Inflammatory Bowel Diseases and Coexisting Spondyloarthritis: A Neglected and too Often Under-Reported Association by Radiologists. A Multicenter Study by Italian Research Group of Imaging in Rheumatology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Biopsy Criteria for Diagnosis of CD
2.3. Scanning Protocols
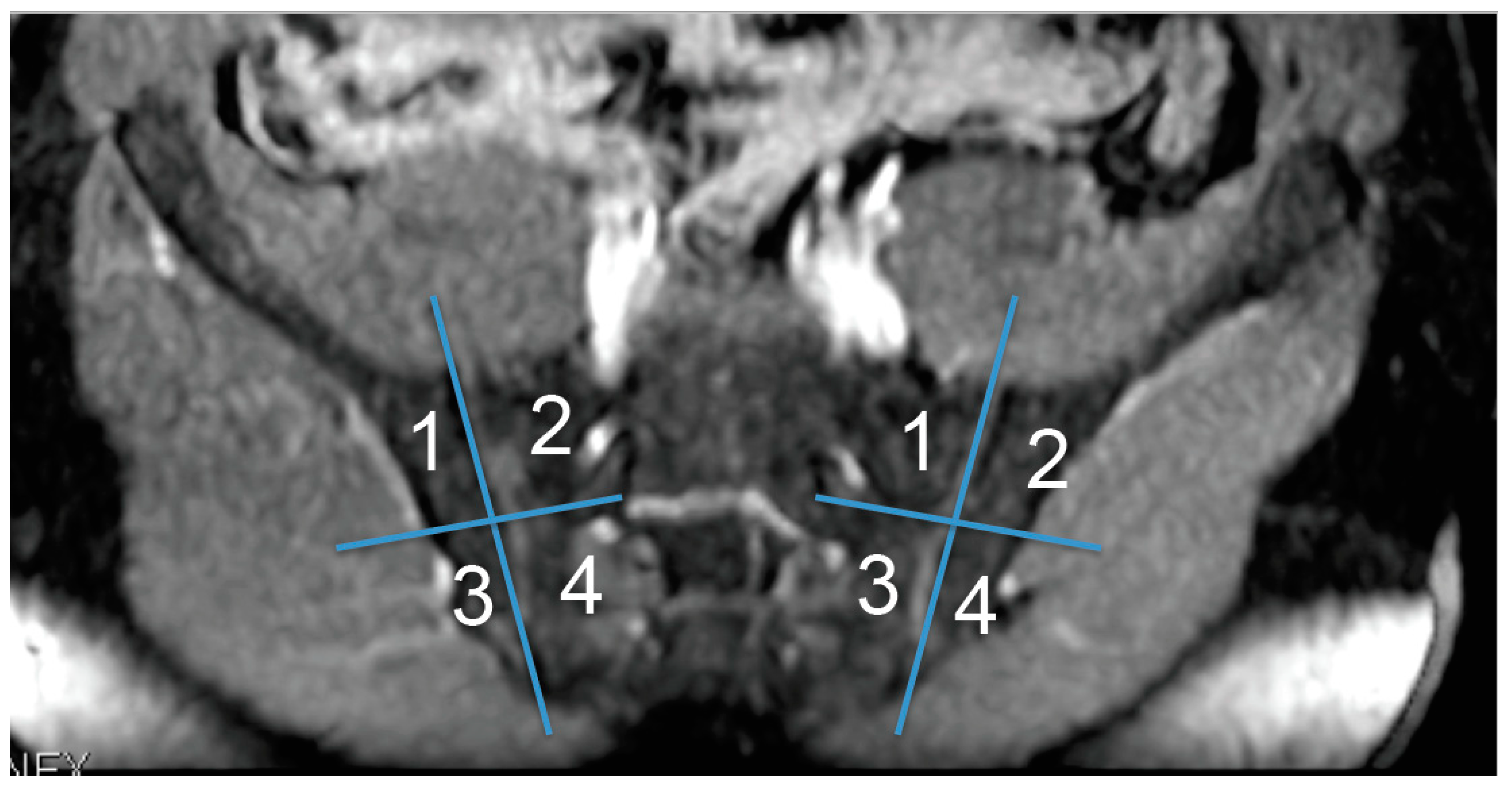
2.4. Image Evaluation
2.5. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wills, J.S.; Lobis, I.F.; Denstman, F.J. Crohn disease: State of the art. Radiology 1997, 202, 597–610. [Google Scholar] [CrossRef]
- De Vos, M. Joint involvement associated with inflammatory bowel disease. Dig. Dis. 2009, 27, 511–515. [Google Scholar] [CrossRef]
- Farraye, F.A.; Odze, R.D.; Eaden, J.; Itzkowitz, S.H.; McCabe, R.P.; Dassopoulos, T.; Lewis, J.D.; Ullman, T.A.; James, T., 3rd; McLeod, R.; et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology 2010, 138, 738–745. [Google Scholar] [CrossRef]
- Akgul, O.; Ozgocmen, S. Classification criteria for spondyloarthropaties. World J. Orthop. 2011, 2, 107–115. [Google Scholar] [CrossRef]
- Guerrini, S.; Giordano, N.; Volterrani, L.; Frediani, B.; Mazzei, M.A. Sonozaki syndrome in the spotlight of imaging. Clin. Rheumatol. 2020. [Google Scholar] [CrossRef]
- Guerrini, S.; Bagnacci, G.; Barile, A.; La Paglia, E.; Gentili, F.; Luzzi, L.; Giordano, N.; Fioravanti, A.; Bellisai, F.; Cantarini, L.; et al. Anterior chest wall non-traumatic diseases: A road map for the radiologist. Acta Biomed. 2020, 91, 43–50. [Google Scholar] [CrossRef]
- Olivieri, I.; Cantini, F.; Castiglione, F.; Felice, C.; Gionchetti, P.; Orlando, A.; Salvarani, C.; Scarpa, R.; Vecchi, M.; Armuzzi, A. Italian Expert Panel on the management of patients with coexisting spondyloarthritis and inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 822–830. [Google Scholar] [CrossRef]
- Mandl, P.; Navarro-Compán, V.; Terslev, L.; Aegerter, P.; van der Heijde, D.; D’Agostino, M.A.; Baraliakos, X.; Pedersen, S.J.; Jurik, A.G.; Naredo, E.; et al. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann. Rheum. Dis. 2015, 74, 1327–1339. [Google Scholar] [CrossRef]
- Fianyo, E.; Wendling, D.; Poulain, C.; Farrenq, V.; Claudepierre, P. Non-radiographic axial spondyloarthritis: What is it? Clin. Exp. Rheumatol. 2014, 32, 1–4. [Google Scholar]
- Gentili, F.; Cantarini, L.; Fabbroni, M.; Nigri, A.; Mazzei, F.G.; Frediani, B.; Galeazzi, M.; Volterrani, L.; Mazzei, M.A. Magnetic resonance imaging of the sacroiliac joints in SpA: With or without intravenous contrast media? A preliminary report. Radiol. Med. 2019, 124, 1142–1150. [Google Scholar] [CrossRef]
- Marchesoni, A.; D’Angelo, S.; Anzidei, M.; Borlotti, R.; Cantini, F.; Caramella, D.; Carotti, M.; Chimenti, M.; Delle Sedie, A.; Egan, C.; et al. Radiologist-rheumatologist multidisciplinary approach in the management of axial spondyloarthritis: A Delphi consensus statement. Clin. Exp. Rheumatol. 2019, 37, 575–584. [Google Scholar]
