Abstract
The consequences of inflammatory bowel disease (IBD) in children are connected to possible detrimental impacts on growth, development, psychosocial function, and general well-being. Therefore, the primary management plan in pediatric IBD is to achieve the long-term control of intestinal inflammation while also monitoring potential disease complications and therapeutic adverse effects, where nutritional management is of utmost importance. This review explores the role of dietary supplements as concentrated sources of nutrients with nutritional and/or physiological effects on children with IBD. While dietary supplements are commonly used in pediatric IBD management, their efficacy and, for some of them, safety remain subjects of debate. We provide an overview of the types of dietary supplements available and their potential benefits and risks in pediatric IBD patients. Additionally, we discuss the evidence supporting the use of specific supplements, their mechanisms of action, and considerations for clinical practice. Understanding the role of dietary supplements in pediatric IBD management is crucial for optimizing patient care and outcomes.
1. Introduction
Inflammatory bowel disease (IBD) is a group of inflammatory conditions of the digestive tract with unknown etiology and a chronic relapsing course [1]. The implications of IBD are significant in children due to the possible detrimental impacts on growth, development, psychosocial function, and general well-being. The primary clinical management plan is to achieve long-term control of intestinal inflammation while monitoring for potential disease complications and adverse effects of the medicine treatment [1]. Overall, there is less information on the care of IBD in children than in adults, although some high-quality multicenter studies, guidelines, and society consensus statements are available [2].
The incidence and prevalence of IBD are rising, with 20–30% of cases occurring before the age of 20 [3,4]. Childhood IBD, usually referred to as development between 1 and 18 years of age, is associated with more widespread disease, increased disease activity, and a more convoluted course than adult-onset IBD [5]. The average time from onset to diagnosis for new onset pediatric IBD is 11 months. Prior to 2000, anti-inflammatory medications and corticosteroids were the primary treatments, but armamentarium has since been extended to include immunomodulators and biologics [6].
As our understanding of IBD’s genotypic–phenotypic characteristics grows, so will personalized treatment for pediatric individuals. Shortening the time to diagnosis and treatment protocols should result in better clinical results. The management goal should be to control intestinal inflammation and optimize nutrition, growth, and quality of life (QOL) while avoiding disease or treatment-related problems [7].
However, the treatment of IBD is complex and requires a multidisciplinary approach, including the participation of a nutritionist. Although there is much speculation about the role of specific supplements in the treatment of patients with IBD, its importance in general should not be overlooked [1]. In line with this, there are many nutritional concerns in pediatric patients with IBD, starting with delayed growth and pubertal development [8].
Consequences of IBD include deficient meal intake due to fear of symptom aggravation, loss of nutrients due to impaired intestinal mucosal functions, increased nutritional requirement and chronic catabolic status, pharmacologic treatment (i.e., corticosteroids), which can inhibit the insulin-like growth factor-I (IGF-I), and disease activity with increased proinflammatory cytokine production which depresses linear growth, defined as a child’s change in length between two time points divided by the number of months between the two time points (cm per month). These are the main reasons why delayed linear growth is more common in IBD, especially Crohn’s disease (CD) [9].
Based on this background, this review aims to give comprehensive and up-to-date data on the importance of nutrition and food in pediatric IBD, with a focus on the supplements available for nutritional disease management.
2. Nutritional Concerns in Children with IBD
Poor nutrition, disease severity, genotype, and cytokines (i.e., TNF-α, IL-6, IL-1β2, IL-6, IL-17, IL-23) are the primary players in IBD’s detrimental effects on children’s health [10]. Proinflammatory cytokines released from inflammation sites interfere with patients’ linear growth. TNF-α is a critical cytokine that causes growth retardation. The effect on growth is more aggressive as the condition progresses and worsens. Anti-TNFα medication has been shown to increase patient growth and address clinical issues [11]. In addition, cytokines contribute to chronic inflammation in the gastrointestinal (GI) tract, worsening the nutrition and well-being of children with IBD.
Diagnosing IBD before full growth (defined as the end of growth when the child reaches a genetically predetermined final height) causes the patient to have a usually weak appetite due to GI discomfort, avoid eating, and suffer from nutritional loss through GI mucosa despite adequate nutrient intake [8]. Additionally, protein-losing enteropathy, mucosal bleeding, fistula, and hepatopathy all contribute to low protein levels during the active stage of CD. Chronic mucosal hemorrhage produces iron shortage, and severe diarrhea causes electrolyte (potassium, magnesium) loss and a zinc deficit, resulting in further GI problems such as worsened diarrhea, impaired immunity, and poor mucosal integrity [12].
Additionally, pediatric IBD is also characterized by increased energy requirements and decreased energy expenditure (especially in the active state of the disease) and growth hormone (GH) resistance (related to acquired GH resistance at the GH receptor), leading to reduced lean body mass associated with decreased IGF-I [13]. We have to emphasize that studies confirm that children with IBD show reduced lean body mass and reduced muscle mass compared to healthy children. Therefore, nutrient deficiencies can occur in children with lean body mass both with and without IBD, though the mechanisms may differ [14,15].
Nevertheless, corticosteroid medication may directly cause low growth when administered for an extended period [8]. Glucocorticoids directly inhibit the chondrocytes of the bone development plate. However, short-term therapy is not directly associated with growth retardation. The patient is already below the standard height percentile for their age at the time of initial IBD diagnosis. However, disease complications such as fistulas are not related to poor growth [8].
Inflammation in the small intestine causes poor digestion and absorption of meals. As poorly digested food travels into the colon, diarrhea and nutritional loss worsen [16]. This is why CD has a higher incidence of malnutrition and growth retardation than ulcerative colitis (UC). Because the UC lesion site is restricted to the colon and the patient has normal small intestine mucosa, malnutrition is less severe than in CD. However, if the lesions are more extensive and profound, diarrhea is exacerbated when water is reabsorbed into the colon. For these reasons, malnutrition is common in IBD patients, particularly CD patients, because various areas are impacted in comparison to UC (small bowel versus colon–rectum disorders). The pathophysiology of malnutrition is diverse, comprising numerous components, including (i) anorexia, food avoidance, and self-imposed hypocaloric diets, and (ii) increased energy and nutritional losses as a result of malabsorption and gastrointestinal losses [16,17]. Data on the prevalence of malnutrition in IBD children are broadly accessible among newly diagnosed patients, accounting for around 60% of newly diagnosed CD children and 35% of UC [17].
For all these reasons, it is essential to address the nutritional status of children with IBD during their management and follow-up.
