Pharmacogenomics of Pediatric Cardiac Arrest: Cisplatin Treatment Worsened by a Ryanodine Receptor 2 Gene Mutation
Abstract
:1. Background
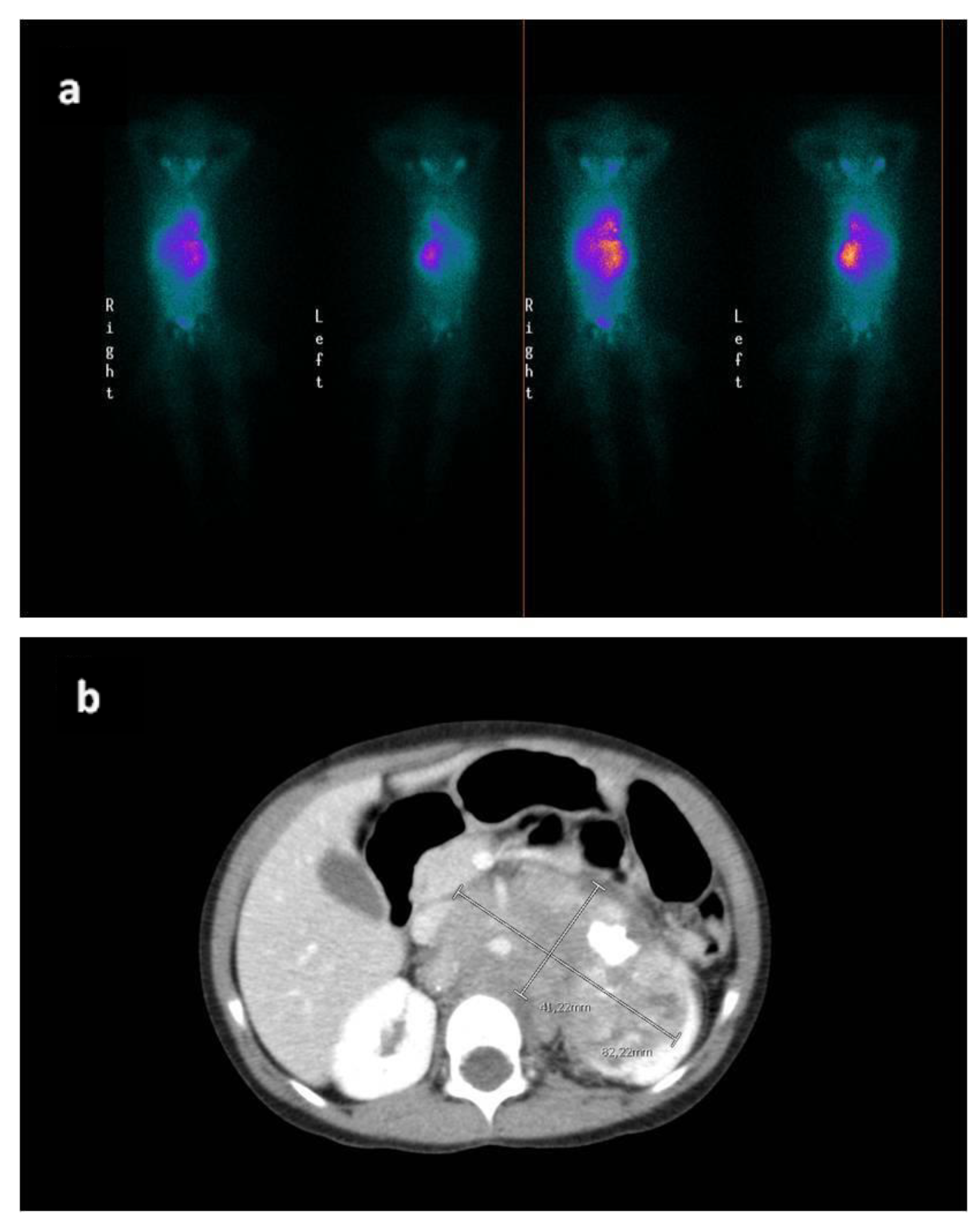
2. Case Report
3. Methods
3.1. DNA Extraction, Libraries Preparation and Next-Generation Sequencing (NGS)
3.2. Sanger Sequencing
3.3. Bioinformatics Analyses (vedi ref SGS)
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Trosman, J.R.; Carlos, R.C.; Simon, M.A.; Madden, D.L.; Gradishar, W.J.; Benson, A.B., III; Rapkin, B.D.; Weiss, E.S.; Gareen, I.F.; Wagner, L.I.; et al. Carefora Patient with Cancerasa Project: Management of Complex Task Interdependencein Cancer Care Delivery. J. Oncol. Pract. 2016, 12, 1101–1103. Available online: https://pubmed.ncbi.nlm.nih.gov/27577619/?from_term=patient+centered+care+fragmented+complexity&from_page=2&from_pos=4 (accessed on 19 April 2020). [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; RodriguezMuñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.; Lyon, A.R.; et al. 2016 ESC Position Paperon Cancer Treatments and Cardiovascular Toxicity Developed under the Auspices of the ESC Committee for Practice Guidelines: The Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. Available online: https://pubmed.ncbi.nlm.nih.gov/27567406/?from_term=2016+ESC+Position+Paper+on+cancer+treatments (accessed on 19 April 2020). [CrossRef] [PubMed]
- Joyce, J. Ulysses, Scylla and Charybdis; Wordsworth Editions: London, UK, 2010; Volume 9. [Google Scholar]
- Ehrhardt, M.J.; Fulbright, J.M.; Armenian, S.H. Cardiomyopathy in Childhood Cancer Survivors: Lessons from the Pastand Challenges for the Future. Curr. Oncol. Rep. 2016, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Stram, D.O.; Chatten, J.; Joshi, V.V.; Hachitanda, Y.; Brodeur, G.M.; Lukens, J.N.; Matthay, K.K.; Seeger, R.C. Identification of subsets of neuroblastomas by combined histopathologic and N-mycanalysis. J. Natl. Cancer Inst. 1995, 87, 1470–1476. [Google Scholar] [CrossRef]