- La Paglia, E.; Zawaideh, J.P.; Lucii, G.; Mazzei, M.A. MRI of the axial skeleton: Differentiating non-inflammatory diseases and axial spondyloarthritis: A review of current concepts and applications : Special issue on “musculoskeletal imaging of the inflammatory and degenerative joints: Current status and perspectives”. Radiol. Med. 2019, 124, 1151–1166. [Google Scholar] [CrossRef]
- Baraliakos, X.; van der Heijde, D.; Braun, J.; Landewé, R.B. OMERACT magnetic resonance imaging initiative on structural and inflammatory lesions in ankylosing spondylitis-report of a special interest group at OMERACT 10 on sacroiliac joint and spine lesions. J. Rheumatol. 2011, 38, 2051–2054. [Google Scholar] [CrossRef]
- Bredella, M.A.; Steinbach, L.S.; Morgan, S.; Ward, M.; Davis, J.C. MRI of the sacroiliac joints in patients with moderate to severe ankylosing spondylitis. AJR Am. J. Roentgenol. 2006, 187, 1420–1426. [Google Scholar] [CrossRef]
- Hermann, K.G.; Bollow, M. Magnetic resonance imaging of sacroiliitis in patients with spondyloarthritis: Correlation with anatomy and histology. Rofo 2014, 186, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Falsetti, P.; Conticini, E.; Mazzei, M.A.; Baldi, C.; Sota, J.; Bardelli, M.; Gentileschi, S.; D’Alessandro, R.; Al Khayyat, S.G.; Acciai, C.; et al. Power and spectral Doppler ultrasound in suspected active sacroiliitis: A comparison with magnetic resonance imaging as gold standard. Rheumatology 2020. [Google Scholar] [CrossRef]
- Volterrani, L.; Mazzei, M.A.; Giordano, N.; Nuti, R.; Galeazzi, M.; Fioravanti, A. Magnetic resonance imaging in Tietze’s syndrome. Clin. Exp. Rheumatol. 2008, 26, 848–853. [Google Scholar]
- Barile, A.; Arrigoni, F.; Bruno, F.; Guglielmi, G.; Zappia, M.; Reginelli, A.; Ruscitti, P.; Cipriani, P.; Giacomelli, R.; Brunese, L.; et al. Computed tomography and MR imaging in rheumatoid arthritis. Radiol. Clin. N. Am. 2017, 55, 997–1007. [Google Scholar] [CrossRef]
- Diekhoff, T.; Hermann, K.G.; Greese, J.; Schwenke, C.; Poddubnyy, D.; Hamm, B.; Sieper, J. Comparison of MRI with radiography for detecting structural lesions of the sacroiliac joint using CT as standard of reference: Results from the SIMACT study. Ann. Rheum. Dis. 2017, 76, 1502–1508. [Google Scholar] [CrossRef]
- Toussirot, E. New treatment options and emerging drugs for axial spondyloarthritis: Biological and targeted synthetic agents. Exp. Opin. Pharmacother. 2017, 18, 275–282. [Google Scholar] [CrossRef]
- Keat, A.; Bennett, A.N.; Gaffney, K.; Marzo-Ortega, H.; Sengupta, R.; Everiss, T. Should axial spondyloarthritis without radiographic changes be treated with anti-TNF agents? Rheumatol. Int. 2017, 37, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Marchesoni, A.; Olivieri, I.; Salvarani, C.; Pipitone, N.; D’Angelo, S.; Mathieu, A.; Cauli, A.; Punzi, L.; Ramonda, R.; Scarpa, R.; et al. Recommendations for the use of biologics and other novel drugs in the treatment of psoriatic arthritis: 2017 update from the Italian Society of Rheumatology. Clin. Exp. Rheumatol. 2017, 35, 991–1010. [Google Scholar] [PubMed]