3. Nutritional Management of Pediatric IBD Patients
Several studies have been undertaken to determine the precise function of nutrition (including malnutrition) and food composition in immune-mediated illness risks, forming a new profession known as “clinical nutrition” [18]. Clinical nutrition also refers to the effect of diet on the overall health of the consumer.
The European Society of Clinical Nutrition and Metabolism (ESPEN) defines clinical nutrition as “the discipline that deals with the prevention, diagnosis, and management of nutritional and metabolic changes related to acute and chronic diseases and conditions caused by a lack or excess of energy and nutrients” [19].
Moreover, scientific data support the relationship between diet composition and the onset, course, and management of IBD [20,21]. Among them, (i) epidemiological studies have found an association between specific dietary habits and nutrients and an increased risk of IBD; (ii) some foods and dietary components have the potential to either enhance or reduce the severity of inflammation; (iii) for some pediatric patients with an established diagnosis of CD, exclusive enteral nutrition can be considered a primary induction treatment with efficacy in achieving mucosal healing. (iv) exclusion diets could treat or prevent disease flares; (v) in IBD children, especially in CD children, malnutrition and nutrient deficits are frequently evident at diagnosis; and (vi) early nutritional measures can lead to better disease control, as well as catch-up growth, bone mineral density increase, and proper pubertal development [20,21,22].
Therefore, the ultimate goals of nutritional concern in children with IBD are as follows: (1) improving nutritional status, (2) reducing disease activity, (3) reducing surgery complications and preventing postoperative complications, and (4) correcting pubertal growth retardation [8]. Aside from that, the non-pharmacological care of juvenile IBD has evolved, and dietary alterations are now regarded as critical therapeutic interventions [22,23,24]. A nutritional assessment is an essential component in the management of IBD patients because a poor nutritional status is strongly linked to growth failure, poor bone accrual, anemia, disrupted pubertal development over time, short stature in adulthood, increased complication rates, and poor prognosis [25,26,27].
The recommended approach for IBD patients should include (i) a periodic dietary history through a 3- to 5-day record of qualitative and quantitative food intake (macro and micronutrients) and (ii) the evaluation of nutritional status with assessments of weight-for-age, height-for-age, and BMI z-scores at each visit, and height velocity every 6 months [25,26].
Nutritional management may start before the diagnosis of IBD since some meals can intensify the symptoms of the patients without knowing that they have a medical condition, but this does not necessarily mean that the condition is worsening. To determine the current disease activity and nutritional condition, physicians must examine weight and height and monitor the aforementioned laboratory tests [27]. Therefore, nutritional management includes a balanced diet with proteins, fats, micronutrients, and minerals.
Micronutrients and vitamins are typically deficient in IBD due to the malabsorption caused by inflammation and/or suboptimal nutritional intake. Micronutrient deficiency can be present at diagnosis and develop during the clinical course of the disease. Hence, IBD patients should frequently be examined for micronutrient levels, and specific shortfalls should be promptly treated [27,28,29]. In line with this, an appropriate diet can reduce the chance of IBD flares, while an age-appropriate nutritional status can reduce the risk of problems and surgery later in the disease’s course. However, supplementation could benefit IBD pediatric patients due to a high incidence of micronutrient deficiency [30].
According to Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002, food supplements are concentrated sources of nutrients or other substances with a nutritional or physiological effect to supplement a standard diet. They can correct dietary deficiencies or maintain an adequate intake of certain nutrients [31]. Food supplements include vitamins, minerals, fibers, fatty acids, amino acids, etc. [32].
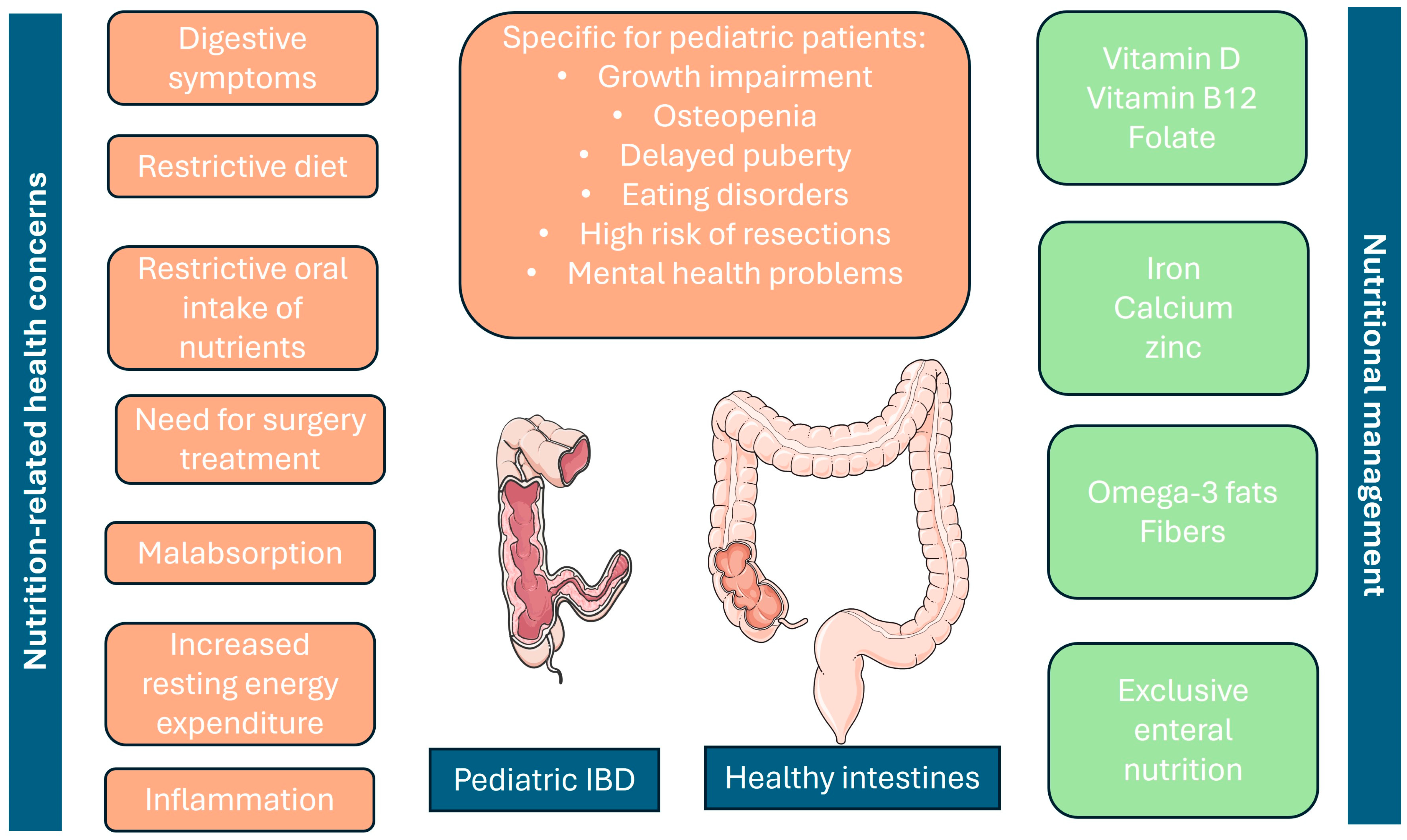
In Figure 1, we present the main nutritional concerns regarding pediatric patients with IBD and how they can be managed.
