The Roles of Platelet-Activating Factor and Magnesium in Pathophysiology of Hypertension, Atherogenesis, Cardiovascular Disease, Stroke and Aging
Abstract
:1. Introduction
2. Hypertension: Risk Factors and Link to Atherosclerosis
3. PAF in the Pathophysiology of Atherosclerosis
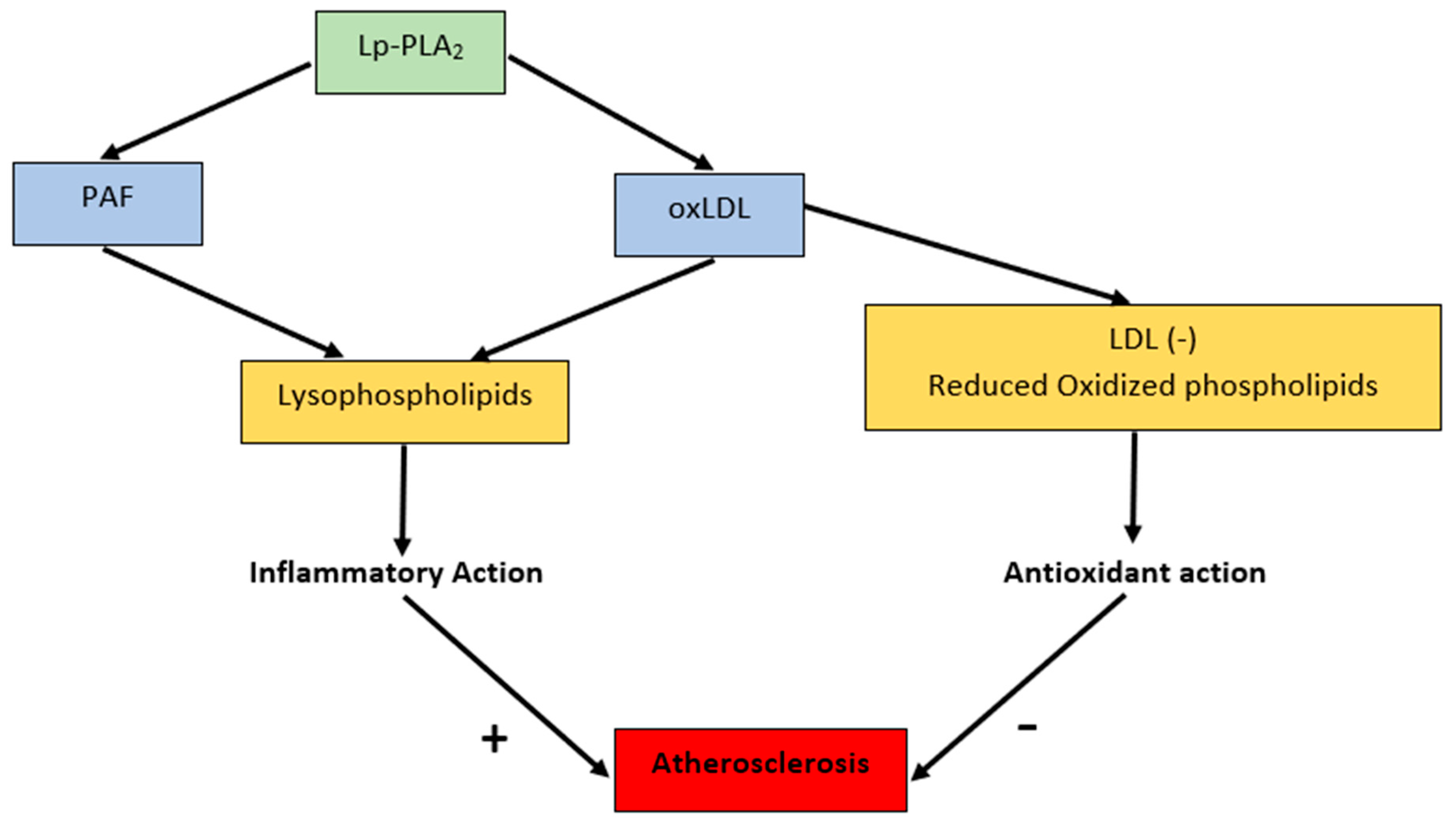
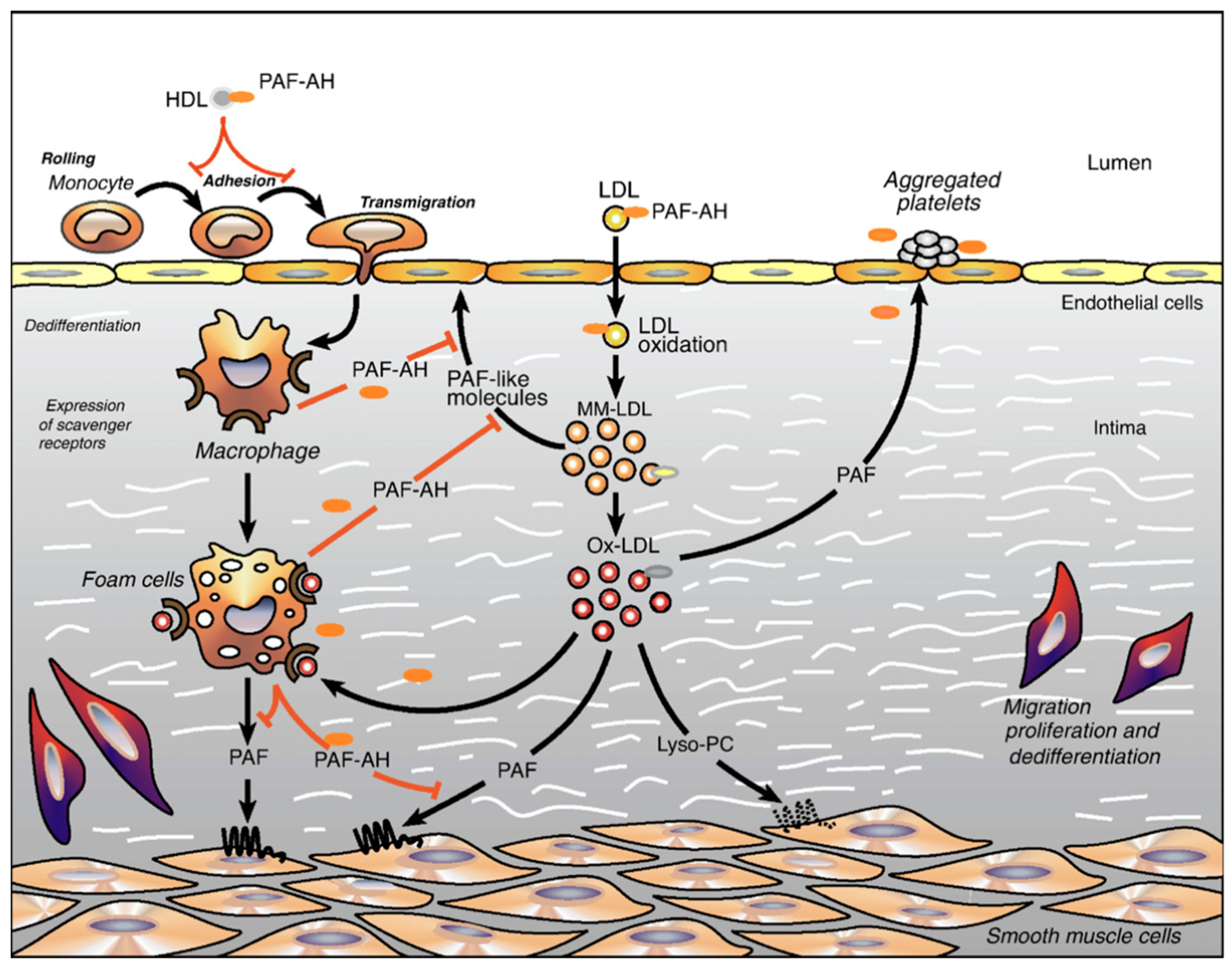
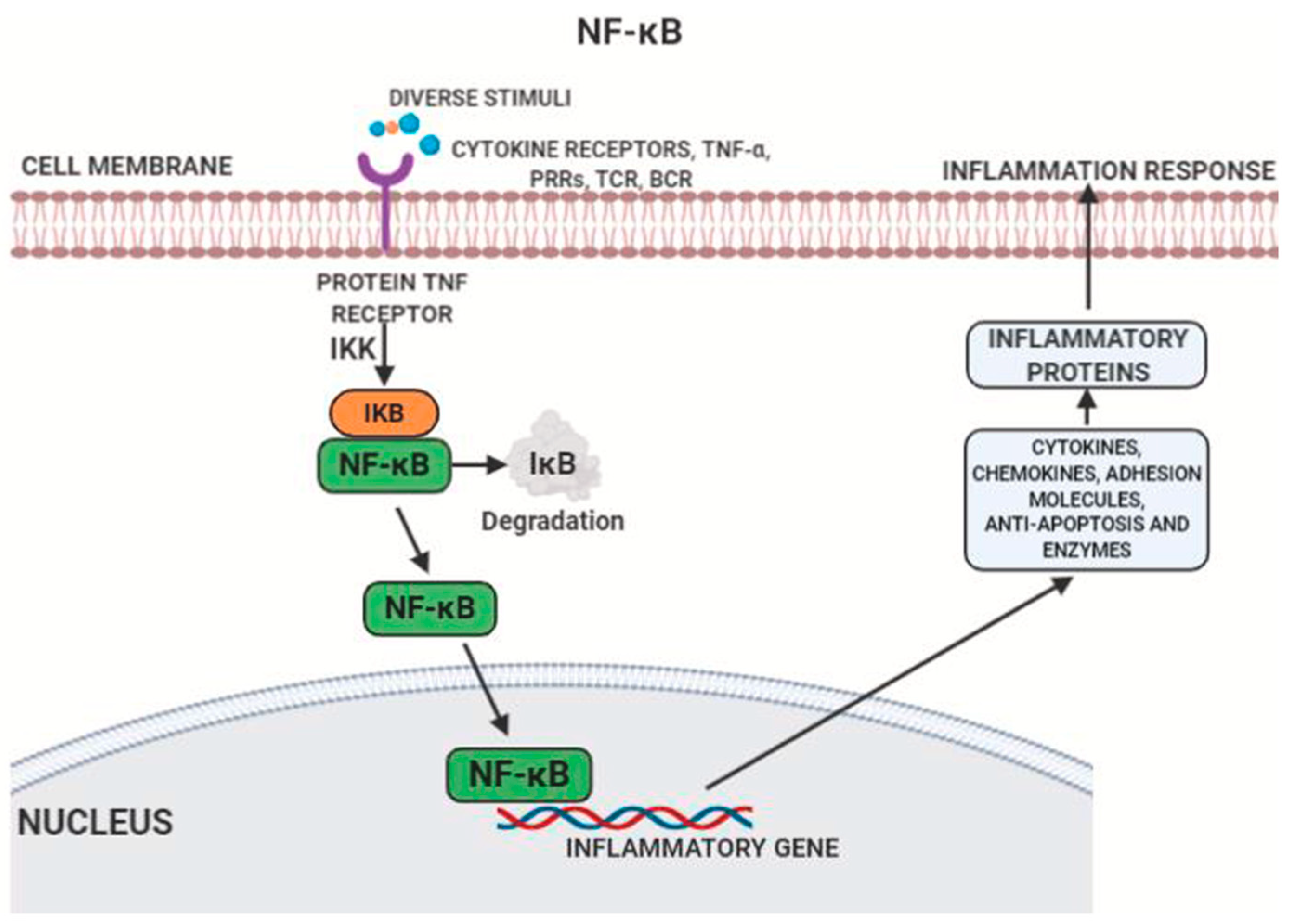
