Left Ventricular Non-Compaction Spectrum in Adults and Children: From a Morphological Trait to a Structural Muscular Disease
Abstract
:1. Introduction
Search Strategy and Selection Criteria
2. Morphological Features and Pathogenesis
3. Etiology: A Genetic Disease of the Cardiac Muscle
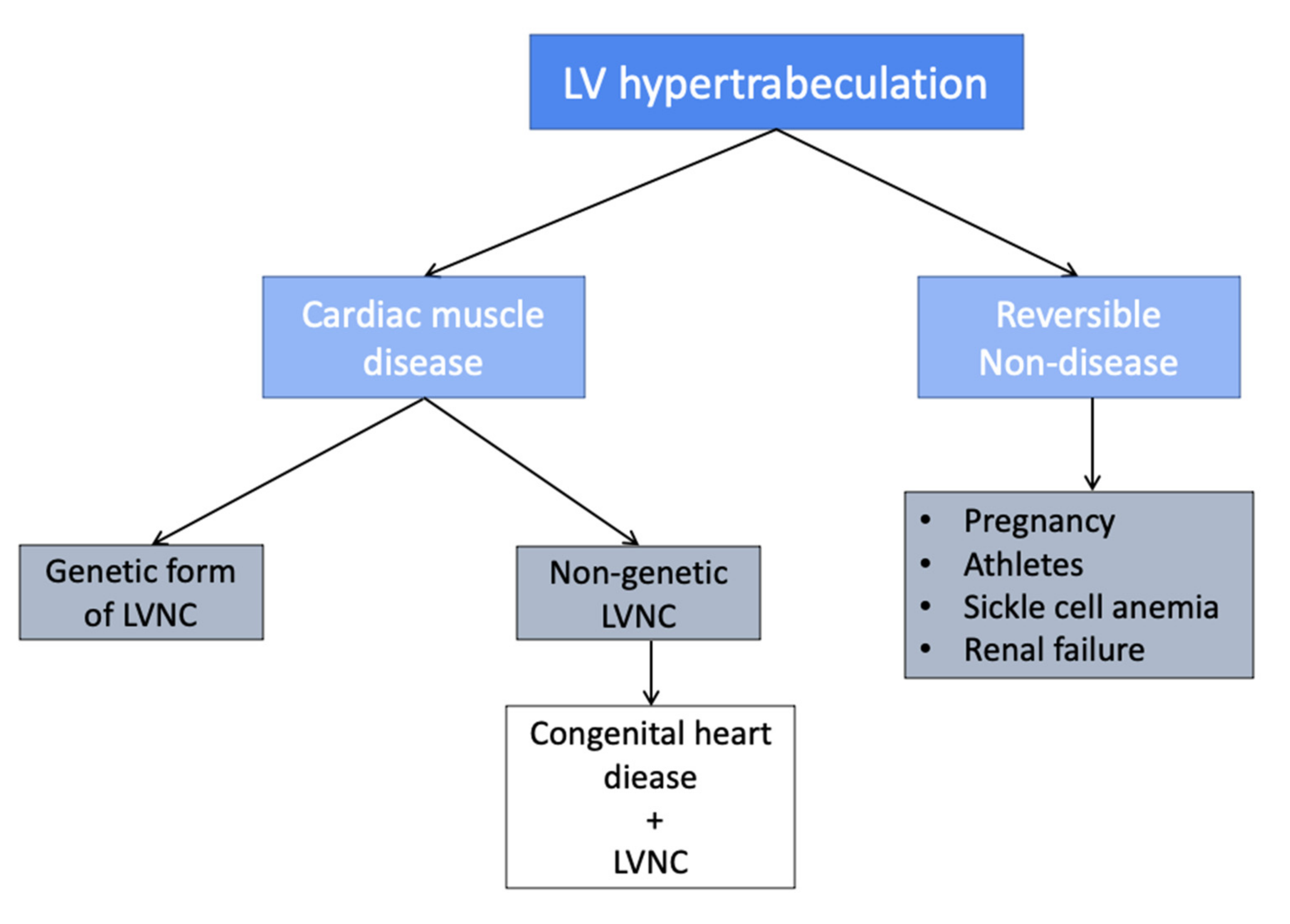
4. LVNC as a Spectrum: The Issue of the Reversible Hypertrabeculated Phenotype
5. LVNC Classification
6. Epidemiology
7. LVNC and Congenital Heart Disease
8. Clinical Presentation and Instrumental Findings at Diagnosis
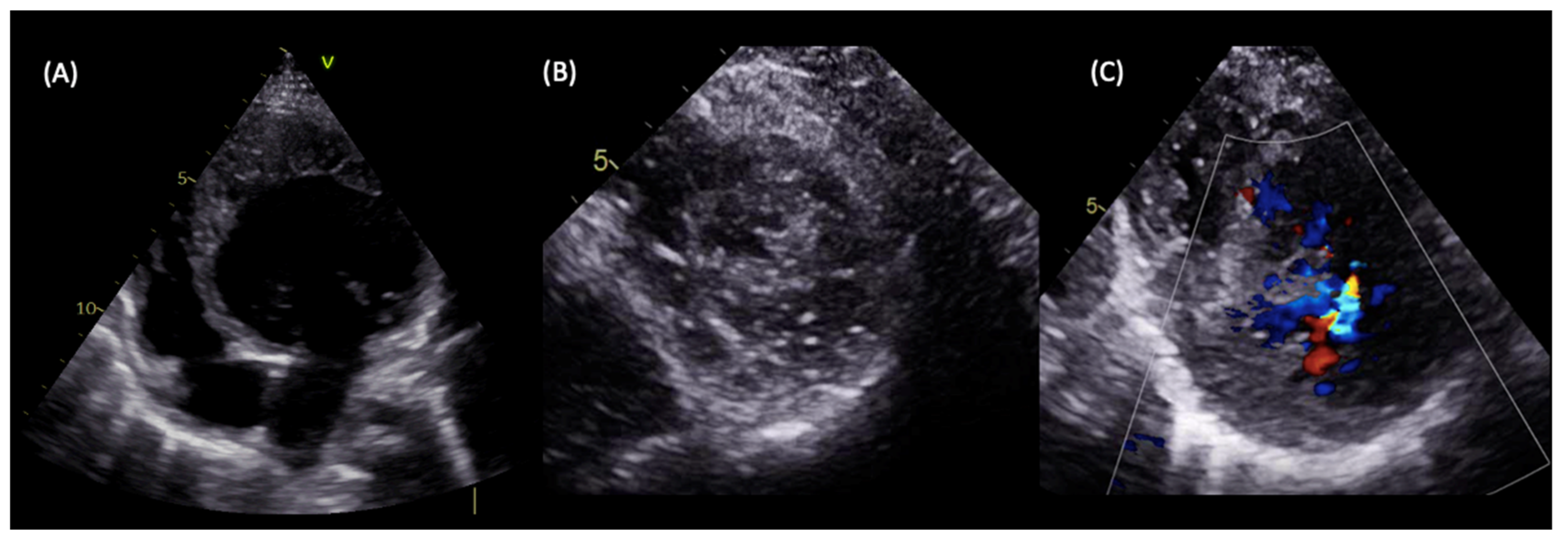
9. Diagnosis
9.1. LVNC Diagnostic Criteria
9.2. Limitations of the Current Diagnostic Criteria
10. Outcome
11. Management
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Freedom, R.M.; Yoo, S.-J.; Perrin, D.; Taylor, G.; Petersen, S.; Anderson, R.H. The morphological spectrum of ventricular noncompaction. Cardiol. Young 2005, 15, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, E.N.; Attenhofer Jost, C.H.; Rojas, J.R.; Kaufmann, P.A.; Jenni, R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: A distinct cardiomyopathy with poor prognosis. J. Am. Coll. Cardiol. 2000, 36, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Ichida, F.; Hamamichi, Y.; Miyawaki, T.; Ono, Y.; Kamiya, T.; Akagi, T.; Hamada, H.; Hirose, O.; Isobe, T.; Yamada, K.; et al. Clinical features of isolated noncompaction of the ventricular myocardium: Long-term clinical course, hemodynamic properties, and genetic background. J. Am. Coll. Cardiol. 1999, 34, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Jenni, R.; Wyss, C.A.; Oechslin, E.N.; Kaufmann, P.A. Isolated ventricular noncompaction is associated with coronary microcirculatory dysfunction. J. Am. Coll. Cardiol. 2002, 39, 450–454. [Google Scholar] [CrossRef] [Green Version]
- Dusek, J.; Ostádal, B.; Duskova, M. Postnatal persistence of spongy myocardium with embryonic blood supply. Arch. Pathol. 1975, 99, 312–317. [Google Scholar]
- Kohli, S.K.; Pantazis, A.A.; Shah, J.S.; Adeyemi, B.; Jackson, G.; McKenna, W.J.; Sharma, S.; Elliott, P.M. Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: Time for a reappraisal of diagnostic criteria? Eur. Hear. J. 2007, 29, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Gati, S.; Papadakis, M.; Papamichael, N.D.; Zaidi, A.; Sheikh, N.; Reed, M.; Sharma, R.; Thilaganathan, B.; Sharma, S. Reversible De Novo Left Ventricular Trabeculations in Pregnant Women. Circulation 2014, 130, 475–483. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Pelliccia, A.; Natali, B.M.; Bonifazi, M.; Mondillo, S. Exercise-induced left-ventricular hypertrabeculation in athlete’s heart. Int. J. Cardiol. 2015, 181, 320–322. [Google Scholar] [CrossRef]
- Probst, S.; Oechslin, E.; Schuler, P.; Greutmann, M.; Boyé, P.; Knirsch, W.; Berger, F.; Thierfelder, L.; Jenni, R.; Klaassen, S. Sarcomere Gene Mutations in Isolated Left Ventricular Noncompaction Cardiomyopathy Do Not Predict Clinical Phenotype. Circ. Cardiovasc. Genet. 2011, 4, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Protonotarios, A.; Elliott, P.M. Left ventricular non-compaction: Have we reached the limits of conventional imaging? Eur. Hear. J. 2019, 41, 1437–1438. [Google Scholar] [CrossRef]