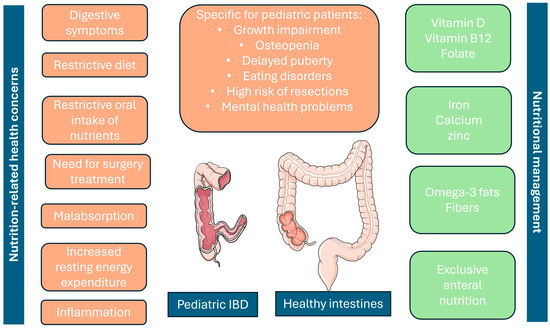
Figure 1.
Nutrition-related concerns in pediatric IBD patients (on the left) and nutritional management options, including supplements (on the right). Additionally, the most distinctive features of pediatric IBD are presented in the middle, paying attention to the fact that they are also significantly related to poor nutrition in children. Parts of the figure were adapted from Servier Medical Art, which is licensed under CC BY 4.0, https://creativecommons.org/licenses/by/4.0/ Accessed on 25 July 2024.
4. Vitamin Supplements for Children with IBD
4.1. Vitamin D
Vitamin D occurs in nature in four primary forms. Ergocalciferol (vitamin D2) is synthesized by ultraviolet radiation from ergosterol in plants. Cholecalciferol (vitamin D3) is synthesized under the action of ultraviolet radiation from 7-dehydrocholesterol in the skin and then is hydroxylated in the liver to its main circulatory form 25-hydroxyvitamin D (25(OH)D) (calcidiol) and in the kidneys to its physiologically active form 1,25-dihydroxyvitamin D (1,25(OH)2D) (calcitriol) [33]. Calcitriol binds to the vitamin D receptor (VDR), stimulating the transcription of vitamin D-dependent genes. The wide occurrence of the vitamin D receptor in the body, including in the colon, small intestine, bones, breast, pancreas, brain, pituitary gland, and muscles, can also explain the growing number of diseases associated with vitamin D deficiency [34].
Vitamin D is a recognized regulator of the immune system. In recent years, several studies have demonstrated a link between the supply of vitamin D through ultraviolet light or diet and the occurrence of immune-mediated diseases, including IBD [35]. According to some authors, the existing north–south gradient in IBD is due to the reduced ultraviolet exposure north of the equator and the reduced synthesis of vitamin D in the body. Vitamin D is essential for maintaining intestinal homeostasis, which is impaired in IBD. It enhances the expression of tight junction proteins in intestinal epithelial cells, thereby reducing intestinal permeability and the entry of bacterial antigens into the lamina propria. This, in turn, reduces the preconditions for inflammation at the intestinal mucosa level and has a beneficial effect if inflammation already exists.
In addition, calcitriol reduces the production of proinflammatory cytokines, such as IL-1, IL-2, IL-5, IL-6, IL-8, IL-17, INF-gamma, and TNF-alpha, which are involved in the pathogenesis of IBD [36]. In addition to its anti-inflammatory effect, which results in a reduction in disease activity, the use of vitamin D in patients with IBD is associated with some other benefits [35,36]. Patients with IBD usually have decreased bone density, both primarily as a result of the disease itself and secondarily as a result of corticosteroid treatment. Vitamin D intake has a beneficial effect on this symptom. Depression and low mood are common in patients with IBD. As some studies have linked vitamin D deficiency to an increased predisposition to the development of depression and mood swings, it is believed that its prophylactic use can have a beneficial effect on this complaint.
Last but not least, vitamin D intake reduces the risk of developing colorectal cancer (CRC), which in patients with ulcerative colitis (UC) is twice as high as in the general population. Furthermore, studies show that higher circulating 25(OH)D levels are associated with a minimized risk of CRC and improved survival [37]. If we take this observation into pediatric IBD, it is far more crucial to avoid vitamin D deficiency in children with IBD since their risk of CRC development is already higher.
The recommended vitamin D supplementation in pediatric patients with IBD is up to 1000 IU/day. Higher doses require close monitoring because of the risk of toxicity [36].
4.2. Vitamin B12
Vitamin B12 is a water-soluble vitamin involved in the formation and regeneration of red blood cells, the normal functioning of the nervous system, and the metabolism of proteins, fats, and carbohydrates. As vitamin B12 is absorbed in the ileum, patients with Crohn’s disease with the isolated involvement of the terminal ileum (L1), ileocolonic form (L3), or surgical resection may suffer from vitamin B12 deficiency [38]. In such patients, prophylaxis with vitamin B12 is recommended, and the dose depends on the form of administration used.
ESPGHAN recommends that either serum cobalamin or methylmalonic acid levels in blood or urine can be assessed in children with active ileal CD, children with an ileal resection of >20 cm, and UC children who undergo ileal pouch surgery at least annually (EL 4) [25]. The position paper also defines the group at greatest risk for vitamin B12 deficiency: CD patients with terminal ileal resection of >20 cm. Additionally, patients with distal ileal resection of >60 cm require lifelong B12 supplementation.
In the event of proven vitamin B12 deficiency, it is recommended to start timely treatment with the venous form of this vitamin. In the absence of neurological symptoms, the dose for the pediatric population is 250 to 1000 μg cyanocobalamin every other day for 2 weeks. The treatment should continue with a dose of 250 μg/week until blood counts return to normal, after which a maintenance dose of 1000 μg/month should be administered. In the presence of neurological symptoms, treatment should start with 1000 μg cyanocobalamin every other day until there is a change in clinical presentation and laboratory parameters. Then, it should continue with a maintenance dose of 1000 μg/month [38,39].