- Chitul, A.; Voiosu, A.M.; Marinescu, M.; Caraiola, S.; Nicolau, A.; Badea, G.C.; Pârvu, M.I.; Ionescu, R.A.; Mateescu, B.R.; Voiosu, M.R.; et al. Different effects of anti-TNF-alpha biologic drugs on the small bowel macroscopic inflammation in patients with ankylosing spondylitis. Rom. J. Intern Med. 2017, 55, 44–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fioravanti, A.; Cantarini, L.; Burroni, L.; Mazzei, M.A.; Volterrani, L.; Galeazzi, M. Efficacy of alendronate in the treatment of the SAPHO syndrome. J. Clin. Rheumatol. 2008, 14, 183–184. [Google Scholar] [CrossRef]
- Armuzzi, A.; Felice, C.; Lubrano, E.; Cantini, F.; Castiglione, F.; Gionchetti, P.; Orlando, A.; Salvarani, C.; Scarpa, R.; Marchesoni, A.; et al. Multidisciplinary management of patients with coexisting inflammatory bowel disease and spondyloarthritis: A Delphi consensus among Italian experts. Dig. Liver Dis. 2017, 49, 1298–1305. [Google Scholar] [CrossRef]
- Bargagli, E.; Bellisai, F.; Mazzei, M.A.; Conticini, E.; Alderighi, L.; Cameli, P.; Biasi, G.; Bergantini, L.; Guerrini, S.; d’Alessandro, M.; et al. Interstitial lung disease associated with psoriatic arthritis: A new disease entity? Intern Emerg. Med. 2020. [Google Scholar] [CrossRef]
- Yoon, K.; Chang, K.T.; Lee, H.J. MRI for Crohn’s disease: present and future. Biomed. Res Int. 2015, 2015, 786802. [Google Scholar] [CrossRef] [Green Version]
- Bruining, D.H.; Bhatnagar, G.; Rimola, J.; Taylor, S.; Zimmermann, E.M.; Fletcher, J.G. CT and MR enterography in Crohn’s disease: Current and future applications. Abdom. Imaging. 2015, 40, 965–974. [Google Scholar] [CrossRef]
- Grassi, R.; Pinto, A.; Mannelli, L.; Marin, D.; Mazzei, M.A. New Imaging in Gastrointestinal Tract. Gastroenterol. Res. Pract. 2016, 2016, 5785871. [Google Scholar] [CrossRef] [Green Version]
- Iacobellis, F.; Berritto, D.; Fleischmann, D.; Gagliardi, G.; Brillantino, A.; Mazzei, M.A.; Grassi, R. CT findings in acute, subacute, and chronic ischemic colitis: Suggestions for diagnosis. Biomed. Res. Int. 2014, 2014, 895248. [Google Scholar] [CrossRef]
- Alabiso, M.E.; Iasiello, F.; Pellino, G.; Iacomino, A.; Roberto, L.; Pinto, A.; Riegler, G.; Selvaggi, F.; Reginelli, A. 3D-EAUS and MRI in the Activity of Anal Fistulas in Crohn’s Disease. Gastroenterol. Res. Pract. 2016, 1895694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzei, M.A.; Guerrini, S.; Cioffi Squitieri, N.; Cagini, L.; Macarini, L.; Coppolino, F.; Melchiore, G.; Volterrani, L. The role of US examination in the management of acute abdomen. Crit. Ultrasound J. 2013, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzei, M.A.; Cioffi Squitieri, N.; Guerrini, S.; Stabile Ianora, A.A.; Cagini, L.; Macarini, L.; Giganti, M.; Volterrani, L. Sigmoid diverticulitis: US findings. Crit. Ultrasound J. 2013, 5 (Suppl. S1), S5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzei, M.A.; Guerrini, S.; Cioffi Squitieri, N.; Imbriaco, G.; Chieca, R.; Civitelli, S.; Savelli, V.; Mazzei, F.G.; Volterrani, L. Magnetic resonance imaging: Is there a role in clinical management for acute ischemic colitis? World J. Gastroenterol. 2013, 19, 1256–1263. [Google Scholar] [CrossRef]