- Monclair, T.; Brodeur, G.M.; Ambros, P.F.; Brisse, H.J.; Cecchetto, G.; Holmes, K.; Kaneko, M.; London, W.B.; Matthay, K.K.; Nuchtern, J.G.; et al. The International Neuroblastoma Risk Group (INRG) staging system. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 298–303. [Google Scholar] [CrossRef] [Green Version]
- Peinemann, F.; Kahangire, D.A.; van Dalen, E.C.; Berthold, F. Rapid COJEC versus standard induction therapies for high-risk neuroblastoma. Cochrane Database Syst. Rev. 2015, 5. [Google Scholar] [CrossRef]
- Sandroni, C.; Nolan, J.; European Resuscitation Council. ERC2010 guidelines for adult and pediatric resuscitation: Summary of major changes. Minerva Anestesiol. 2011, 77, 220–226. [Google Scholar]
- Eftekharian, A.; Mahani, M.H. Jervell and Lange-Nielsen syndrome in cochlear implanted patients: Our experience and a review of literature. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1544–1547. [Google Scholar] [CrossRef]
- Estivill, X.; Fortina, P.; Surrey, S.; Rabionet, R.; Melchionda, S.; D’Agruma, L.; Mansfield, E.; Rappaport, E.; Govea, N.; Milà, M.; et al. Connexin-26 mutations in sporadic and inherited sensor In eural deafness. Lancet 1998, 351, 394–398. [Google Scholar] [CrossRef]
- Kim, C.W.; Aronow, W.S.; Dutta, T.; Frenkel, D.; Frishman, W.H. Catecholaminergic Polymorphic Ventricular Tachycardia. Cardiol. Rev. 2020, 28, 325–331. [Google Scholar] [CrossRef]
- Sait, S.; Modak, S.I. Anti-GD2 immuno therapy for neuroblastomas. Expert Rev. Anticancer Ther. 2017, 17, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Babraham Bioinformatics- FastQC a Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 3 November 2020).