4.3. Folate/Folic Acid
Folic acid is a water-soluble vitamin of group B (B9). The naturally occurring form of vitamin B9, folate, and its active form, 5-methyltetrahydrofolate (5-MTHF), is necessary for cell division and growth and involves several metabolic processes. During pregnancy, folic acid reduces the risk of congenital abnormalities in the fetus and the risk of premature birth and is especially important for the condition of the placenta. Some of the drugs used to treat IBD disrupt folic acid absorption. Therefore, an 800 µg to 1 mg/day supplementation is recommended in all patients treated with sulfasalazine or methotrexate, including children. The folic acid supplementation dose in pregnant women is 5 mg/day [39,40,41,42].
Although in adults, folic acid deficiency has been estimated in 92% of CD patients and 94% of UC patients [38], in the pediatric population, 10–13% of CD and 3.8–9.7% of UC patients suffer vitamin B9 deficiency [43].
Interestingly, Heyman et al. demonstrated higher levels of folate in newly diagnosed untreated IBD children than in healthy children, indicating that IBD treatment strongly influences folate metabolism and causes long-term clinical implications regarding folate concentrations [44].
Further, Temtem et al., in their single-arm observational study with 2–21-year-IBD patients, showed that once-weekly folic acid supplementation at a dose similar to that seen in a multivitamin may be sufficient to maintain normal folate levels without the development of adverse effects in juvenile patients with IBD on methotrexate [45].
4.4. Other Vitamins
Other vitamins, such as vitamins A, B1, B6, E, and K, are also discussed in pediatric patients with IBD. However, studies show that their supplementation is of benefit for these patients if elevated dosages are administered to make up for deficiencies in absorptive processes or signaling pathways adversely impacted by the inflammatory process in order to restore normal levels of the chosen vitamins and minerals [46,47].
We include summarized information on vitamins in Table 1 [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50].

Table 1.
Vitamins in pediatric IBD.
5. Mineral Supplements for Pediatric IBD
5.1. Calcium
Calcium supplements are recommended in all patients with IBD with a history of calcium deficiency, low bone density, or those who have received corticosteroid therapy. The recommended daily intake, children included, is 1200–1500 mg/day, divided into 2–3 doses. It is essential to remember that the body cannot absorb more than 500–600 mg of calcium at a time and that vitamin D is critical for calcium absorption [47].
Due to insufficient data, the position paper of ESPGHAN does not recommend a specific dose for calcium supplementation but suggests following the European Food Safety Authority’s (EFSA’s) recommendations for the general pediatric population (i.e., 450–1150 mg) [25].
5.2. Iron
Iron deficiency is a common condition in patients with IBD. Its etiology is different. This may be due to chronic blood loss from the ulcerated intestinal mucosa, impaired iron absorption in the duodenum, or chronic inflammation. Regardless of the cause, iron supplementation at a dose of 6 mg/kg/day for 2–3 months is recommended in all patients (including pediatric) with IBD and iron deficiency. Oral iron therapy has been demonstrated to change the intestinal microbiome and may worsen intestinal inflammation. Due to the known proinflammatory effect of oral forms, intravenous administration is preferred. Liquid medications are preferred if oral forms should be used [47,50].
Furthermore, high-molecular-weight iron dextran, the first generation of IV iron products, has been linked to severe allergic reactions and should not be administered, whereas the safety profiles of the second generation of IV iron products—such as ferric carboxymaltose and iron sucrose—are superior [25].
5.3. Zinc
Zinc deficiency is common in patients with Crohn’s disease (CD), especially in those with the more common form of the disease, which involves the small intestine. It is clinically manifested by rashes, changes in taste, smell, and vision, and difficulty healing wounds. Supplementation at a dose of 5–10 mg/day is recommended [47,51].
We present the summarized information in Table 2, adding information for magnesium, where the data on pediatric IBD are scarce [25,47,50].

Table 2.
Mineral nutrients in pediatric patients with IBD.
6. Other Supplements under Investigation for Pediatric IBD Nutritional Management
The supplementation mentioned above was validated and approved for clinical use in children with IBD. However, other supplements are being explored for the pediatric IBD population, where safety and efficacy should be further assessed.
6.1. Omega-3 Fatty Acids
Omega-3 fatty acids are essential polyunsaturated fatty acids that play an important role in some biochemical processes in the human body. There are over 10 different types of omega-3 fatty acids, but three of them are the most important: alpha-linolenic, eicosapentaenoic, and docosahexaenoic acid. Omega-3 fatty acids have anti-inflammatory properties [52,53]. They reduce the synthesis of proinflammatory eicosanoids (arachidonic acid), reduce the synthesis of proinflammatory cytokines (TNF-alpha, IL-1ß, IL-6, IL-8), and reduce leukocyte chemotaxis and T-cell activity [54]. In this regard, it is considered that their use in patients with IBD could be beneficial. Unfortunately, evidence from studies evaluating the role of omega-3 fatty acids in inducing and maintaining remission in patients with IBD is contradictory. For this reason, no specific recommendations can be made in this regard, but their role as a form of adjuvant therapy in the treatment of IBD is indisputable [54].
6.2. Boswellia serrata
Boswellia is an herbal extract taken from the Boswellia serrata tree. Resin from the bark of this tree is purported to have anti-inflammatory properties derived primarily from the two boswellia acids, namely 11-keto-β-boswellic acid and acetyl-11-keto-β-boswellic acid [55]. The Boswellia acids are specific, non-redox, and noncompetitive inhibitors of 5-lipoxygenase, the key enzyme of leukotriene biosynthesis, and this is the primary mechanism for their anti-inflammatory properties. Clinical studies in adults have suggested that Boswellia serrata resin could be effective in IBD. Gupta et al. evaluated the utility of Boswellia in inducing remission in patients who have UC compared to sulfasalazine. The study showed Boswellia to be similar to sulfasalazine in the UC remission rate [56]. A non-inferiority study by Gerhardt et al. of 102 patients with CD concluded that therapy with the Boswellia serrata extract H15 is not inferior to mesalazine, and considering both the safety and efficacy of Boswellia serrata extract H15, it appears to be superior to mesalazine in terms of a benefit–risk evaluation [57].
6.3. Curcumin
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is a natural polyphenol found in the rhizome of curcuma longa (turmeric) and in another curcuma spp. [58]. Curcumin has a broad spectrum of pharmacologic actions, including anti-inflammatory, antioxidant, and anti-tumor effects [59]. The anti-inflammatory action of curcumin is caused by the blockade of the transcriptional nuclear factor-kappa B (NF-kappa B) with the subsequent inhibition of tumor necrosis factors, IL-12 and IL-2 [59,60,61,62]. Studies have shown curcumin to be a natural anti-inflammatory agent that may benefit IBD patients [63,64]. A pilot study in adult patients with UC and CD demonstrated the improvement of symptoms and reductions in concomitant medications with an oral intake of 550 mg of curcumin three times a day [65].