- Shan, L.; Makita, N.; Xing, Y.; Watanabe, S.; Futatani, T.; Ye, F.; Saito, K.; Ibuki, K.; Watanabe, K.; Hirono, K. SCN5A variants in Japanese patients with left ventricular noncompaction and arrhythmia. Mol. Genet. Metab. 2008, 93, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, E.; Jenni, R. Left ventricular non-compaction revisited: A distinct phenotype with genetic heterogeneity? Eur. Hear. J. 2011, 32, 1446–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirono, K.; Hata, Y.; Nakazawa, M.; Momoi, N.; Tsuji, T.; Matsuoka, T.; Ayusawa, M.; Abe, Y.; Hayashi, T.; Tsujii, N.; et al. Clinical and Echocardiographic Impact of Tafazzin Variants on Dilated Cardiomyopathy Phenotype in Left Ventricular Non-Compaction Patients in Early Infancy. Circ. J. 2018, 82, 2609–2618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finsterer, J.; Slany, J. Is left ventricular hypertrabeculation/ noncompaction a cardiac manifestation of Fabry?s disease? Z. Kardiol. 2003, 92, 966–969. [Google Scholar] [CrossRef]
- Towbin, J.A.; Lorts, A.; Jefferies, J.L. Left ventricular non-compaction cardiomyopathy. Lancet 2015, 386, 813–825. [Google Scholar] [CrossRef]
- Ackerman, M.J.; Priori, S.G.; Willems, S.; Berul, C.; Brugada, R.; Calkins, H.; Camm, A.J.; Ellinor, P.T.; Gollob, M.H.; Hamilton, R.M.; et al. HRS/EHRA Expert Consensus Statement on the State of Genetic Testing for the Channelopathies and Cardiomyopathies: This document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Europace 2011, 13, 1077–1109. [Google Scholar] [CrossRef]
- Gati, S.; Chandra, N.; Bennett, R.L.; Reed, M.; Kervio, G.; Panoulas, V.F.; Ghani, S.; Sheikh, N.; Zaidi, A.; Wilson, M.; et al. Increased left ventricular trabeculation in highly trained athletes: Do we need more stringent criteria for the diagnosis of left ventricular non-compaction in athletes? Heart 2013, 99, 401–408. [Google Scholar] [CrossRef]
- Piga, A.; Longo, F.; Musallam, K.M.; Veltri, A.; Ferroni, F.; Chiribiri, A.; Bonamini, R. Left ventricular noncompaction in patients with β-thalassemia: Uncovering a previously unrecognized abnormality. Am. J. Hematol. 2012, 87, 1079–1083. [Google Scholar] [CrossRef]
- Markovic, N.; Dimkovic, N.; Damjanovic, T.; Loncar, G. Isolated ventricular noncompaction in patients with chronic renal failure. Clin. Nephrol. 2008, 70, 72–76. [Google Scholar] [CrossRef]
- Zemrak, F.; Ahlman, M.A.; Captur, G.; Mohiddin, S.; Kawel-Boehm, N.; Prince, M.; Moon, J.; Hundley, W.G.; Lima, J.A.; Bluemke, D.; et al. The Relationship of Left Ventricular Trabeculation to Ventricular Function and Structure Over a 9.5-Year Follow-Up. J. Am. Coll. Cardiol. 2014, 64, 1971–1980. [Google Scholar] [CrossRef] [Green Version]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B.; American Heart, A.; et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbustini, E.; Narula, N.; Dec, G.W.; Reddy, K.S.; Greenberg, B.; Kushwaha, S.; Marwick, T.; Pinney, S.; Bellazzi, R.; Favalli, V.; et al. The MOGE(S) Classification for a Phenotype–Genotype Nomenclature of Cardiomyopathy. J. Am. Coll. Cardiol. 2013, 62, 2046–2072. [Google Scholar] [CrossRef] [Green Version]