4. PAF Structure and Molecular Mechanism
5. PAF in Cardiac Pathophysiology and Stroke
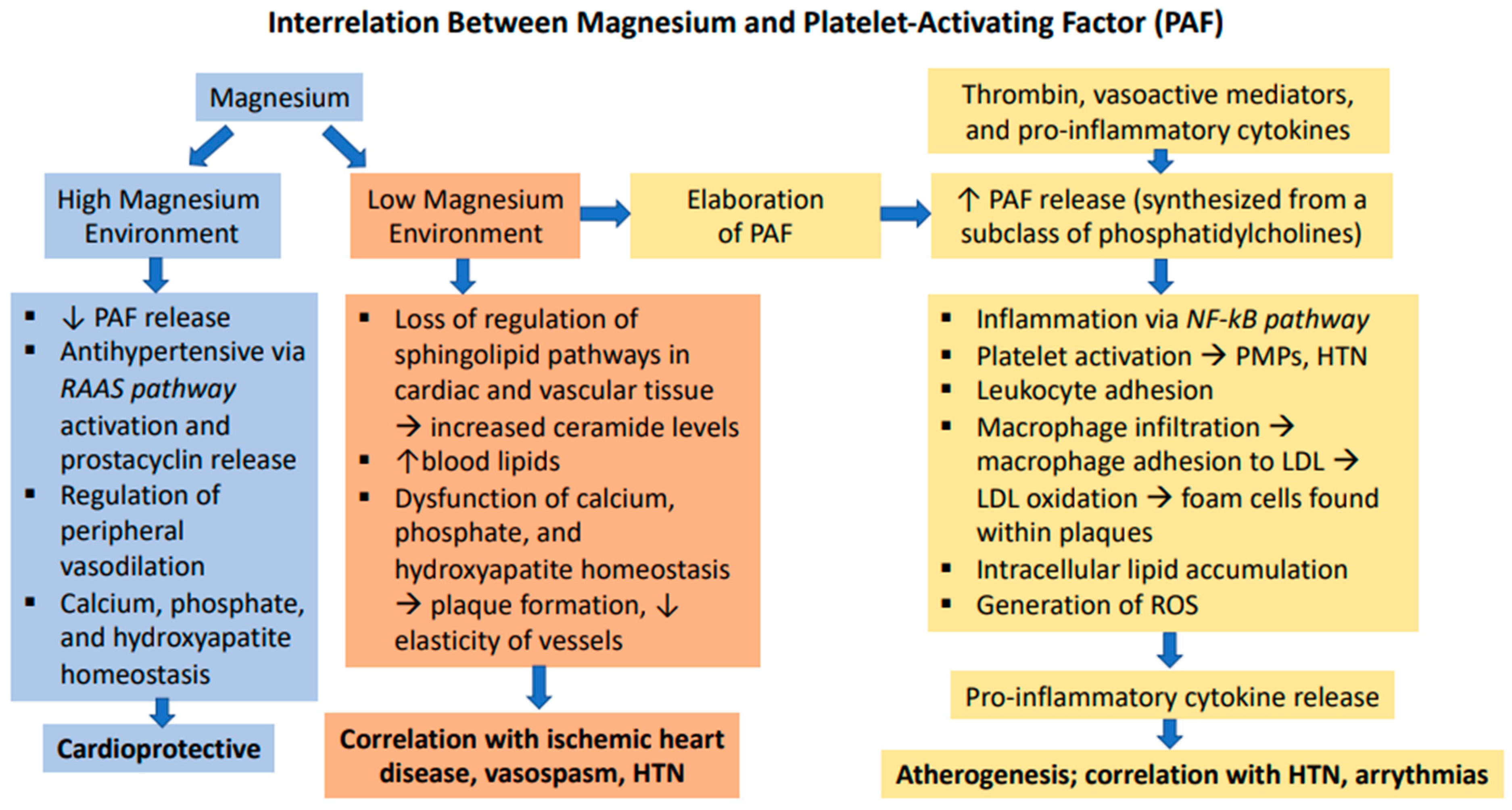
6. Magnesium in the Pathophysiology of Atherosclerosis and Hypertension
7. Magnesium’s Role in Cardiac Pathophysiology
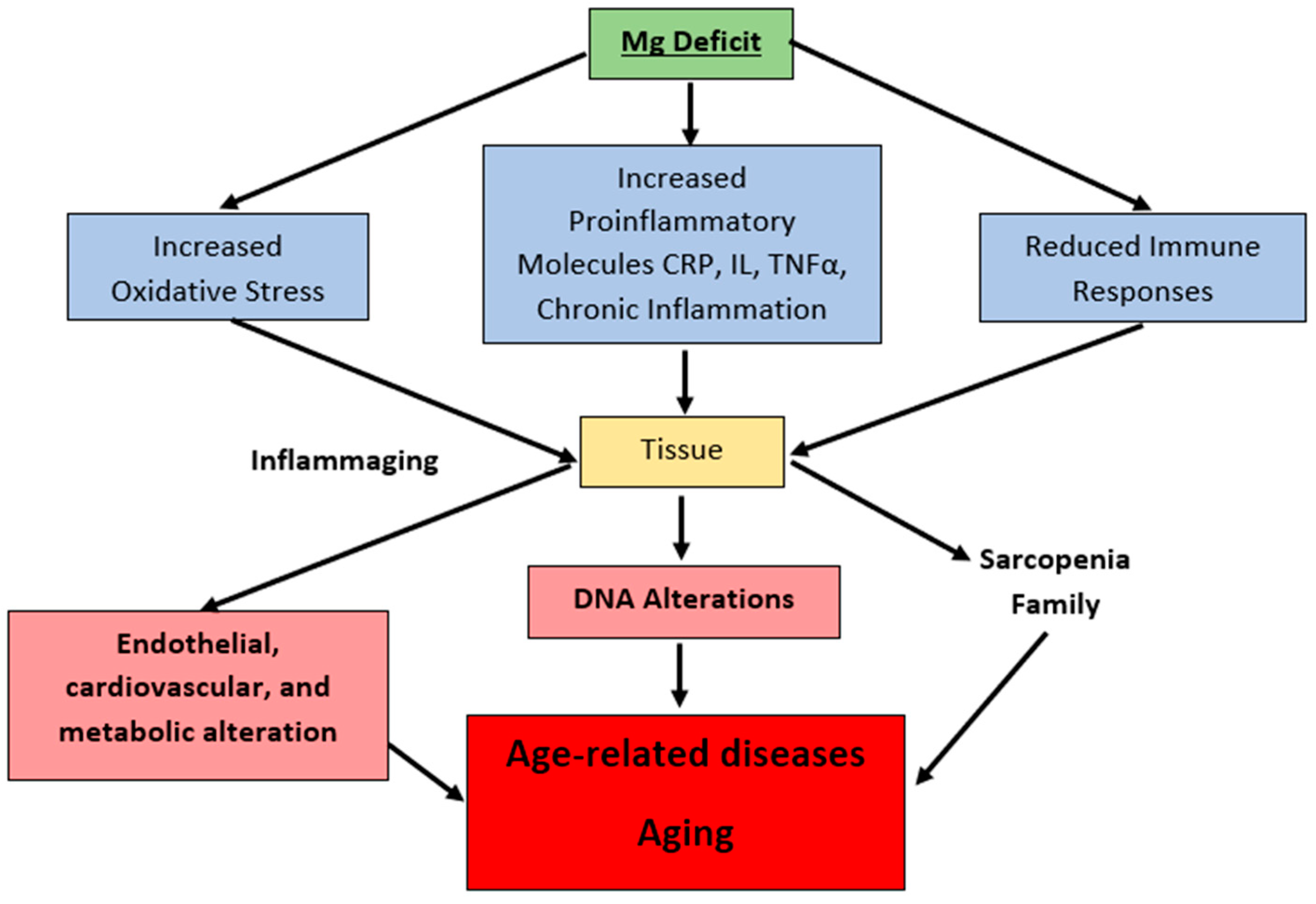
8. Magnesium in the Pathophysiology of Aging
9. Magnesium: An Essential Nutrient