Another study by Hanai et al. showed curcumin as a promising and safe medication for remission in patients with quiescent UC. In this study, the oral intake of 1 g of curcumin for 6 months significantly improved the clinical activity and endoscopic indexes compared to the placebo [66]. In a pilot pediatric study in 11 patients with UC or CD with a forced dose titration design, curcumin was shown to be well tolerated at all study doses, causing a lowering of the PCDAI/PUCAI score in three of the study participants [67].
6.4. Butyric Acid
Butyric acid is one of the short-chain fatty acids produced by the colonic microflora (microbiota) during the fermentation of digestible fiber, such as cereal flour, inulin, and psyllium [68]. Butyric acid has well-known anti-inflammatory effects by inhibiting NF-κB, which reduces the expressions of cytokine genes, including TNFα, IL1β, IL2, IL6, IL8, and IL12 [69]. Butyric acid has also been shown to stimulate mucin production through an increased expression of mucin genes, such as MUC2, and to stimulate the physiological proliferation of normal colonocytes [68,70]. The anti-inflammatory properties and the ability to strengthen the wall of the intestinal cells make butyric acid a promising therapeutic agent, particularly in patients with UC—most patients with UC ulcerative colitis have an increased intestinal wall permeability, a “leaky gut” and a reduced thickness of the mucosal barrier [71].
Several studies evaluate the use of butyric acid as mono- or adjuvant therapy in IBD patients. They have several limitations, including the fact that not all are randomized, the small numbers of patients studied, the different methods of butyric acid administration (oral/enema), and the various criteria for endpoint evaluation, but in summary, all of them report that the use of butyric acid has beneficial effects for the treatment of IBD [72,73,74,75,76,77,78,79].
Table 3 presents the main groups of supplements for pediatric IBD and their main benefits [52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79].

Table 3.
Main groups of supplements and their effects and benefits for pediatric patients with IBD.
6.5. Cannabidiol (CBD) Oil
A review by Nduma et al. (2023) [80] on the use of cannabinoids in the treatment of IBD demonstrated promising outcomes, as reported in most of the chosen studies, which included decreased clinical complications measured by weight gain, an improved patient perception of their health, reduced clinical complications, as measured by Mayo scores, Crohn’s Disease Activity Index (CDAI) score, the Lichtiger Index, Harvey–Bradshaw Index, or general well-being. However, the use of cannabinoids is still debatable because robust data have not yet emerged, particularly when it comes to dosing and delivery methods [80].
However, when we speak of cannabidiol for children with IBD, there is “still a long way to go”, according to Halbmeijer et al. [81]. Although the role of the endocannabinoid system within the GI tract is widely researched, there is a lack of studies to determine the safety and effectiveness of cannabis for pediatric IBD, as well as to define the proper cannabis modalities, their components, applications, and forms.
Valuable information for cannabinoid uses in IBD is still scarce. Survey-based studies have shown that several cannabis products improve appetite, nausea, and abdominal discomfort for the symptomatic control of IBD. However, they may also increase craving, tolerance, dizziness, and drowsiness [82,83,84,85].
Hoffenberg et al. asked several questions while discussing the use of cannabinoids in pediatric IBD: “Is it beneficial? What are the risks? How should we evaluate special considerations in IBD? Do our IBD patients use cannabis differently than those who use it for recreational use? Are IBD patients at greater risk for addiction (i.e., combined use with narcotics after surgery or for chronic pain)? What impact does intestinal inflammation or dysmotility have on the absorption of edibles? And how do the chemicals in cannabis affect other medications used to treat IBD?” [83]. In conclusion, due to an incorrect public perception of the safety of the regular use of cannabis, despite some possible immune-modifying effects of cannabis on IBD, the benefits and significant risks need to be better considered.
7. Probiotics in Pediatric IBD
The ESPGHAN position paper clearly states that due to a limited number of high-quality studies on the effect of probiotics in pediatric IBD, data are limited—only 3 RCTs (2 in UC and 1 in CD). Some extrapolations can be made from adult data, but they can hardly be applied to the pediatric population [86,87,88].
Amon these statements are the following: 1. VSL#3 or Lactobacillus reuteri ATCC 55730 can be used as an adjuvant to standard therapy for the induction of remission in mild-to-moderate pediatric UC (EL 2); 2. VSL#3 or Escherichia coli Nissle can be used as an alternative to 5-ASA therapy in the maintenance of remission in mild-to-moderate pediatric UC, especially in mesalazine (5-ASA) intolerance (EL 2); and 3. VSL#3 has shown efficacy for maintaining antibiotic-induced remission in pouchitis and for preventing it in adults (adult EL 2; pediatric EL 5). It was clearly stated that results from clinical trials are strain-specific and should not be extrapolated to other bacterial strains [25].
Moreover, ESPGHAN does not recommend the use of probiotics in the induction or in the maintenance of remission in pediatric CD (EL 2). Additionally, as a general rule, probiotics should be used with caution in immunocompromised patients and those with a central venous catheter [25].
8. Conclusions
In conclusion, dietary supplements could serve as concentrated sources of nutrients with potential nutritional or physiological effects in children with IBD. Their use is widespread in pediatric IBD management, and their efficacy and safety profiles are intensively researched since the nutritional management of pediatric IBD is of the utmost importance for patient well-being. Moving forward, further research is needed to elucidate the optimal role of dietary supplements in pediatric IBD management, ensuring personalized and evidence-based approaches to improve nutritional status and overall outcomes in affected children.
Author Contributions
Conceptualization, R.S. and T.V.; investigation, A.M.; resources, T.V.; data curation, R.S.; writing—original draft preparation, R.S. and T.V.; writing—review and editing, A.M.; visualization, T.V.; supervision, T.V.; project administration, A.M.; funding acquisition, T.V. All authors have read and agreed to the published version of the manuscript.