- Aras, D.; Tufekcioglu, O.; Ergun, K.; Özeke, Ö.; Yildiz, A.; Topaloglu, S.; Deveci, B.; Sahin, O.; Kisacik, H.L.; Korkmaz, S. Clinical Features of Isolated Ventricular Noncompaction in Adults Long-Term Clinical Course, Echocardiographic Properties, and Predictors of Left Ventricular Failure. J. Card. Fail. 2006, 12, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Ronderos, R.; Avegliano, G.; Borelli, E.; Kuschnir, P.; Castro, F.; Sanchez, G.; Perea, G.; Corneli, M.; Zanier, M.M.; Andres, S.; et al. Estimation of Prevalence of the Left Ventricular Noncompaction Among Adults. Am. J. Cardiol. 2016, 118, 901–905. [Google Scholar] [CrossRef]
- Kovacevic-Preradovic, T.; Jenni, R.; Oechslin, E.; Noll, G.; Seifert, B.; Jost, C.A. Isolated Left Ventricular Noncompaction as a Cause for Heart Failure and Heart Transplantation: A Single Center Experience. Cardiology 2009, 112, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Lilje, C.; Porciani, M.C.; Lilli, A.; Macioce, R.; Cappelli, F.; Demarchi, G.; Pappone, A.; Ricciardi, G.; Padeletti, L. Complications of non-compaction of the left ventricular myocardium in a paediatric population: A prospective study. Eur. Hear. J. 2006, 27, 1855–1860. [Google Scholar] [CrossRef] [Green Version]
- Pignatelli, R.H.; McMahon, C.J.; Dreyer, W.J.; Denfield, S.W.; Price, J.; Belmont, J.; Craigen, W.J.; Wu, J.; El Said, H.; Bezold, L.I.; et al. Clinical Characterization of Left Ventricular Noncompaction in Children. Circulation 2003, 108, 2672–2678. [Google Scholar] [CrossRef]
- Shi, W.Y.; Moreno-Betancur, M.; Nugent, A.W.; Cheung, M.; Colan, S.; Turner, C.; Sholler, G.F.; Robertson, T.; Justo, R.; Bullock, A.; et al. Long-Term Outcomes of Childhood Left Ventricular Noncompaction Cardiomyopathy. Circulation 2018, 138, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Marques, L.C.; Liguori, G.R.; Souza, A.C.A.A.; Aiello, V.D. Left Ventricular Noncompaction Is More Prevalent in Ventricular Septal Defect Than Other Congenital Heart Defects: A Morphological Study. J. Cardiovasc. Dev. Dis. 2020, 7, 39. [Google Scholar] [CrossRef]
- Choudhary, P.; Strugnell, W.; Puranik, R.; Hamilton-Craig, C.; Kutty, S.; Celermajer, D.S. LV non-compaction in patients with coarctation of the aorta: Prevalence and effects on cardiac function. Cardiol. Young 2021, 31, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Stähli, B.E.; Gebhard, C.; Biaggi, P.; Klaassen, S.; Buechel, E.V.; Jost, C.H.A.; Jenni, R.; Tanner, F.C.; Greutmann, M. Left ventricular non-compaction: Prevalence in congenital heart disease. Int. J. Cardiol. 2013, 167, 2477–2481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirono, K.; Hata, Y.; Miyao, N.; Okabe, M.; Takarada, S.; Nakaoka, H.; Ibuki, K.; Ozawa, S.; Yoshimura, N.; Nishida, N.; et al. Left Ventricular Noncompaction and Congenital Heart Disease Increases the Risk of Congestive Heart Failure. J. Clin. Med. 2020, 9, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, P.; Strugnell, W.; Puranik, R.; Hamilton-Craig, C.; Kutty, S.; Celermajer, D.S. Left ventricular non-compaction in patients with single ventricle heart disease. Cardiol. Young 2020, 30, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Khan, R.; Silverman, N.H.; Singh, G.K. Tricuspid Atresia with Non-compaction: An Early Experience with Implications for Surgical Palliation. Pediatr. Cardiol. 2016, 38, 495–505. [Google Scholar] [CrossRef]