- Mazzei, M.A.; Gentili, F.; Mazzei, F.G.; Grassi, R.; Volterrani, L. Non-occlusive mesenteric ischaemia: CT findings, clinical outcomes and assessment of the diameter of the superior mesenteric artery: Don’t forget the reperfusion process! Br. J. Radiol. 2019, 92, 20180736. [Google Scholar] [CrossRef]
- Zeidler, H.; Amor, B. The Assessment in Spondyloarthritis International Society (ASAS) classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general: The spondyloarthritis concept in progress. Ann. Rheum. Dis. 2011, 70, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Leclerc-Jacob, S.; Lux, G.; Rat, A.C.; Laurent, V.; Blum, A.; Chary-Valckenaere, I.; Peyrin-Biroulet, L.; Loeuille, D. The prevalence of inflammatory sacroiliitis assessed on magnetic resonance imaging of inflammatory bowel disease: A retrospective study performed on 186 patients. Aliment Pharmacol. Ther. 2014, 39, 957–962. [Google Scholar] [CrossRef] [Green Version]
- Bandinelli, F.; Terenzi, R.; Giovannini, L.; Milla, M.; Genise, S.; Bagnoli, S.; Biagini, S.; Annese, V.; Matucci-Cerinic, M. Occult radiological sacroiliac abnormalities in patients with inflammatory bowel disease who do not present signs or symptoms of axial spondylitis. Clin. Exp. Rheumatol. 2014, 32, 949–952. [Google Scholar]
- Gotler, J.; Amitai, M.M.; Lidar, M.; Aharoni, D.; Flusser, G.; Eshed, I. Utilizing MR enterography for detection of sacroiliitis in patients with inflammatory bowel disease. J. Magn. Reason. Imaging. 2015, 42, 121–127. [Google Scholar] [CrossRef]
- Chan, J.; Sari, I.; Salonen, D.; Silverberg, M.S.; Haroon, N.; Inman, R.D. Prevalence of Sacroiliitis in Inflammatory Bowel Disease Using a Standardized CT Scoring System. Arthritis Care Res. 2018, 70, 807–810. [Google Scholar] [CrossRef] [Green Version]
- McEniff, N.; Eustace, S.; McCarthy, C.; O’Malley, M.; O’Morain, C.A.; Hamilton, S. Asymptomatic sacroiliitis in inflammatory bowel disease. Assessment by computed tomography. Clin. Imaging. 1995, 19, 258–262. [Google Scholar] [CrossRef]
- Hwangbo, Y.; Kim, H.J.; Park, J.S.; Ryu, K.N.; Kim, N.H.; Shim, J.; Jang, J.Y.; Dong, S.H.; Kim, B.H.; Chang, Y.W.; et al. Sacroiliitis is common in Crohn’s disease patients with perianal or upper gastrointestinal involvement. Gut Liver 2010, 4, 338–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fauny, M.; Cohen, N.; Morizot, C.; Leclerc-Jacob, S.; Wendling, D.; Lux, G.; Laurent, V.; Blum, A.; Netter, P.; Baumann, C.; et al. Low back pain and sacroiliitis on cross-sectional abdominal imaging for axial spondyloarthritis diagnosis in inflammatory Bowel diseases. Inflamm. Intest. Dis. 2020, 5, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Sostegni, R.; Daperno, M.; Scaglione, N.; Lavagna, A.; Rocca, R.; Pera, A. Crohn’s disease: Monitoring disease activity. Aliment Pharmacol. Ther. 2003, 17, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Lo Re, G.; Cappello, M.; Tudisca, C.; Galia, M.; Randazzo, C.; Craxì, A.; Cammà, C.; Giovagnoni, A.; Midiri, M. CT enterography as a powerful tool for the evaluation of inflammatory activity in Crohn’s disease: Relationship of CT findings with CDAI and acute-phase reactants. Radiol. Med. 2014, 119, 658–666. [Google Scholar] [CrossRef]