Funding
This study is financed by the European Union-NextGenerationEU through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No BG-RRP-2.004-0008.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Oliveira, S.B.; Monteiro, I.M. Diagnosis and management of inflammatory bowel disease in children. BMJ 2017, 357, j2083. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, W.S.; Arai, K.; Alex, G.; Treepongkaruna, S.; Kim, K.M.; Choong, C.L.; Mercado, K.C.; Darma, A.; Srivastava, A.; Aw, M.M.; et al. Management and monitoring of pediatric inflammatory bowel disease in the Asia-Pacific region: A position paper by the Asian Pan-Pacific Society for Pediatric Gastroenterology, Hepatology, and Nutrition (APPSPGHAN) PIBD Working Group: Surgical management, disease monitoring, and special considerations. J. Gastroenterol. Hepatol. 2023, 38, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Pigneur, B.; Seksik, P.; Viola, S.; Viala, J.; Beaugerie, L.; Girardet, J.-P.; Ruemmele, F.M.; Cosnes, J. Natural history of Crohn’s disease: Comparison between childhood- and adult-onset disease. Inflamm. Bowel Dis. 2010, 357, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Buderus, S.; Scholz, D.; Behrens, R.; Classen, M.; De Laffolie, J.; Keller, K.M.; Zimmer, K.P.; Koletzko, S. Inflammatory bowel disease in pediatric patients: Characteristics of newly diagnosed patients from the CEDATA-GPGE Registry. Dtsch. Arztebl. Int. 2015, 357, 121–127. [Google Scholar]
- Benchimol, E.I.; Fortinsky, K.J.; Gozdyra, P.; Van den Heuvel, M.; Van Limbergen, J.; Griffiths, A.M. Epidemiology of pediatric inflammatory bowel disease: A systematic review of international trends. Inflamm. Bowel Dis. 2011, 357, 423–439. [Google Scholar] [CrossRef]
- Légeret, C.; Furlano, R.; Köhler, H. Therapy Strategies for Children Suffering from Inflammatory Bowel Disease (IBD)-A Narrative Review. Children 2022, 9, 617. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Day, A.S.; Ledder, O.; Leach, S.T.; Lemberg, D.A. Crohn’s and colitis in children and adolescents. World J. Gastroenterol. 2012, 357, 5862–5869. [Google Scholar] [CrossRef]
- Kim, Y.J. Nutritional concerns in pediatric inflammatory bowel disease. Korean J. Pediatr. 2016, 59, 247–251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Vortia, E.; Kay, M.; Wyllie, R. The role of growth hormone and insulin-like growth factor-1 in Crohn’s disease: Implications for therapeutic use of human growth hormone in pediatric patients. Curr. Opin. Pediatr. 2011, 23, 545–551. [Google Scholar] [CrossRef]
- Shamir, R.; Phillip, M.; Levine, A. Growth retardation in pediatric Crohn’s disease: Pathogenesis and interventions. Inflamm. Bowel Dis. 2007, 13, 620–628. [Google Scholar] [CrossRef]
- Malik, S.; Ahmed, S.F.; Wilson, M.L.; Shah, N.; Loganathan, S.; Naik, S.; Bourke, B.; Thomas, A.; Akobeng, A.K.; Fagbemi, A.; et al. The effects of anti-TNF-α treatment with adalimumab on growth in children with Crohn’s disease (CD). J. Crohns Colitis 2012, 6, 337–344. [Google Scholar] [CrossRef]
- Gavin, J.; Anderson, C.E.; Bremner, A.R.; Beattie, R.M. Energy intakes of children with Crohn’s disease treated with enteral nutrition as primary therapy. J. Hum. Nutr. Diet. 2005, 18, 337–342. [Google Scholar] [CrossRef]
- Smith, W.J.; Underwood, L.E.; Clemmons, D.R. Effects of caloric or protein restriction on insulin-like growth factor-I (IGF-I) and IGF-binding proteins in children and adults. J. Clin. Endocrinol. Metab. 1995, 80, 443–449. [Google Scholar]
- Burnham, J.; Shults, J.; Semeao, E.; Foster, B.J.; Zemel, B.S.; Stallings, V.A.; Leonard, M.B. Body-composition alterations consistent with cachexia in children and young adults with Crohn disease. Am. J. Clin. Nutr. 2005, 82, 413–420. [Google Scholar] [CrossRef]
- Werkstetter, K.J.; Ullrich, J.; Schatz, S.B.; Prell, C.; Koletzko, B.; Koletzko, S. Lean body mass, physical activity and quality of life in paediatric patients with inflammatory bowel disease and in healthy controls. J. Crohns Colitis 2012, 6, 665–673. [Google Scholar] [CrossRef]
- Gavin, J.; Ashton, J.J.; Heather, N.; Marino, L.V.; Beattie, R.M. Nutritional support in pediatric Crohn’s disease: Outcome at 12 months. Acta Paediatr. 2018, 107, 156–162. [Google Scholar] [CrossRef]
- Gerasimidis, K.; McGrogan, P.; Edwards, C.A. The aetiology and impact of malnutrition in paediatric inflammatory bowel disease. J. Hum. Nutr. Diet. 2011, 24, 313–326. [Google Scholar] [CrossRef]
- Cucinotta, U.; Romano, C.; Dipasquale, V. Diet and Nutrition in Pediatric Inflammatory Bowel Diseases. Nutrients 2021, 13, 655. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef]
- Schreiner, P.; Martinho-Grueber, M.; Studerus, D.; Vavricka, S.R.; Tilg, H.; Biedermann, L. On behalf of Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology Nutrition in Inflammatory Bowel Disease. Digestion 2020, 101, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Forbes, A.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef]
- Levine, A.; Sigall Boneh, R.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef]
- Khalili, H.; Chan, S.S.; Lochhead, P.; Ananthakrishnan, A.N.; Hart, A.R.; Chan, A.T. The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 525–535. [Google Scholar] [CrossRef]
- Miele, E.; Shamir, R.; Aloi, M.; Assa, A.; Braegger, C.; Bronsky, J.; De Ridder, L.; Escher, J.C.; Hojsak, I.; Kolacek, S.; et al. Nutrition in pediatric inflammatory bowel disease: A position paper on behalf of the Porto Inflammatory Bowel Disease Group of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 687–708. [Google Scholar] [CrossRef] [PubMed]
- Wiskin, A.E.; Wootton, S.A.; Hunt, T.M.; Cornelius, V.R.; Afzal, N.A.; Jackson, A.A.; Beattie, R.M. Body composition in childhood inflammatory bowel disease. Clin. Nutr. 2011, 30, 112–115. [Google Scholar] [CrossRef]
- Massironi, S.; Rossi, R.E.; Cavalcoli, F.A.; Della Valle, S.; Fraquelli, M.; Conte, D. Nutritional deficiencies in inflammatory bowel disease: Therapeutic approaches. Clin. Nutr. 2013, 32, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.; Walia, C.; Elkadri, A.; Pipkorn, R.; Dunn, R.K.; Sieracki, R.; Goday, P.S.; Mariano Cabrera, J. A systematic review of micronutrient deficiencies in pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 2019, 25, 445–459. [Google Scholar] [CrossRef]
- Weisshof, R.; Chermesh, I. Micronutrient deficiencies in inflammatory bowel disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 576–581. [Google Scholar] [CrossRef]
- Kaenkumchorn, T.; Kesavan, A. Dietary Management of Pediatric Inflammatory Bowel Disease. J. Med. Food 2019, 22, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. Off. J. L 2002, 183, 51–57.