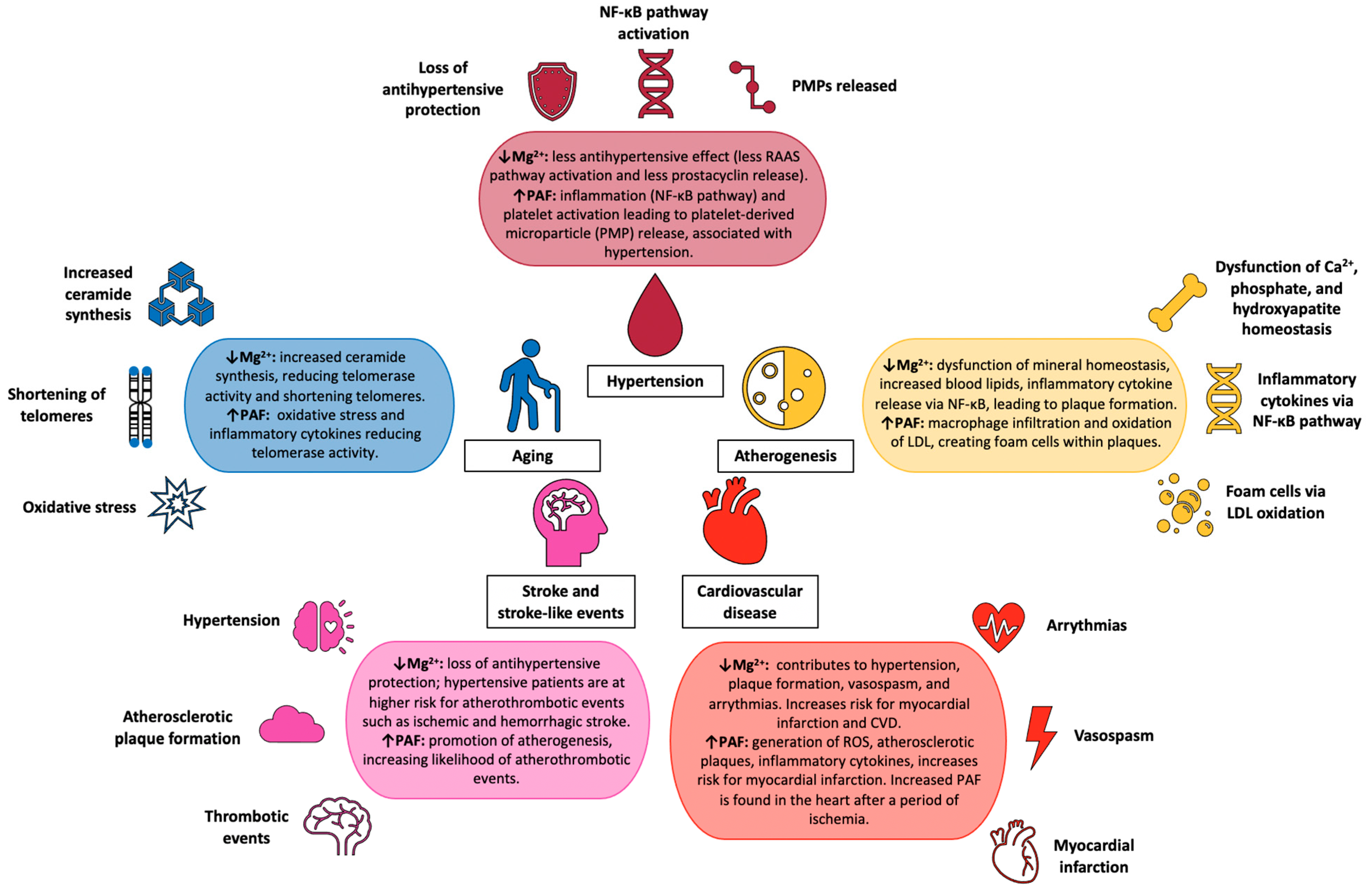
10. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dominguez, L.; Veronese, N.; Barbagallo, M. Magnesium and Hypertension in Old Age. Nutrients 2020, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Wegner, M.; Araszkiewicz, A.; Zozulińska-Ziółkiewicz, D.; Wierusz-Wysocka, B.; Pioruńska-Mikołajczak, A.; Pioruńska-Stolzmann, M. The relationship between concentrations of magnesium and oxidized low density lipoprotein and the activity of platelet activating factor acetylhydrolase in the serum of patients with type 1 diabetes. Magnes. Res. 2010, 23, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 1993, 362, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Ostchega, Y.; Fryar, C.D.; Nwankwo, T.; Nguyen, D.T. Hypertension Prevalence among Adults Aged 18 and Over: United States, 2017–2018; NCHS Data Brief, No. 364; National Center for Health Statistics: Hyattsville, MD, USA, 2020.
- High Blood Pressure: Also Known as Hypertension. U.S. Department of Health & Human Services: National Heart, Lung and Blood. Available online: https://www.nhlbi.nih.gov/health-topics/high-blood-pressure (accessed on 2 June 2021).