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinform. Oxf. Engl. 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hummel, M.; Bonnin, S.; Lowy, E.; Roma, G. TEQC: An R package for quality control in target capture experiments. Bioinform. Oxf. Engl. 2011, 27, 1316–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; DelAngel, G.; Rivas, M.A.; Hanna, M.; et al. A frame work for variation discovery and generation DNA sequencing data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-through put sequencing data. Nucleic Acids Res. 2010, 38, ne164. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef] [Green Version]
- Sherry, S.T.; Ward, M.-H.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.M.; Sirotkin, K. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001, 29, 308–311. [Google Scholar] [CrossRef] [Green Version]
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Analysis of protein-coding genetic variation in 60, 706 humans. Nature 2016, 536, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Jian, X.; Boerwinkle, E. dbNSFP: Alight weight database of human nonsynonymous SNPs and the irfunctional predictions. Hum. Mutat. 2011, 32, 894–899. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. Off. J. Am. Coll. Med. Genet. 2015, 17, 405–424. [Google Scholar] [CrossRef] [Green Version]
- Van den Berg, H.A. Occam’s Razor: From Ockham’s via Moderna to Modern Data Science. Sci. Prog. 2018, 101, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Sanecka, A.; Biernacka, E.K.; Sosna, M.; Mueller-Malesinska, M.; Ploski, R.; Skarzynski, H.; Piotrowicz, R. Evaluation of electrocardiographic parameters in patients with hearing loss genotyped for the connexin 26 gene (GJB2) mutations. Braz. J. Otorhinolaryngol. 2017, 83, 176–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priori, S.G.; Wilde, A.A.; Horie, M.; Cho, Y.; Behr, E.R.; Berul, C.; Blom, N.; Brugada, J.; Chiang, C.E.; Huikuri, H.; et al. Executive Summary: HRS/EHRA/APHRS Expert Consensus Statement on the Diagnosis and Management of Patients with Inherited Primary Arrhythmia Syndromes. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2013, 15, 1389–1409. Available online: https://pubmed.ncbi.nlm.nih.gov/23994779/?from_term=on+the+diagnosis+and+management+of+patients+with+inherited+primary+priori&from_pos=2 (accessed on 22 April 2020).
- Marks, A.R.; Priori, S.; Memmi, M.; Kontula, K.; Laitinen, P.J. Involvement of the Cardiac Ryanodine Receptor/Calcium Release Channel in Catecholaminergic Polymorphic Ventricular Tachycardia. J. Cell. Physiol. 2002, 190, 1–6. Available online: https://pubmed.ncbi.nlm.nih.gov/11807805/?from_term=ryanodine+cpvt+calcium&from_filter=pubt.review&from_pos=7 (accessed on 22 April 2020). [CrossRef] [PubMed]
- Chakraborty, A.D.; Gonano, L.A.; Munro, M.L.; Smith, L.J.; Thekkedam, C.; Staudacher, V.; Gamble, A.B.; Macquaide, N.; Dulhunty, A.; Jones, P.P. Activation of RyR2 by class Ikinase inhibitors. Br. J. Pharmacol. 2019, 176, 773–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadini-Buoninsegni, F.; Sordi, G.; Smeazzetto, S.; Natile, G.; Arnesano, F. Effect of cisplatin on the transport activity of PII-type ATPases. Metallomics 2017, 9, 960–968. [Google Scholar] [CrossRef]
- Khalil, R.B. Emotional Chemobrain’: A new concept for chemotherapy adverse drug effect? L’Encephale 2021, 47, 613–615. [Google Scholar] [CrossRef]
- Eisner, D.A.; Kashimura, T.; Venetucci, L.A.; Trafford, A.W. From the ryanodine receptor to cardiac arrhythmias. Circ. J. Off. J. Jpn. Circ. Soc. 2009, 73, 1561–1567. [Google Scholar] [CrossRef] [Green Version]