- Tian, T.; Yang, Y.; Zhou, L.; Luo, F.; Li, Y.; Fan, P.; Dong, X.; Liu, Y.; Cui, J.; Zhou, X. Left Ventricular Non-Compaction: A Cardiomyopathy With Acceptable Prognosis in Children. Hear. Lung Circ. 2018, 27, 28–32. [Google Scholar] [CrossRef]
- Ramachandran, P.; Woo, J.G.; Ryan, T.D.; Bryant, R.; Heydarian, H.C.; Jefferies, J.L.; Towbin, J.A.; Lorts, A. The Impact of Concomitant Left Ventricular Non-compaction with Congenital Heart Disease on Perioperative Outcomes. Pediatr. Cardiol. 2016, 37, 1307–1312. [Google Scholar] [CrossRef]
- Habib, G.; Charron, P.; Eicher, J.C.; Giorgi, R.; Donal, E.; Laperche, T.; Boulmier, D.; Pascal, C.; Logeart, D.; et al.; Working Groups ‘Heart Failure and Cardiomyopathies’ and ‘Echocardiography’ of the French Society of Cardiology. Isolated left ventricular non-compaction in adults: Clinical and echocardiographic features in 105 patients. Results from a French registry. Eur. J. Heart Fail. 2011, 13, 177–185. [Google Scholar] [CrossRef]
- Murphy, R.T.; Thaman, R.; Blanes, J.G.; Ward, D.; Sevdalis, E.; Papra, E.; Kiotsekolglou, A.; Tome, M.T.; Pellerin, D.; McKenna, W.J.; et al. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur. Hear. J. 2005, 26, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Lofiego, C.; Biagini, E.; Pasquale, F.; Ferlito, M.; Rocchi, G.; Perugini, E.; Reggiani, M.L.B.; Boriani, G.; Leone, O.; Caliskan, K.; et al. Wide spectrum of presentation and variable outcomes of isolated left ventricular non-compaction. Heart 2007, 93, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Peters, F.; Khandheria, B.K.; dos Santos, C.; Matioda, H.; Maharaj, N.; Libhaber, E.; Mamdoo, F.; Essop, M.R. Isolated Left Ventricular Noncompaction in Sub-Saharan Africa: A Clinical and Echocardiographic Perspective. Circ. Cardiovasc. Imaging 2012, 5, 187–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, T.; Liu, Y.; Gao, L.; Wang, J.; Sun, K.; Zou, Y.; Wang, L.; Zhang, L.; Li, Y.; Xiao, Y.; et al. Isolated left ventricular noncompaction: Clinical profile and prognosis in 106 adult patients. Hear. Vessel. 2014, 29, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Howard, T.S.; Valdes, S.O.; Hope, K.D.; Morris, S.A.; Landstrom, A.P.; Schneider, A.E.; Miyake, C.Y.; Denfield, S.W.; Pignatelli, R.H.; Wang, Y.; et al. Association of Wolff-Parkinson-White With Left Ventricular Noncompaction Cardiomyopathy in Children. J. Card. Fail. 2019, 25, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, A.; Ganesan, G.; Sangareddi, V.; Pillai, A.P.; Ramasamy, A. Isolated Noncompaction of Right Ventricle-A Case Report. Echocardiography 2012, 29, E169–E172. [Google Scholar] [CrossRef]
