrossRef] [Green Version]
- Dalen, H.; Thorstensen, A.; Aase, S.A.; Ingul, C.B.; Torp, H.; Vatten, L.J.; Stoylen, A. Segmental and global longitudinal strain and strain rate based on echocardiography of 1266 healthy individuals: The HUNT study in Norway. Eur. Heart J. Cardiovasc. Imaging 2009, 11, 176–183. [Google Scholar] [CrossRef] [Green Version]
- Klein, A.L.; Hatle, L.K.; Taliercio, C.P.; Taylor, C.L.; Kyle, R.A.; Bailey, K.R.; Seward, J.B.; Tajik, A. Serial Doppler echocardiographic follow-up of left ventricular diastolic function in cardiac amyloidosis. J. Am. Coll. Cardiol. 1990, 16, 1135–1141. [Google Scholar] [CrossRef] [Green Version]
- Koyama, J.; Ray-Sequin, P.A.; Falk, R.H. Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue Doppler echocardiography in patients with AL (primary) cardiac amyloidosis. Circulation 2003, 107, 2446–2452. [Google Scholar] [CrossRef]
- Gunaratnam, K.; Wong, L.H.; Nasis, A.; Ellims, A.; Nandurkar, D.; Soo, G.; Cameron, J.; Troupis, J. Review of cardiomyopathy imaging. Eur. J. Radiol. 2013, 82, 1763–1775. [Google Scholar] [CrossRef]
- Koyama, J.; Falk, R.H. Prognostic significance of strain Doppler imaging in light-chain amyloidosis. JACC Cardiovasc. Imaging 2010, 3, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Chengode, S. Left ventricular global systolic function assessment by echocardiography. Ann. Card. Anaesth. 2016, 19, S26–S34. [Google Scholar] [CrossRef]
- Lima, J.A.C.; Desai, M.Y. Cardiovascular magnetic resonance imaging: Current and emerging applications. J. Am. Coll. Cardiol. 2004, 44, 1164–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotschy, A.; von Deuster, C.; van Gorkum, R.J.H.; Gastl, M.; Vintschger, E.; Schwotzer, R.; Flammer, A.J.; Manka, R.; Stoeck, C.T.; Kozerke, S. Characterizing cardiac involvement in amyloidosis using cardiovascular magnetic resonance diffusion tensor imaging. J. Cardiovasc. Magn. Reson. 2019, 21, 56. [Google Scholar] [CrossRef] [Green Version]
- Pagourelias, E.D.; Mirea, O.; Duchenne, J.; Van Cleemput, J.; Delforge, M.; Bogaert, J.; Kuznetsova, T.; Voigt, J.U. Echo Parameters for Differential Diagnosis in Cardiac Amyloidosis: A Head-to-Head Comparison of Deformation and Nondeformation Parameters. Circ. Cardiovasc. Imaging 2017, 10, e005588. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, T.; Dulgheru, R.; Bernard, A.; Ilardi, F.; Contu, L.; Addetia, K.; Caballero, L.; Akhaladze, N.; Athanassopoulos, G.D.; Barone, D.; et al. Echocardiographic reference ranges for normal left ventricular 2D strain: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Bodez, D.; Ternacle, J.; Guellich, A.; Galat, A.; Lim, P.; Radu, C.; Guendouz, S.; Bergoend, E.; Couetil, J.P.; Hittinger, L.; et al. Prognostic value of right ventricular systolic function in cardiac amyloidosis. Amyloid 2016, 23, 158–167. [Google Scholar] [CrossRef]
- Wan, K.; Sun, J.; Han, Y.; Luo, Y.; Liu, H.; Yang, D.; Cheng, W.; Zhang, Q.; Zeng, Z.; Chen, Y. Right ventricular involvement evaluated by cardiac magnetic resonance imaging predicts mortality in patients with light chain amyloidosis. Heart Vessel. 2018, 33, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hu, K.; Stork, S.; Herrmann, S.; Kramer, B.; Cikes, M.; Gaudron, P.D.; Knop, S.; Ertl, G.; Bijnens, B.; et al. Predictive value of assessing diastolic strain rate on survival in cardiac amyloidosis patients with preserved ejection fraction. PLoS ONE 2014, 9, e115910. [Google Scholar] [CrossRef] [PubMed]
- Gale, E.; Wey, H.; Ramsay, I.; Yen, Y.; Sosnovik, D.; Caravan, P. A Manganese-based alternative to Gadolinium: Contrast-enhanced MR Angiography, Excretion, Pharmacokinetics and Metabolism. Radiology 2018, 286, 865–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]

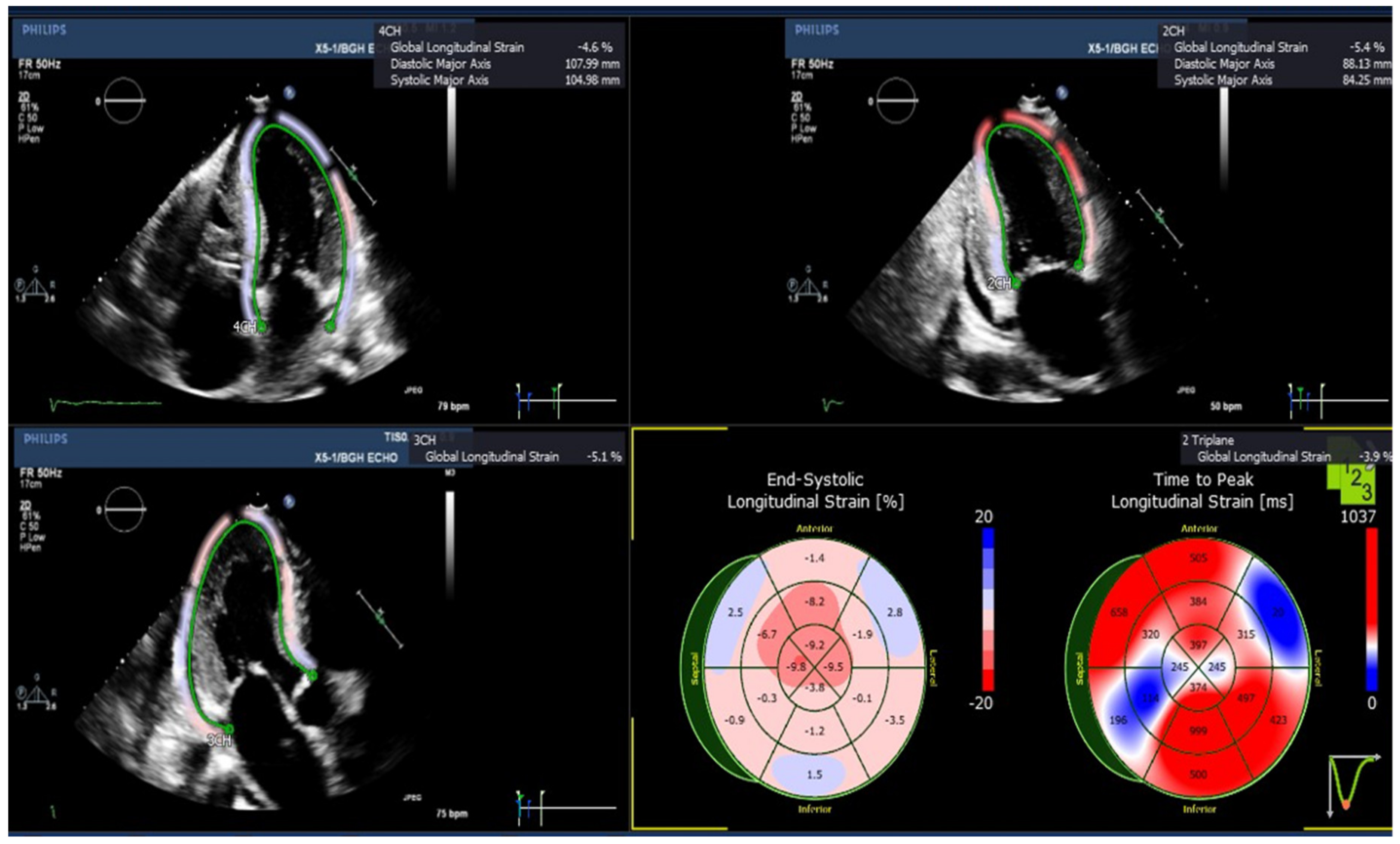



| GLS (%), Mean (SEM) | Amyloid Patients (n = 5) | Normal Subjects (n = 8) |
|---|---|---|
| GLS, triplane | −7.96 (1.45) * | −19.44 (0.33) |
| GLS, apical four chamber | −9.10 (1.42) * | −21.51 (0.62) |
| GLS, apical two chamber | −8.12 (1.72) * | −19.35 (0.77) |
| GLS, apical three chamber | −8.0 (1.11) * | −18.45 (0.64) |
| Basal anterior | −4.54 (1.60) * | −26.65 (1.57) |
| Basal anteroseptal | −2.42 (2.10) * | −15.78 (3.38) |
| Basal inferoseptal | −5.96 (1.66) | −16.85 (4.61) |
| Basal inferior | −3.44 (2.17) * | −20.26 (3.65) |
| Basal inferolateral | −8.5 (2.92) * | −32.54 (3.13) |
| Basal anterolateral | −5.88 (3.09) * | −34.56 (2.77) |
| Mid anterior | −6.62 (1.10) * | −15.23 (1.80) |
| Mid anteroseptal | −6.88 (1.94) * | −16.83 (2.50) |
| Mid inferoseptal | −5.74 (1.97) * | −18.15 (2.34) |
| Mid inferior | −6.40 (1.5) * | −16.15 (2.20) |
| Mid inferolateral | −1.96 (1.91) * | −10.74 (1.97) |