- El-Matary, W. Advances in Nutritional Management of Pediatric Inflammatory Bowel Disease. Nutrients 2021, 13, 324. [Google Scholar] [CrossRef] [PubMed]
- Holick, M. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Alpert, P.; Shaikh, U. The effects of vitamin D deficiency and insufficiency on the endocrine and paracrine systems. Biol. Res. Nurs. 2007, 9, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, I.; Dalzell, A.M.; El-Matary, W. Vitamin D as a therapy for colitis: A systematic review. J. Crohns Colitis 2012, 6, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Marshall, J.K. Management of inflammatory bowel disease with vitamin D: Beyond bone health. J. Crohns Colitis 2012, 6, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Na, S.Y.; Kim, K.B.; Lim, Y.J.; Song, H.J. Vitamin D and Colorectal Cancer: Current Perspectives and Future Directions. J. Cancer Prev. 2022, 27, 147–156. [Google Scholar] [CrossRef]
- Ehrlich, S.; Mark, A.G.; Rinawi, F.; Shamir, R.; Assa, A. Micronutrient Deficiencies in Children with Inflammatory Bowel Diseases. Nutr. Clin. Pract. 2020, 35, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, Y.; Guo, H.; Jabir, M.S.; Liu, X.; Cui, W.; Li, D. Associations between Folate and Vitamin B12 Levels and Inflammatory Bowel Disease: A Meta-Analysis. Nutrients 2017, 9, 382. [Google Scholar] [CrossRef]
- Aghdassi, E.; Wendland, B.E.; Stapleton, M.; Raman, M.; Allard, J.P. Adequacy of nutritional intake in a Canadian population of patients with Crohn’s disease. J. Am. Diet. Assoc. 2007, 107, 1575–1580. [Google Scholar] [CrossRef]
- Cimpoca, B.A.; Nedelea, F.; Furtuna, M.; Peltecu, G.; Panaitescu, A.M. Managing Crohn’s Disease during Pregnancy. Maedica 2016, 11, 221–226. [Google Scholar]
- Crohn’s & Colitis Foundation of America. Diet, Nutrition and Inflammatory Bowel Disease; Crohn’s & Colitis Foundation of America: New York, NY, USA, 2019. [Google Scholar]
- Ratajczak, A.E.; Szymczak-Tomczak, A.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Does Folic Acid Protect Patients with Inflammatory Bowel Disease from Complications? Nutrients 2021, 13, 4036. [Google Scholar] [CrossRef]
- Heyman, M.B.; Garnett, E.A.; Shaikh, N.; Huen, K.; Jose, F.A.; Harmatz, P.; Winter, H.S.; Baldassano, R.N.; Cohen, S.A.; Gold, B.D.; et al. Folate concentrations in pediatric patients with newly diagnosed inflammatory bowel disease. Am. J. Clin. Nutr. 2009, 89, 545–550. [Google Scholar] [CrossRef]
- Temtem, T.A.; Vickers, M.; Whitworth, J. Weekly Folic Acid Is a Convenient and Well-Tolerated Alternative to Daily Dosing in Pediatric Patients with Inflammatory Bowel Disease on Methotrexate. Nutrients 2023, 15, 1586. [Google Scholar] [CrossRef]
- Ghishan, F.K.; Kiela, P.R. Vitamins and Minerals in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 797–808. [Google Scholar] [CrossRef]
- Valvano, M.; Capannolo, A.; Cesaro, N.; Stefanelli, G.; Fabiani, S.; Frassino, S.; Monaco, S.; Magistroni, M.; Viscido, A.; Latella, G. Nutrition, Nutritional Status, Micronutrients Deficiency, and Disease Course of Inflammatory Bowel Disease. Nutrients 2023, 15, 3824. [Google Scholar] [CrossRef]
- Dignass, A.; Gasche, C.; Dominik Bettenworth, D.; Birgegård, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, K.; Koutroubakis, I.; et al. European Consensus on the Diagnosis and Management of Iron Deficiency and Anaemia in Inflammatory Bowel Diseases. J. Crohn’s Colitis 2015, 9, 211–222. [Google Scholar] [CrossRef]
- Dunleavy, K.A.; Ungaro, R.C.; Manning, L.; Gold, S.; Novak, J.; Colombel, J.F. Vitamin C Deficiency in Inflammatory Bowel Disease: The Forgotten Micronutrient. Crohns Colitis 2021, 360, otab009. [Google Scholar] [CrossRef]
- Micronutrient Requirements of Children Ages 4 to 13 Years. Linus Pauling Institute, Oregon State University. Available online: https://lpi.oregonstate.edu/mic/life-stages/children#vitamin-A (accessed on 17 July 2024).