- Maestrini, V.; Torlasco, C.; Hughes, R.; Moon, J.C. Cardiovascular Magnetic Resonance and Sport Cardiology: A Growing Role in Clinical Dilemmas. J. Cardiovasc. Transl. Res. 2020, 13, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Saglam, M.; Saygin, H.; Kozan, H.; Ozturk, E.; Mutlu, H. Noncompaction of Ventricular Myocardium Involving the Right Ventricle. Kor. Circ. J. 2015, 45, 439–441. [Google Scholar] [CrossRef] [Green Version]
- Ulusoy, R.E.; Kucukarslan, N.; Kirilmaz, A.; Demiralp, E. Noncompaction of ventricular myocardium involving both ventricles. Eur. J. Echocardiogr. 2006, 7, 457–460. [Google Scholar] [CrossRef] [Green Version]
- Sirin, B.; Kurdal, A.; Iskesen, I.; Cerrahoglu, M. Right Ventricular Outflow Obstruction of the Patient with Biventricular Non-Compaction. Thorac. Cardiovasc. Surg. 2010, 58, 364–366. [Google Scholar] [CrossRef]
- Martinez, H.R.; Miller, E.; Mead, R.; Osher, J.; Almasri, M.; Parent, J.J. Biventricular noncompaction cardiomyopathy with severe dilated phenotype in a family with a novel MYH7 gene variant. Prog. Pediatr. Cardiol. 2020, 59, 101205. [Google Scholar] [CrossRef]
- Miura, F.; Shimada, J.; Kitagawa, Y.; Otani, K.; Sato, T.; Toki, T.; Takahashi, T.; Yonesaka, S.; Mizukami, H.; Ito, E. MYH7 mutation identified by next-generation sequencing in three infant siblings with bi-ventricular noncompaction presenting with restrictive hemodynamics. J. Cardiol. Cases 2019, 19, 140–143. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Fanjul, J.; Tubio-Gómez, S.; Bellón, J.M.C.; Bautista-Rodríguez, C.; Sanchez-De-Toledo, J. Neonatal Non-compacted Cardiomyopathy: Predictors of Poor Outcome. Pediatr. Cardiol. 2019, 41, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Stanton, C.; Bruce, C.; Connolly, H.; Brady, P.; Syed, I.; Hodge, D.; Asirvatham, S.; Friedman, P. Isolated Left Ventricular Noncompaction Syndrome. Am. J. Cardiol. 2009, 104, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Stöllberger, C.; Finsterer, J.; Blazek, G. Left ventricular hypertrabeculation/noncompaction and association with additional cardiac abnormalities and neuromuscular disorders. Am. J. Cardiol. 2002, 90, 899–902. [Google Scholar] [CrossRef]
- Brescia, S.T.; Rossano, J.W.; Pignatelli, R.; Jefferies, J.L.; Price, J.F.; Decker, J.A.; Denfield, S.W.; Dreyer, W.J.; Smith, O.; Towbin, J.A.; et al. Mortality and sudden death in pediatric left ventricular noncompaction in a tertiary referral center. Circulation 2013, 127, 2202–2208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, S.B.; Jones, K.; Blanch, B.; Puranik, R.; McGeechan, K.; Barratt, A.; Semsarian, C. A systematic review and meta-analysis of the prevalence of left ventricular non-compaction in adults. Eur. Hear. J. 2020, 41, 1428–1436. [Google Scholar] [CrossRef]
- Dodd, J.D.; Holmvang, G.; Hoffmann, U.; Ferencik, M.; Abbara, S.; Brady, T.J.; Cury, R.C. Quantification of Left Ventricular Noncompaction and Trabecular Delayed Hyperenhancement with Cardiac MRI: Correlation with Clinical Severity. Am. J. Roentgenol. 2007, 189, 974–980. [Google Scholar] [CrossRef]