| Mid anterolateral | −3.30 (1.33) * | −16.23 (2.11) |
| Apical anterior | −12.94 (4.27) | −15.95 (2.49) |
| Apical septal | −14.50 (2.83) * | −20.86 (1.29) |
| Apical inferior | −11.76 (3.60) | −19.20 (1.70) |
| Apical lateral | −13.32 (1.55) | −17.23 (2.66) |
| Parameters | Controls (N = 5) | Amyloidosis (N = 5) | p Value |
|---|---|---|---|
| LV Systolic Circumferential Strain (%) | |||
| Basal | −18.133 ± 1.742 | −6.063 ± 1.739 | 0.001 * |
| Midventricular | −17.014 ± 0.987 | −8.928 ± 1.545 | 0.002 * |
| Apical | −20.159 ± 2.630 | −9.944 ± 2.645 | 0.025 * |
| Septal | −19.778 ± 1.756 | −9.248 ± 1.936 | 0.004 * |
| Global | −18.220 ± 1.535 | −8.108 ± 1.734 | 0.002 * |
| LV Systolic Radial Strain (%) | |||
| Basal | 41.404 ± 5.938 | 10.347 ± 4.655 | 0.003 * |
| Midventricular | 40.361 ± 1.523 | 17.909 ± 4.964 | 0.003 * |
| Apical | 36.224 ± 4.916 | 12.045 ± 7.220 | 0.024 * |
| Septal | 30.588 ± 2.199 | 5.628 ± 2.657 | <0.001 * |
| Global | 39.718 ± 3.533 | 13.607 ± 5.138 | 0.003 * |
| LV Systolic Longitudinal Strain (%) | |||
| Basal | −18.525 ± 0.735 | −8.859 ± 2.741 | 0.009 * |
| Midventricular | −14.884 ± 1.120 | −6.496 ± 1.532 | 0.002 * |
| Apical | −10.998 ± 1.367 | −3.543 ± 0.965 | 0.002 * |
| Septal | −14.036 ± 1.276 | −7.395 ± 1.437 | 0.009 * |
| Global | −15.278 ± 0.929 | −6.644 ± 1.398 | 0.001 * |
| LV Peak Systolic Circumferential Strain Rate (s−1) | |||
| Basal | −0.793 ± 0.091 | −0.246 ± 0.080 | 0.002 * |
| Midventricular | −0.756 ± 0.077 | −0.348 ± 0.069 | 0.004 * |
| Apical | −1.027 ± 0.256 | −0.432 ± 0.128 | 0.071 |
| Septal | −0.868 ± 0.102 | −0.374 ± 0.086 | 0.006 * |
| Global | −0.837 ± 0.122 | −0.331 ± 0.080 | 0.009 * |
| LV Peak Systolic Radial Strain Rate (s−1) | |||
| Basal | 1.278 ± 0.210 | 0.452 ± 0.175 | 0.017 * |
| Midventricular | 1.276 ± 0.028 | 0.619 ± 0.152 | 0.003 * |
| Apical | 1.077 ± 0.133 | 0.403 ± 0.223 | 0.032 * |
| Septal | 0.971 ± 0.105 | 0.243 ± 0.069 | <0.001 * |
| Global | 1.227 ± 0.111 | 0.502 ± 0.172 | 0.008 * |
| LV Peak Systolic Longitudinal Strain Rate (s−1) | |||
| Basal | −0.740 ± 0.068 | −0.390 ± 0.150 | 0.066 |
| Midventricular | −0.645 ± 0.060 | −0.258 ± 0.051 | 0.001 * |
| Apical | −0.498 ± 0.088 | −0.141 ± 0.048 | 0.007 * |
| Septal | −0.561 ± 0.074 | −0.305 ± 0.061 | 0.029 * |
| Global | −0.644 ± 0.062 | −0.278 ± 0.065 | 0.004 * |