- Valverde, C.A.; Mazzocchi, G.; DiCarlo, M.N.; Pardo, A.C.; Salas, N.; Ragone, M.I.; IFelice, J.; Cely-Ortiz, A.; E Consolini, A.; Portiansky, E.; et al. Ablation of phospholamban rescues reperfusion arrhythmias but exacerbates myocardium in farction in heart swith Ca2+/calmodulinkinaseIIconstitutivephosphorylationofryanodinereceptors. Cardiovasc. Res. 2019, 115, 556–569. [Google Scholar] [CrossRef]
- Federico, M.; Valverde, C.A.; Mattiazzi, A.; Palomeque, J. Unbalance between Sarcoplasmic Reticulum Ca2+ Uptake and Release: A First Step Toward Ca2+ Triggered Arrhythmias and Cardiac Damage. Front. Physiol. 2020, 10, 1630. Available online: https://pubmed.ncbi.nlm.nih.gov/32038301/?from_single_result=valverde+Unbalance+Between+Sarcoplasmic&expanded_search_query=valverde+Unbalance+Between+Sarcoplasmic (accessed on 22 April 2020). [CrossRef]
- Bellamy, D.; Nuthall, G.; Dalziel, S.; Skinner, J.R. Catecholaminergic Polymorphic Ventricular Tachycardia: The Cardiac Arrest Where Epinephrine Is Contraindicated. Pediatr. Crit. Care Med. 2019, 20, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Circulation 2018, 138, e272–e391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.K.; Sharma, R.; Agrawal, A.; Varma, A. Vasopressin in the pediatric cardiac intensive care unit: Mythorreality. Ann. Pediatr. Cardiol. 2009, 2, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Baskar, S.; Bao, H.; Minges, K.E.; Spar, D.S.; Czosek, R.J. Characteristics and Outcomes of Pediatric Patients Who Undergo Placement of Implantable Cardioverter Defibrillators. Circ. Arrhythm. Electrophysiol. 2018, 11, e006542. [Google Scholar] [CrossRef] [PubMed]
- Bartholmew, J.; Washington, T.; Bergeron, S.; Nielson, D.; Saggio, J.; Quirk, L. Dinutuximab: A Novel Immunotherapy in the Treatment of Pediatric Patients with High-Risk Neuroblastoma. J. Pediatr. Oncol. Nurs. 2017, 34, 5–12. [Google Scholar] [CrossRef]
- Krebs, K.; Milani, L. Translating pharmacogenomics into clinical decisions: Do not let the perfect be the enemy of the good. Hum. Genom. 2019, 13, 39. [Google Scholar] [CrossRef] [Green Version]
- Barrot, C.-C.; Woillard, J.-B.; Picard, N. Big data in pharmacogenomics: Current applications, perspectives and pitfalls. Pharmacogenomics 2019, 20, 609–620. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggio, A.; Mastroianno, S.; Di Stolfo, G.; Castellana, S.; Palumbo, P.; Leone, M.P.; Spirito, A.; Potenza, D.R.; Ladogana, S.; Castori, M.; et al. Pharmacogenomics of Pediatric Cardiac Arrest: Cisplatin Treatment Worsened by a Ryanodine Receptor 2 Gene Mutation. Cardiogenetics 2022, 12, 80-88. https://doi.org/10.3390/cardiogenetics12010007
Maggio A, Mastroianno S, Di Stolfo G, Castellana S, Palumbo P, Leone MP, Spirito A, Potenza DR, Ladogana S, Castori M, et al. Pharmacogenomics of Pediatric Cardiac Arrest: Cisplatin Treatment Worsened by a Ryanodine Receptor 2 Gene Mutation. Cardiogenetics. 2022; 12(1):80-88. https://doi.org/10.3390/cardiogenetics12010007
Chicago/Turabian StyleMaggio, Angela, Sandra Mastroianno, Giuseppe Di Stolfo, Stefano Castellana, Pietro Palumbo, Maria Pia Leone, Anita Spirito, Domenico Rosario Potenza, Saverio Ladogana, Marco Castori, and et al. 2022. "Pharmacogenomics of Pediatric Cardiac Arrest: Cisplatin Treatment Worsened by a Ryanodine Receptor 2 Gene Mutation" Cardiogenetics 12, no. 1: 80-88. https://doi.org/10.3390/cardiogenetics12010007
APA StyleMaggio, A., Mastroianno, S., Di Stolfo, G., Castellana, S., Palumbo, P., Leone, M. P., Spirito, A., Potenza, D. R., Ladogana, S., Castori, M., Carella, M., Villella, M., & Salvatori, M. P. (2022). Pharmacogenomics of Pediatric Cardiac Arrest: Cisplatin Treatment Worsened by a Ryanodine Receptor 2 Gene Mutation. Cardiogenetics, 12(1), 80-88. https://doi.org/10.3390/cardiogenetics12010007