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef]
- Poulter, N.R.; Prabhakaran, D.; Caulfield, M. Hypertension. Lancet Lond Engl. 2015, 386, 801–812. [Google Scholar] [CrossRef]
- Charles, L.; Triscott, J.; Dobbs, B. Secondary Hypertension: Discovering the Underlying Cause. Am. Fam. Physician 2017, 96, 453–461. [Google Scholar]
- Nguyen, B.; Bauman, A.; Ding, D. Association between lifestyle risk factors and incident hypertension among middle-aged and older Australians. Prev. Med. 2019, 118, 73–80. [Google Scholar] [CrossRef]
- Jamrozik, K. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [CrossRef]
- Konukoglu, D.; Uzun, H. Endothelial Dysfunction and Hypertension. Adv. Exp. Med. Biol. 2017, 956, 511–540. [Google Scholar] [CrossRef]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [Green Version]
- Marketou, M.; Kontaraki, J.; Papadakis, J.; Kochiadakis, G.; Vrentzos, G.; Maragkoudakis, S.; Fragkiadakis, K.; Katsouli, E.; Plataki, M.; Patrianakos, A.; et al. Platelet microRNAs in hypertensive patients with and without cardiovascular disease. J. Hum. Hypertens 2019, 33, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Barale, C.; Russo, I. Influence of Cardiometabolic Risk Factors on Platelet Function. Int. J. Mol. Sci. 2020, 21, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 21, 7023. [Google Scholar] [CrossRef] [Green Version]
- Monaco, C.; Andreakos, E.; Kiriakidis, S.; Mauri, C.; Bicknell, C.; Foxwell, B.; Cheshire, N.; Paleolog, E.; Feldmann, M. Canonical pathway of nuclear factor B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc. Natl. Acad. Sci. USA 2004, 101, 5634–5639. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.K.; Singhi, E.K.; Arroyo, J.P.; Ikizler, T.A.; Gould, E.R.; Brown, J.; Beckman, J.A.; Harrison, D.G.; Moslehi, J. Mechanisms of VEGF (Vascular Endothelial Growth Factor) Inhibitor–Associated Hypertension and Vascular Disease. Hypertension 2018, 71, e1–e8. [Google Scholar] [CrossRef]
- Honda, Z.I.; Ishii, S.; Shimizu, T. Platelet-Activating Factor Receptor. J. Biochem. 2002, 131, 773–779. [Google Scholar] [CrossRef]
- Kumar, A.; Sawhney, G.; Kumar Nagar, R.; Chauhan, N.; Gupta, N.; Kaul, A.; Ahmed, Z.; Sangwan, P.L.; Satheesh Kumar, P.; Yadav, G. Evaluation of the immunomodulatory and anti-inflammatory activity of Bakuchiol using RAW 264.7 macrophage cell lines and in animal models stimulated by lipopolysaccharide (LPS). Int. Immunopharmacol. 2021, 91, 107264. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [Green Version]
- Mueller, H.W.; Haught, C.A.; McNatt, J.M.; Cui, K.; Gaskell, S.J.; Johnston, D.A.; Willerson, J.T. Measurement of platelet-activating factor in a canine model of coronary thrombosis and in endarterectomy samples from patients with advanced coronary artery disease. Circ. Res. 1995, 77, 54–63. [Google Scholar] [CrossRef]
- Garrido, D.; Chanteloup, N.K.; Trotereau, A.; Lion, A.; Bailleul, G.; Esnault, E.; Trapp, S.; Quéré, P.; Schouler, C.; Guabiraba, R. Characterization of the Phospholipid Platelet-Activating Factor As a Mediator of Inflammation in Chickens. Front. Vet. Sci. 2017, 4, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouis, M.; Nigon, F.; John Chapman, M. Platelet activating factor is a potent stimulant of the production of active oxygen species by human monocyte-derived macrophages. Biochem. Biophys. Res. Commun. 1988, 156, 1293–1301. [Google Scholar] [CrossRef]
- Dentan, C.; Lesnik, P.; Chapman, M.J.; Ninio, E. Phagocytic Activation Induces Formation of Platelet-Activating Factor in Human Monocyte-Derived Macrophages and in Macrophage-Derived foam Cells. Relevance to the Inflammatory Reaction in Atherogenesis. Eur. J. Biochem. 1996, 236, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Kamata, K.; Mori, T.; Shigenobu, K.; Kasuya, Y. Endothelium-dependent vasodilator effects of platelet activating factor on rat resistance vessels. Br. J. Pharmacol. 1989, 98, 1360–1364. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.K.; Zhao, S.P.; Yu, P.L.; Shi, J.; Gu, C.D.; Sun, H.T.; Zhang, G.Q. Elevated platelet activating factor level in ischemia-related arrhythmia and its electrophysiological effect on myocardium. Biomed. Environ. Sci. 2013, 26, 365–370. [Google Scholar] [CrossRef]