| LV Peak Diastolic Circumferential Strain Rate (s−1) | |||
| Basal | 0.689 ± 0.072 | 0.180 ± 0.042 | <0.001 * |
| Midventricular | 0.678 ± 0.044 | 0.271 ± 0.061 | 0.001 * |
| Apical | 0.803 ± 0.172 | 0.346 ± 0.095 | 0.049 * |
| Septal | 0.812 ± 0.104 | 0.286 ± 0.063 | 0.002 * |
| Global | 0.713 ± 0.072 | 0.255 ± 0.046 | 0.001 * |
| LV Peak Diastolic Radial Strain Rate (s−1) | |||
| Basal | −1.294 ± 0.149 | −0.317 ± 0.082 | <0.001 * |
| Midventricular | −1.350 ± 0.215 | −0.494 ± 0.118 | 0.008 * |
| Apical | −1.264 ± 0.167 | −0.415 ± 0.210 | 0.013 * |
| Septal | −1.112 ± 0.148 | −0.290 ± 0.035 | 0.001 * |
| Global | −1.307 ± 0.137 | −0.408 ± 0.109 | 0.001 * |
| LV Peak Diastolic Longitudinal Strain Rate (s−1) | |||
| Basal | 0.598 ± 0.059 | 0.296 ± 0.076 | 0.014 * |
| Midventricular | 0.504 ± 0.072 | 0.166 ± 0.030 | 0.002 * |
| Apical | 0.380 ± 0.068 | 0.114 ± 0.044 | 0.011 * |
| Septal | 0.461 ± 0.081 | 0.211 ± 0.025 | 0.019 * |
| Global | 0.508 ± 0.059 | 0.202 ± 0.026 | 0.001 * |
| Parameters | Controls (N = 5) | Amyloidosis (N = 5) | p Value |
|---|---|---|---|
| RV Systolic Circumferential Strain (%) | |||
| Basal | −11.376 ± 1.208 | −3.564 ± 0.949 | 0.001 * |
| Midventricular | −10.269 ± 0.509 | −9.079 ± 1.482 | 0.469 |
| Apical | −14.775 ± 3.606 | −8.064 ± 1.815 | 0.135 |
| Lateral | −8.463 ± 2.193 | −6.999 ± 1.210 | 0.575 |
| Septal | −15.817 ± 1.715 | −6.805 ± 1.433 | 0.004 * |
| Global | −12.140 ± 1.606 | −6.902 ± 1.258 | 0.033 * |
| RV Systolic Longitudinal Strain (%) | |||
| Basal | −17.883 ± 1.621 | −12.600 ± 3.009 | 0.161 |
| Midventricular | −16.811 ± 2.586 | −10.758 ± 2.433 | 0.127 |
| Apical | −12.337 ± 3.113 | −2.168 ± 1.414 | 0.018 * |
| Lateral | −19.283 ± 3.066 | −11.309 ± 2.138 | 0.065 |
| Septal | −12.070 ± 1.395 | −5.709 ± 1.119 | 0.007 * |
| Global | −15.677 ± 2.114 | −8.509 ± 1.471 | 0.024 * |
| RV Peak Systolic Circumferential Strain Rate (s−1) | |||
| Basal | −0.501 ± 0.050 | −0.131 ± 0.037 | <0.001 * |
| Midventricular | −0.484 ± 0.028 | −0.349 ± 0.071 | 0.116 |
| Apical | −0.753 ± 0.162 | −0.328 ± 0.084 | 0.049 * |
| Lateral | −0.436 ± 0.077 | −0.271 ± 0.060 | 0.128 |
| Septal | −0.723 ± 0.093 | −0.269 ± 0.061 | 0.004 * |
| Global | −0.579 ± 0.074 | −0.270 ± 0.058 | 0.011 * |
| RV Peak Systolic Longitudinal Strain Rate (s−1) | |||
| Basal | −0.647 ± 0.052 | −0.586 ± 0.168 | 0.739 |