- Andreini, D.; Pontone, G.; Bogaert, J.; Roghi, A.; Barison, A.; Schwitter, J.; Mushtaq, S.; Vovas, G.; Sormani, P.; Aquaro, G.D.; et al. Long-Term Prognostic Value of Cardiac Magnetic Resonance in Left Ventricle Noncompaction. J. Am. Coll. Cardiol. 2016, 68, 2166–2181. [Google Scholar] [CrossRef]
- Laat, L.E.D.G.-D.; Krenning, B.J.; Cate, F.J.T.; Roelandt, J.R. Usefulness of Contrast Echocardiography for Diagnosis of Left Ventricular Noncompaction. Am. J. Cardiol. 2005, 95, 1131–1134. [Google Scholar] [CrossRef]
- Ari, M.E.; Cetin, I.I.; Kocabas, A.; Ekici, F.; Ceylan, O.; Surucu, M. Decreased Deformation in Asymptomatic Children with Isolated Left Ventricular Non-compaction and Normal Ejection Fraction. Pediatr. Cardiol. 2016, 37, 201–207. [Google Scholar] [CrossRef]
- Bellavia, D.; Michelena, H.I.; Martinez, M.; Pellikka, P.A.; Bruce, C.J.; Connolly, H.M.; Villarraga, H.R.; Veress, G.; Oh, J.K.; Miller, F.A. Speckle myocardial imaging modalities for early detection of myocardial impairment in isolated left ventricular non-compaction. Heart 2009, 96, 440–447. [Google Scholar] [CrossRef]
- Arunamata, A.; Stringer, J.; Balasubramanian, S.; Tacy, T.A.; Silverman, N.H.; Punn, R. Cardiac Segmental Strain Analysis in Pediatric Left Ventricular Noncompaction Cardiomyopathy. J. Am. Soc. Echocardiogr. 2019, 32, 763–773.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huttin, O.; Venner, C.; Frikha, Z.; Voilliot, D.; Marie, P.-Y.; Aliot, E.; Sadoul, N.; Juillière, Y.; Brembilla-Perrot, B.; Selton-Suty, C. Myocardial deformation pattern in left ventricular non-compaction: Comparison with dilated cardiomyopathy. IJC Heart Vasc. 2014, 5, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Tarando, F.; Coisne, D.; Galli, E.; Rousseau, C.; Viera, F.; Bosseau, C.; Habib, G.; Lederlin, M.; Schnell, F.; Donal, E. Left ventricular non-compaction and idiopathic dilated cardiomyopathy: The significant diagnostic value of longitudinal strain. Int. J. Cardiovasc. Imaging 2016, 33, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Haland, T.F.; Saberniak, J.; Leren, I.S.; Edvardsen, T.; Haugaa, K.H. Echocardiographic comparison between left ventricular non-compaction and hypertrophic cardiomyopathy. Int. J. Cardiol. 2016, 228, 900–905. [Google Scholar] [CrossRef] [Green Version]
- Peters, F.; Khandheria, B.K.; Libhaber, E.; Maharaj, N.; Dos Santos, C.; Matioda, H.; Essop, M.R. Left ventricular twist in left ventricular noncompaction. Eur. Hear. J. Cardiovasc. Imaging 2014, 15, 48–55. [Google Scholar] [CrossRef] [PubMed]
- van Dalen, B.M.; Caliskan, K.; Soliman, O.I.; Kauer, F.; van der Zwaan, H.B.; Vletter, W.B.; van Vark, L.C.; Cate, F.J.T.; Geleijnse, M.L. Diagnostic Value of Rigid Body Rotation in Noncompaction Cardiomyopathy. J. Am. Soc. Echocardiogr. 2011, 24, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.K.; Perloff, J.K.; Williams, R.G.; Jue, K.; Mohrmann, R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 1990, 82, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Jenni, R.; Oechslin, E.; Schneider, J.; Jost, C.A.; Kaufmann, P.A. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: A step towards classification as a distinct cardiomyopathy. Heart 2001, 86, 666–671. [Google Scholar] [CrossRef] [Green Version]