- Tanaka, M.; Riddell, R.H.; Saito, H.; Soma, Y.; Hidaka, H.; Kudo, H. Morphologic criteria applicable to biopsy specimens for effective distinction of inflammatory bowel disease from other forms of colitis and of Crohn’s disease from ulcerative colitis. Scand. J. Gastroenterol. 1999, 34, 55–67. [Google Scholar]
- van der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef]
- Maksymowych, W.P.; Inman, R.D.; Salonen, D.; Dhillon, S.S.; Williams, M.; Stone, M. Spondyloarthritis research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum. 2005, 53, 703–709. [Google Scholar] [CrossRef]
- Orchard, T.R.; Holt, H.; Bradbury, L.; Hammersma, J.; McNally, E.; Jewell, D.P.; Wordsworth, B.P. The prevalence, clinical features and association of HLA-B27 in sacroiliitis associated with established Crohn’s disease. Aliment Pharmacol Ther. 2009, 29, 193–197. [Google Scholar] [CrossRef]
- Scott, W.W., Jr.; Fishman, E.K.; Kuhlman, J.E.; Caskey, C.I.; O’Brien, J.J.; Walia, G.S.; Bayless, T.M. Computed tomography evaluation of the sacroiliac joints in Crohn disease. Radiologic/clinical correlation. Skelet. Radiol. 1990, 19, 207–210. [Google Scholar] [CrossRef]
- Paparo, F.; Bacigalupo, L.; Garello, I.; Biscaldi, E.; Cimmino, M.A.; Marinaro, E.; Rollandi, G.A. Crohn’s disease: Prevalence of intestinal and extraintestinal manifestations detected by computed tomography enterography with water enema. Abdom. Imaging. 2012, 37, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.R.; Wordsworth, B.P.; Jewell, D.P. Peripheral arthropathies in inflammatory bowel disease: Their articular distribution and natural history. Gut 1998, 42, 387–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turkcapar, N.; Toruner, M.; Soykan, I.; Aydintug, O.T.; Cetinkaya, H.; Duzgun, N.; Ozden, A.; Duman, M. The prevalence of extraintestinal manifestations and HLA association in patients with inflammatory bowel disease. Rheumatol. Int. 2006, 26, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Steer, S.; Jones, H.; Hibbert, J.; Kondeatis, E.; Vaughan, R.; Sanderson, J.; Gibson, T. Low back pain, sacroiliitis, and the relationship with HLA-B27 in Crohn’s disease. J. Rheumatol. 2013, 30, 518–522. [Google Scholar]
- Peeters, H.; Vander, C.B.; Laukens, D.; Coucke, P.; Marichal, D.; Van Den Berghe, M.; Cuvelier, C.; Remaut, E.; Mielants, H.; De Keyser, F.; et al. Radiological sacroiliitis, a hallmark of spondylitis, is linked with CARD15 gene polymorphisms in patients with Crohn’s disease. Ann. Rheum. Dis. 2004, 63, 1131–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, O.B.; Li, N.; Smith, M.; Chan, J.; Inman, R.D.; Silverberg, M.S. The Prevalence and Clinical Associations of Subclinical Sacroiliitis in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Brakenhoff, L.K.; van der Heijde, D.M.; Hommes, D.W.; Huizinga, T.W.; Fidder, H.H. The joint-gut axis in inflammatory bowel diseases. J. Crohns Colitis. 2010, 4, 257–268. [Google Scholar] [CrossRef] [Green Version]