- Silva, I.T.; Mello, A.P.; Damasceno, N.R. Antioxidant and inflammatory aspects of lipoprotein-associated phospholipase A2(Lp-PLA2): A review. Lipids Health Dis. 2011, 10, 170. [Google Scholar] [CrossRef] [Green Version]
- Karabina, S.-A.; Ninio, E. Plasma PAF-acetylhydrolase: An unfulfilled promise? Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2006, 1761, 1351–1358. [Google Scholar] [CrossRef]
- Fruhwirth, G.O.; Loidl, A.; Hermetter, A. Oxidized phospholipids: From molecular properties to disease. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2007, 1772, 718–736. [Google Scholar] [CrossRef] [Green Version]
- Ishii, S.; Nagase, T.; Shimizu, T. Platelet-activating factor receptor. Prostaglandins Other Lipid Mediat. 2002, 68–69, 599–609. [Google Scholar] [CrossRef]
- Chao, W.; Olson, M.S. Platelet-activating factor: Receptors and signal transduction. Biochem. J. 1993, 292, 617–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feuerstein, G.; Rabinovici, R.; Leor, J.; Winkler, J.D.; Vonhof, S. Platelet-activating factor and cardiac diseases: Therapeutic potential for PAF inhibitors. J. Lipid Mediat. Cell Signal. 1997, 15, 255–284. [Google Scholar] [CrossRef]
- Palur Ramakrishnan, A.V.; Varghese, T.P.; Vanapalli, S.; Nair, N.K.; Mingate, M.D. Platelet activating factor: A potential biomarker in acute coronary syndrome? Cardiovasc. Ther. 2017, 35, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Lindsberg, P.J.; Hallenbeck, J.M.; Feuerstein, G. Platelet-activating factor in stroke and brain injury. Ann. Neurol. 1991, 30, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Imaizumi, T.; Yoshida, H.; Hiramoto, M.; Takamatsu, S. Increased levels of blood platelet-activating factor (PAF) and PAF-like lipids in patients with ischemic stroke. Acta Neurol. Scand. 1992, 85, 122–127. [Google Scholar] [CrossRef]
- Morrill, G.A.; Gupta, R.K.; Kostellow, A.B.; Ma, G.Y.; Zhang, A.; Altura, B.T.; Altura, B.M. Mg2+ modulates membrane lipids in vascular smooth muscle: A link to atherogenesis. FEBS Lett. 1997, 408, 191–194. [Google Scholar] [CrossRef] [Green Version]
- Altura, B.M.; Li, W.; Zhang, A.; Zheng, T.; Shah, N.C.; Shah, G.J.; Altura, B.T. The Expression of Platelet-Activating Factor is Induced by Low Extracellular Mg2+ in Aortic, Cerebral and Neonatal Coronary Vascular Smooth Muscle; Cross Talk with Ceramide Production, NF–kB and Proto-Oncogenes: Possible Links to Atherogenesis and Sudden Cardiac Death in Children and Infants, and Aging; Hypothesis, Review and Viewpoint. Int. J. Cardiol. Res. 2016, 3, 47–67. [Google Scholar] [CrossRef]
- Rayssiguier, Y.; Gueux, E.; Bussière, L.; Durlach, J.; Mazur, A. Dietary magnesium affects susceptibility of lipoproteins and tissues to peroxidation in rats. J. Am. Coll. Nutr. 1993, 12, 133–137. [Google Scholar] [CrossRef]
- Kostov, K.; Halacheva, L. Role of Magnesium Deficiency in Promoting Atherosclerosis, Endothelial Dysfunction, and Arterial Stiffening as Risk Factors for Hypertension. Int. J. Mol. Sci. 2018, 19, 1724. [Google Scholar] [CrossRef] [Green Version]
- Tangvoraphonkchai, K.; Davenport, A. Magnesium and Cardiovascular Disease. Adv. Chronic Kidney Dis. 2018, 25, 251–260. [Google Scholar] [CrossRef]
- ter Braake, A.D.; Shanahan, C.M.; de Baaij, J.H.F. Magnesium Counteracts Vascular Calcification: Passive Interference or Active Modulation? Arter. Thromb Vasc Biol. 2017, 37, 1431–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swaminathan, R. Magnesium metabolism and its disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar] [PubMed]
- Yolcu, M.; Ipek, E.; Turkmen, S.; Ozen, Y.; Yıldırım, E.; Sertcelik, A.; Ulusoy, F.; Mustafa, Y.; Emrah, I.; Erkan, Y.; et al. The relationship between elevated magnesium levels and coronary artery ectasia. Cardiovasc. J. Afr. 2016, 27, 294–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, A.R.; Umbelino, B.; Correia, M.L.; Neves, M.F. Magnesium and vascular changes in hypertension. Int. J. Hypertens. 2012, 2012, 754250. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.C.; Shah, G.J.; Li, Z.; Jiang, X.C.; Altura, B.T.; Altura, B.M. Short-term magnesium deficiency downregulates telomerase, upregulates neutral sphingomyelinase and induces oxidative DNA damage in cardiovascular tissues: Relevance to atherogenesis, cardiovascular diseases and aging. Int. J. Clin. Exp. Med. 2014, 74, 97–514. [Google Scholar]