- Gebhard, C.; Stähli, B.E.; Greutmann, M.; Biaggi, P.; Jenni, R.; Tanner, F.C. Reduced Left Ventricular Compacta Thickness: A Novel Echocardiographic Criterion for Non-Compaction Cardiomyopathy. J. Am. Soc. Echocardiogr. 2012, 25, 1050–1057. [Google Scholar] [CrossRef]
- Petersen, S.E.; Selvanayagam, J.B.; Wiesmann, F.; Robson, M.D.; Francis, J.M.; Anderson, R.H.; Watkins, H.; Neubauer, S. Left Ventricular Non-Compaction: Insights From Cardiovascular Magnetic Resonance Imaging. J. Am. Coll. Cardiol. 2005, 46, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Jacquier, A.; Thuny, F.; Jop, B.; Giorgi, R.; Cohen, F.; Gaubert, J.Y.; Vidal, V.; Bartoli, J.M.; Habib, G.; Moulin, G. Measurement of trabeculated left ventricular mass 52 using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur. Heart J. 2010, 31, 1098–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stacey, R.B.; Andersen, M.M.; Clair, M.S.; Hundley, W.G.; Thohan, V. Comparison of Systolic and Diastolic Criteria for Isolated LV Noncompaction in CMR. JACC Cardiovasc. Imaging 2013, 6, 931–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Captur, G.; Muthurangu, V.; Cook, C.; Flett, A.S.; Wilson, R.; Barison, A.; Sado, D.M.; Anderson, S.; McKenna, W.J.; Mohun, T.J.; et al. Quantification of left ventricular trabeculae using fractal analysis. J. Cardiovasc. Magn. Reson. 2013, 15, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernabé, G.; Casanova, J.; González-Carrillo, J.; Gimeno-Blanes, J. Towards an Enhanced Tool for Quantifying the Degree of LV Hyper-Trabeculation. J. Clin. Med. 2021, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Bernabé, G.; Casanova, J.D.; Cuenca, J.; González-Carrillo, J. A self-optimized software tool for quantifying the degree of left ventricle hyper-trabeculation. J. Supercomput. 2018, 75, 1625–1640. [Google Scholar] [CrossRef]
- Ivanov, A.; Dabiesingh, D.S.; Bhumireddy, G.P.; Mohamed, A.; Asfour, A.; Briggs, W.M.; Ho, J.; Khan, S.A.; Grossman, A.; Klem, I.; et al. Prevalence and Prognostic Significance of Left Ventricular Noncompaction in Patients Referred for Cardiac Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2017, 10, e006174. [Google Scholar] [CrossRef] [Green Version]
- Stöllberger, C.; Gerecke, B.; Engberding, R.; Grabner, B.; Wandaller, C.; Finsterer, J.; Gietzelt, M.; Balzereit, A. Interobserver Agreement of the Echocardiographic Diagnosis of LV Hypertrabeculation/Noncompaction. JACC Cardiovasc. Imaging 2015, 8, 1252–1257. [Google Scholar] [CrossRef] [Green Version]
- Stöllberger, C.; Finsterer, J. Thrombi in the left ventricular hypertrabeculation/noncompaction. Acta Cardiol. 2004, 59, 341–344. [Google Scholar] [CrossRef]
- Aung, N.; Doimo, S.; Ricci, F.; Sanghvi, M.M.; Pedrosa, C.; Woodbridge, S.P.; Al-Balah, A.; Zemrak, F.; Khanji, M.Y.; Munroe, P.B.; et al. Prognostic Significance of Left Ventricular Noncompaction. Circ. Cardiovasc. Imaging 2020, 13, e009712. [Google Scholar] [CrossRef]
- Wang, W.; Chen, W.; Lin, X.; Fang, L. Influence of Right Ventricular Dysfunction on Outcomes of Left Ventricular Non-compaction Cardiomyopathy. Front. Cardiovasc. Med. 2022, 9, 816404. [Google Scholar] [CrossRef]