| Midventricular | −0.691 ± 0.121 | −0.416 ± 0.094 | 0.111 |
| Apical | −0.500 ± 0.099 | −0.125 ± 0.070 | 0.015 * |
| Lateral | −0.762 ± 0.103 | −0.494 ± 0.103 | 0.102 |
| Septal | −0.464 ± 0.055 | −0.258 ± 0.045 | 0.020 * |
| Global | −0.613 ± 0.072 | −0.376 ± 0.072 | 0.049 * |
| RV Peak Diastolic Circumferential Strain Rate (s−1) | |||
| Basal | 0.379 ± 0.061 | 0.130 ± 0.049 | 0.013 * |
| Midventricular | 0.402 ± 0.075 | 0.346 ± 0.068 | 0.599 |
| Apical | 0.576 ± 0.164 | 0.236 ± 0.069 | 0.092 |
| Lateral | 0.336 ± 0.110 | 0.305 ± 0.073 | 0.819 |
| Septal | 0.569 ± 0.091 | 0.170 ± 0.042 | 0.004 * |
| Global | 0.452 ± 0.088 | 0.238 ± 0.055 | 0.072 |
| RV Peak Diastolic Longitudinal Strain Rate (s−1) | |||
| Basal | 0.454 ± 0.084 | 0.412 ± 0.115 | 0.778 |
| Midventricular | 0.586 ± 0.180 | 0.352 ± 0.122 | 0.312 |
| Apical | 0.420 ± 0.103 | 0.034 ± 0.037 | 0.008 * |
| Lateral | 0.624 ± 0.151 | 0.381 ± 0.092 | 0.206 |
| Septal | 0.349 ± 0.081 | 0.151 ± 0.037 | 0.057 |
| Global | 0.487 ± 0.109 | 0.266 ± 0.054 | 0.107 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, A.; Singh, V.; Karthikeyan, B.; Jiang, L.; Kristo, S.; Kattel, S.; Amuthan, R.; Pokharel, S.; Sharma, U.C. Biventricular Strain Imaging with Cardiac MRI in Genotyped and Histology Validated Amyloid Cardiomyopathy. Cardiogenetics 2021, 11, 98-110. https://doi.org/10.3390/cardiogenetics11030011
Reddy A, Singh V, Karthikeyan B, Jiang L, Kristo S, Kattel S, Amuthan R, Pokharel S, Sharma UC. Biventricular Strain Imaging with Cardiac MRI in Genotyped and Histology Validated Amyloid Cardiomyopathy. Cardiogenetics. 2021; 11(3):98-110. https://doi.org/10.3390/cardiogenetics11030011
Chicago/Turabian StyleReddy, Abhinay, Vasvi Singh, Badri Karthikeyan, Leyi Jiang, Silva Kristo, Sharma Kattel, Ram Amuthan, Saraswati Pokharel, and Umesh C. Sharma. 2021. "Biventricular Strain Imaging with Cardiac MRI in Genotyped and Histology Validated Amyloid Cardiomyopathy" Cardiogenetics 11, no. 3: 98-110. https://doi.org/10.3390/cardiogenetics11030011
APA StyleReddy, A., Singh, V., Karthikeyan, B., Jiang, L., Kristo, S., Kattel, S., Amuthan, R., Pokharel, S., & Sharma, U. C. (2021). Biventricular Strain Imaging with Cardiac MRI in Genotyped and Histology Validated Amyloid Cardiomyopathy. Cardiogenetics, 11(3), 98-110. https://doi.org/10.3390/cardiogenetics11030011