- Killilea, D.W.; Maier, J.A.M. A connection between magnesium deficiency and aging: New insights from cellular studies. Magnes. Res. 2008, 21, 77–82. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J. Magnesium and aging. Curr. Pharm. Des. 2010, 16, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press: Washington, DC, USA, 1997. [CrossRef]
- Ceremuzyński, L.; Gebalska, J.; Wolk, R.; Makowska, E. Hypomagnesemia in heart failure with ventricular arrhythmias. Beneficial effects of magnesium supplementation. J. Intern. Med. 2000, 247, 78–86. [Google Scholar] [CrossRef]
- Bailey, R.L.; Fulgoni, V.L.; Keast, D.R.; Dwyer, J.T. Dietary supplement use is associated with higher intakes of minerals from food sources. Am. J. Clin. Nutr. 2011, 94, 1376–1381. [Google Scholar] [CrossRef] [Green Version]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W. Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis. Open Heart 2018, 5, e000668. [Google Scholar] [CrossRef]
- Burton, A. Cardiovascular Health: Hard Data for Hard Water. Environ. Health Perspect. 2008, 116, A114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenberg, M.J. Magnesium deficiency and sudden death. Am. Heart J. 1992, 124, 544–549. [Google Scholar] [CrossRef]
- Han, H.; Fang, X.; Wei, X.; Liu, Y.; Jin, Z.; Chen, Q.; Fan, Z.; Aaseth, J.; Hiyoshi, A.; He, J.; et al. Dose-response relationship between dietary magnesium intake, serum magnesium concentration and risk of hypertension: A systematic review and meta-analysis of prospective cohort studies. Nutr. J. 2017, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, K.; Han, D.; He, X.; Wei, J.; Zhao, L.; Imam, M.U.; Ping, Z.; Li, Y.; Xu, Y.; et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: A dose–response meta-analysis of prospective cohort studies. BMC Med. 2016, 14, 210. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, H.S.; Suenson, M.; Mcnair, P.; Nørregård, P.; Balslev, S. Magnesium infusion reduces the incidence of arrhythmias in acute myocardial infarction. A double-blind placebo-controlled study. Clin. Cardiol. 1987, 10, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.S. Magnesium in the Prevention of Lethal Arrhythmias in Acute Myocardial Infarction. Arch. Intern. Med. 1987, 147, 753. [Google Scholar] [CrossRef]
- Altura, B.T.; Brust, M.; Bloom, S.; Barbour, R.L.; Stempak, J.G.; Altura, B.M. Magnesium dietary intake modulates blood lipid levels and atherogenesis. Proc. Natl. Acad. Sci. USA 1990, 87, 1840–1844. [Google Scholar] [CrossRef] [Green Version]


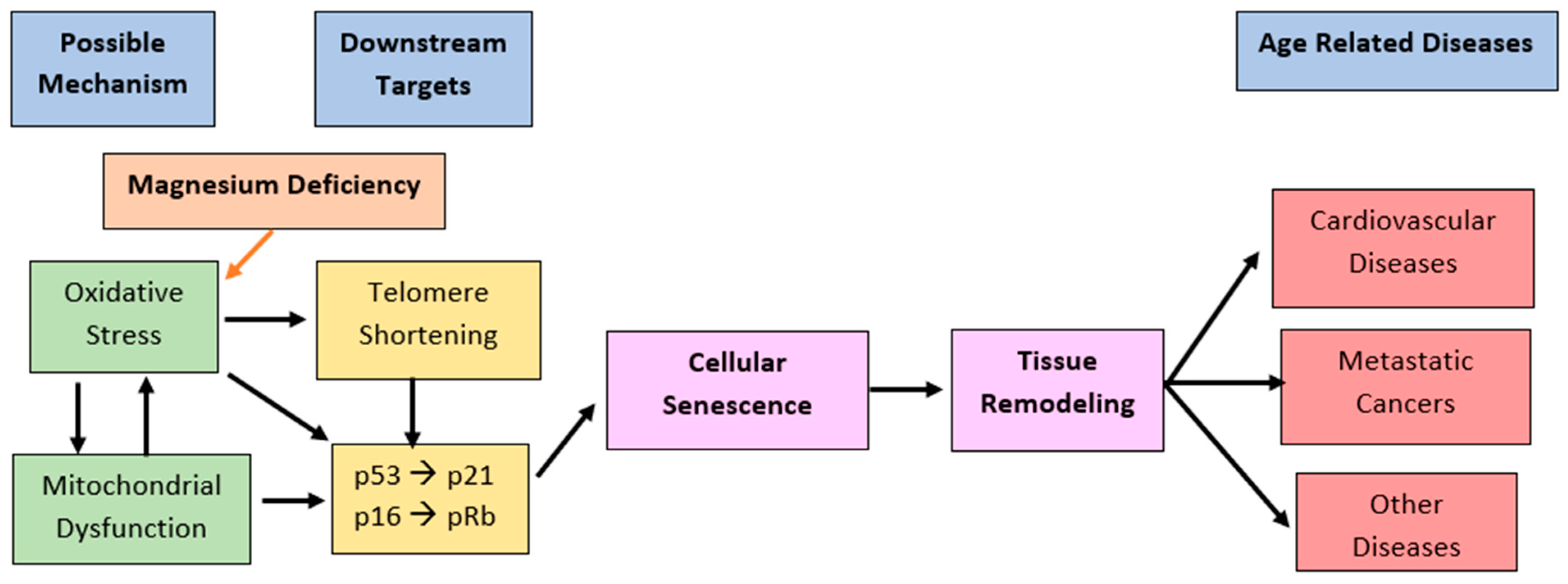
| Blood Pressure | Systolic (mmHg) | Diastolic (mmHg) |
|---|---|---|
| Normal | <120 | <80 |
| Elevated | 129–120 | <80 |
| Stage 1 Hypertension | 139–130 | 89–80 |
| Stage 2 Hypertension | ≥140 | ≥90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, N.; Sethi, R.; Shah, S.; Jafri, K.; Duran, J.; Chang, Y.; Soni, C.; Wollocko, H. The Roles of Platelet-Activating Factor and Magnesium in Pathophysiology of Hypertension, Atherogenesis, Cardiovascular Disease, Stroke and Aging. Cardiogenetics 2022, 12, 49-62. https://doi.org/10.3390/cardiogenetics12010005
Shah N, Sethi R, Shah S, Jafri K, Duran J, Chang Y, Soni C, Wollocko H. The Roles of Platelet-Activating Factor and Magnesium in Pathophysiology of Hypertension, Atherogenesis, Cardiovascular Disease, Stroke and Aging. Cardiogenetics. 2022; 12(1):49-62. https://doi.org/10.3390/cardiogenetics12010005
Chicago/Turabian StyleShah, Nilank, Roshni Sethi, Sachin Shah, Komail Jafri, Jonah Duran, Yong Chang, Chirag Soni, and Hanna Wollocko. 2022. "The Roles of Platelet-Activating Factor and Magnesium in Pathophysiology of Hypertension, Atherogenesis, Cardiovascular Disease, Stroke and Aging" Cardiogenetics 12, no. 1: 49-62. https://doi.org/10.3390/cardiogenetics12010005