- Crousillat, D.R.; Ghoshhajra, B.B.; Scott, N.S. Pregnancy in Familial Left Ventricular Noncompaction-Associated Cardiomyopathy. JACC Case Rep. 2020, 2, 120–124. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Subahi, A.; Hassan, A.I.; Abubakar, H.; Ibrahim, W. Isolated left ventricular non-compaction (LVNC) and recurrent strokes: To anticoagulate or not to anticoagulate, that is the question. BMJ Case Rep. 2017, 2017, bcr2017220954. [Google Scholar] [CrossRef] [PubMed]



| Echocardiographic Criteria | ||
|---|---|---|
| Chim [67] |
| First set of proposed diagnostic criteria Also known as the California criteria |
| Jenni [68] |
| Globally the most used set of criteria |
| Stöllberger [53] |
| |
| Gebhart [69] | Maximal systolic compact layer thickness <8 mm | Proposed additional diagnostic criteria to allow differentiation from normal myocardial |
| Advanced echocardiography |
| Emerging role of new technologies See the text |
| CMR criteria | ||
| Petersen [70] |
| Sensitivity of 86% and specificity of 99% |
| Jacquier [71] | Trabecular mass is measured as the difference between total LV mass and compacted myocardial A proportion of trabecular mass >20% in SSFP cine short axis view in end-diastole is diagnostic of LVNC | Papillary muscles should be included in the compacted myocardial mass. Sensitivity and a specificity of 93.7%: |
| Stacey [72] | NC/C ratio measured in end-systole on SSFP images | Better correlation with adverse events, heart failure and systolic dysfunction |
| Fractal analysis [73] | An automated software provides a unit-less index expression of the degree of geometrical complexity of the LV cavity. A fractal dimensions >1.392 was used to define LVNC | Better inter-observer reproducibility compared to both Petersen and Jacquier criteria. |
| LV trabeculae quantification [74,75] | An automated software quantifies the volumes of the compacted trabeculated areas, giving the percentage of trabelated LV | High reproducibily Time saving |
| LGE assessment | Prognostic implications | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fusco, F.; Borrelli, N.; Barracano, R.; Ciriello, G.D.; Verrillo, F.; Scognamiglio, G.; Sarubbi, B. Left Ventricular Non-Compaction Spectrum in Adults and Children: From a Morphological Trait to a Structural Muscular Disease. Cardiogenetics 2022, 12, 170-184. https://doi.org/10.3390/cardiogenetics12020016
Fusco F, Borrelli N, Barracano R, Ciriello GD, Verrillo F, Scognamiglio G, Sarubbi B. Left Ventricular Non-Compaction Spectrum in Adults and Children: From a Morphological Trait to a Structural Muscular Disease. Cardiogenetics. 2022; 12(2):170-184. https://doi.org/10.3390/cardiogenetics12020016
Chicago/Turabian StyleFusco, Flavia, Nunzia Borrelli, Rosaria Barracano, Giovanni Domenico Ciriello, Federica Verrillo, Giancarlo Scognamiglio, and Berardo Sarubbi. 2022. "Left Ventricular Non-Compaction Spectrum in Adults and Children: From a Morphological Trait to a Structural Muscular Disease" Cardiogenetics 12, no. 2: 170-184. https://doi.org/10.3390/cardiogenetics12020016