- Bjarnason, I.; Helgason, K.O.; Geirsson, Á.J.; Sigthorsson, G.; Reynisdottir, I.; Gudbjartsson, D.; Einarsdottir, A.S.; Sherwood, R.; Kristjansson, K.; Kjartansson, Ó.; et al. Subclinical intestinal inflammation and sacroiliac changes in relatives of patients with ankylosing spondylitis. Gastroenterology 2003, 125, 1598–1605. [Google Scholar] [CrossRef]
- Mazzei, M.A.; Volterrani, L. Errors in multidetector row computed tomography. Radiol. Med. 2015, 120, 785–794. [Google Scholar] [CrossRef]
- Casciani, E.; De Vincentiis, C.; Mazzei, M.A.; Masselli, G.; Guerrini, S.; Polettini, E.; Pinto, A.; Gualdi, G. Errors in imaging the pregnant patient with acute abdomen. Abdom. Imaging. 2015, 40, 2112–2126. [Google Scholar] [CrossRef]
- Mandato, Y.; Reginelli, A.; Galasso, R.; Iacobellis, F.; Berritto, D.; Cappabianca, S. Errors in the radiological evaluation of the alimentary tract: Part I. Semin. Ultrasound CT MRI 2012, 33, 300–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reginelli, A.; Mandato, Y.; Solazzo, A.; Berritto, D.; Iacobellis, F.; Grassi, R. Errors in the radiological evaluation of the alimentary tract: Part II. Semin. Ultrasound CT MRI 2012, 33, 308–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzei, M.A.; Contorni, F.; Gentili, F.; Guerrini, S.; Mazzei, F.G.; Pinto, A.; Cioffi Squitieri, N.; Sisinni, A.G.; Paolucci, V.; Romeo, R.; et al. Incidental and underreported pleural plaques at chest CT: Do not miss them-asbestos exposure still exists. Biomed. Res. Int. 2017, 6797826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, A.; Brunese, L.; Pinto, F.; Reali, R.; Daniele, S.; Romano, L. The concept of error and malpractice in radiology. Semin. Ultrasound CT MRI 2012, 33, 275–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, D.; Fossi, A.; Refini, R.M.; Gentili, F.; Luzzi, L.; Voltolini, L.; Paladini, P.; Mazzei, M.A.; Rottoli, P. Posttransplant solid organ malignancies in lung transplant recipients: A single-center experience and review of the literature. Tumori 2016, 102, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Mignarri, A.; Gentili, F.; Masia, F.; Genua, A.; Cenciarelli, S.; Brunori, P.; Mazzei, M.A.; Malandrini, A.; Federico, A.; Mazzei, F.G.; et al. Imaging of the thymus in myotonic dystrophy type 1. Neurol. Sci. 2018, 39, 347–351. [Google Scholar] [CrossRef]
- Mazzei, M.A.; Sartorelli, P.; Bagnacci, G.; Gentili, F.; Sisinni, A.G.; Fausto, A.; Mazzei, F.G.; Volterrani, L. Occupational Lung Diseases: Underreported Diagnosis in Radiological Practice. Semin Ultrasound CT MRI 2019, 40, 36–50. [Google Scholar] [CrossRef]
- Guler, E.; Unal, N.G.; Hekimsoy, I.; Kose, T.; Harman, M.; Ozutemiz, A.O.; Elmas, N.Z. Dual-energy CT enterography in evaluation of Crohn’s disease: The role of virtual monochromatic images. Jpn. J. Radiol. 2020. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, S.H.; Ahn, S.J.; Kang, H.J.; Kang, J.H.; Han, J.K. Virtual monoenergetic dual-layer, dual-energy CT enterography: Optimization of keV settings and its added value for Crohn’s disease. Eur. Radiol. 2018, 28, 2525–2534. [Google Scholar] [CrossRef]
- Mazzei, M.A.; Gentili, F.; Volterrani, L. Dual-Energy CT Iodine Mapping and 40-keV Monoenergetic Applications in the Diagnosis of Acute Bowel Ischemia: A Necessary Clarification. AJR Am. J. Roentgenol. 2019, 212, W93–W94. [Google Scholar] [CrossRef]
- Biondi, M.; Vanzi, E.; De Otto, G.; Banci Buonamici, F.; Belmonte, G.M.; Mazzoni, L.N.; Guasti, A.; Carbone, S.F.; Mazzei, M.A.; La Penna, A.; et al. Water/cortical bone decomposition: A new approach in dual energy CT imaging for bone marrow oedema detection. A feasibility study. Phys. Med. 2016, 32, 1712–1716. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzei, M.A.; Gentili, F.; Guerrini, S.; Di Meglio, N.; Lo Re, G.; Carotti, M.; Interlicchia, F.; Reginelli, A.; Barile, A.; Sadotti, G.; et al. Inflammatory Bowel Diseases and Coexisting Spondyloarthritis: A Neglected and too Often Under-Reported Association by Radiologists. A Multicenter Study by Italian Research Group of Imaging in Rheumatology. Gastroenterol. Insights 2020, 11, 47-57. https://doi.org/10.3390/gastroent11020008
Mazzei MA, Gentili F, Guerrini S, Di Meglio N, Lo Re G, Carotti M, Interlicchia F, Reginelli A, Barile A, Sadotti G, et al. Inflammatory Bowel Diseases and Coexisting Spondyloarthritis: A Neglected and too Often Under-Reported Association by Radiologists. A Multicenter Study by Italian Research Group of Imaging in Rheumatology. Gastroenterology Insights. 2020; 11(2):47-57. https://doi.org/10.3390/gastroent11020008
Chicago/Turabian StyleMazzei, Maria Antonietta, Francesco Gentili, Susanna Guerrini, Nunzia Di Meglio, Giuseppe Lo Re, Marina Carotti, Francesca Interlicchia, Alfonso Reginelli, Antonio Barile, Giulia Sadotti, and et al. 2020. "Inflammatory Bowel Diseases and Coexisting Spondyloarthritis: A Neglected and too Often Under-Reported Association by Radiologists. A Multicenter Study by Italian Research Group of Imaging in Rheumatology" Gastroenterology Insights 11, no. 2: 47-57. https://doi.org/10.3390/gastroent11020008
APA StyleMazzei, M. A., Gentili, F., Guerrini, S., Di Meglio, N., Lo Re, G., Carotti, M., Interlicchia, F., Reginelli, A., Barile, A., Sadotti, G., Plastina Romeo, U., La Paglia, E., Maggialetti, N., Lo Scalzo, R., Vinci, A., Capodieci, G., Vacca, G., Bruno, F., Cantarini, L., ... Brunese, L. (2020). Inflammatory Bowel Diseases and Coexisting Spondyloarthritis: A Neglected and too Often Under-Reported Association by Radiologists. A Multicenter Study by Italian Research Group of Imaging in Rheumatology. Gastroenterology Insights, 11(2), 47-57. https://doi.org/10.3390/gastroent11020008