- Owczarek, D.; Rodacki, T.; Domagała-Rodacka, R.; Cibor, D.; Mach, T. Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 895–905. [Google Scholar] [CrossRef]
- Calder, P.C. N-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef]
- Calder, P.C. Fatty acids and immune function: Relevance to inflammatory bowel diseases. Int. Rev. Immunol. 2009, 28, 506–534. [Google Scholar] [CrossRef]
- Cabre, E.; Manosa, M.; Gassull, M.A. Omega-3 fatty acids and inflammatory bowel diseases—A systematic review. Br. J. Nutr. 2012, 107, S240–S252. [Google Scholar] [CrossRef]
- Abdel-Tawab, M.; Werz, O.; Schubert-Zsilavecz, M. Boswellia serrata: An overall assessment of in vitro, preclinical, pharmacokinetic and clinical data. Clin. Pharmacokinet. 2011, 50, 349–369. [Google Scholar] [CrossRef]
- Gupta, I.; Parihar, A.; Malhotra, P.; Singh, G.B.; Lüdtke, R.; Safayhi, H.; Ammon, H.P. Effects of Boswellia serrata gum resin in patients with ulcerative colitis. Eur. J. Med. Res. 1997, 2, 37–43. [Google Scholar]
- Gerhardt, H.; Seifert, F.; Buvari, P.; Vogelsang, H.; Repges, R. Therapy of active Crohn disease with Boswellia serrata extract H 15. Z. Gastroenterol. 2001, 39, 11–17. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Ramsewak, R.S.; DeWitt, D.L.; Nair, M.G. Cytotoxicity, antioxidant and anti-inflammatory activities of curcumins I–III from Curcuma longa. Phytomedicine 2000, 7, 303–308. [Google Scholar] [CrossRef]
- Chan, M.M. Inhibition of tumor necrosis factor by curcumin, a phytochemical. Biochem. Pharmacol. 1995, 49, 1551–1556. [Google Scholar] [CrossRef]
- Kang, B.Y.; Chung, S.W.; Chung, W.; Im, S.; Hwang, S.Y.; Kim, T.S. Inhibition of interleukin-12 production in lipopolysaccharide-activated macrophages by curcumin. Eur. J. Pharmacol. 1999, 384, 191–195. [Google Scholar] [CrossRef]
- Xu, Y.X.; Pindolia, K.R.; Janakiraman, N.; Chapman, R.A.; Gautam, S.C. Curcumin inhibits IL1 alpha and TNF-alpha induction of AP-1 and NF-kB DNA-binding activity in bone marrow stromal cells. Hematopathol. Mol. Hematol. 1997, 11, 49–62. [Google Scholar]
- Taylor, R.A.; Leonard, M.C. Curcumin for inflammatory bowel disease: A review of human studies. Altern. Med. Rev. 2011, 16, 152–156. [Google Scholar]
- Burge, K.; Gunasekaran, A.; Eckert, J.; Chaaban, H. Curcumin and Intestinal Inflammatory Diseases: Molecular Mechanisms of Protection. Int. J. Mol. Sci. 2019, 20, 1912. [Google Scholar] [CrossRef]
- Holt, P.R.; Katz, S.; Kirshoff, R. Curcumin therapy in inflammatory bowel disease: A pilot study. Dig. Dis. Sci. 2005, 50, 2191–2193. [Google Scholar] [CrossRef]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Maruyama, Y.; Andoh, A.; Tsujikawa, T.; Fujiyama, Y.; Mitsuyama, K.; Sata, M.; et al. Curcumin maintenance therapy for ulcerative colitis: Randomized, multicenter, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. 2006, 4, 1502–1506. [Google Scholar] [CrossRef]
- Suskind, D.L.; Wahbeh, G.; Burpee, T.; Cohen, M.; Christie, D.; Weber, W. Tolerability of curcumin in pediatric inflammatory bowel disease: A forced-dose titration study. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 277–279. [Google Scholar] [CrossRef]
- Berni Canani, R.; Di Costanzo, M.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal disease. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.M.A.E.; Vanhoutvin, S.A.L.W.; Troost, F.J.; Rijkers, G.; de Bruine, A.; Bast, A.; Venema, K.; Brummer, R.-J.M. Effect of butyrate enemas on inflammation and antioxidant status in the colonic mucosa of patients with ulcerative colitis in remission. Clin. Nutr. 2010, 29, 738–744. [Google Scholar] [CrossRef]
- Scheppach, W.; Weiler, F. The butyrate story: Old wine in new bottles? Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 563–567. [Google Scholar] [CrossRef]
- Büning, C.; Geissler, N.; Prager, M.; Sturm, A.; Baumgart, D.C.; Büttner, J.; Bühner, S.; Haas, V.; Lochs, H. Increased small intestinal permeability in ulcerative colitis: Rather genetic than environmental and a risk factor for extensive disease? Inflamm. Bowel Dis. 2012, 18, 1932–1939. [Google Scholar] [CrossRef]
- Assisi, R.F.; GISDI Study Group. Combined butyric acid/mesalazine treatment in ulcerative colitis with mild-moderate activity. Minerva Gastroenterol. Dietol. 2008, 54, 231–238. [Google Scholar]
- Di Sabotino, A.; Morera, R.; Ciccocioppo, R.; Cazzola, P.; Gotti, S.; Tinozzi, F.P.; Tinozzi, S.; Corazza, G.R. Oral butyrate for mild to moderately active Crohn’s disease. Aliment. Pharmacol. Ther. 2005, 22, 789–794. [Google Scholar] [CrossRef]
- Vernia, P.; Annese, V.; Bresci, G.; d’Albasio, G.; D’Incà, R.; Giaccari, S.; Ingrosso, M.; Mansi, C.; Riegler, G.; Valpiani, D.; et al. Topical butyrate improbe efficacy of 5-ASA in refractory distal ulcerative colitis: Results of a multicentre trial. Eur. J. Clin. Investig. 2003, 33, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Vernia, P.; Monteleone, G.; Grandinetti, G.; Villotti, G.; Di Giulio, E.; Frieri, G.; Marcheggiano, A.; Pallone, F.; Caprilli, R.; Torsoli, A. Combined oral sodium butyrate and mesalazine treatment compared to oral mesalazine alone in ulcerative colitis. Randomized, double-blind, placebo-controlled pilot study. Dig. Dis. Sci. 2000, 45, 976–981. [Google Scholar] [CrossRef]
- Steinhart, A.H.; Hiruki, T.; Brzezinski, A.; Baker, J.P. Treatment of left-sided ulcerative colitis with butyrate enemas: A controlled trial. Aliment. Pharmacol. Ther. 1996, 10, 729–736. [Google Scholar] [CrossRef]
- Vernia, P.; Marcheggiano, A.; Caprilli, R.; Frieri, G.; Corrao, G.; Valpini, D.; DI Paolo, M.C.; Paoluzi, P.; Torsoli, A. Short-chain fatty acid topical treatment in distal ulcerative colitis. Aliment. Pharmacol. Ther. 1995, 9, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Steinhart, A.H.; Brzezinski, A.; Baker, J.P. Treatment of refractory ulcerative proctosigmoiditis with butyrate enemas. Am. J. Gastroenterol. 1994, 89, 179–183. [Google Scholar]
- Scheppach, W.; Sommer, H.; Kirchner, T.; Paganelli, G.M.; Bartram, P.; Christl, S.; Richter, F.; Dusel, G.; Kasper, H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology 1992, 103, 51–56. [Google Scholar] [CrossRef]
- Nduma, B.N.; Mofor, K.A.; Tatang, J.; Ekhator, C.; Ambe, S.; Fonkem, E. The Use of Cannabinoids in the Treatment of Inflammatory Bowel Disease (IBD): A Review of the Literature. Cureus 2023, 15, e36148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Halbmeijer, N.; Groeneweg, M.; De Ridder, L. Cannabis, a potential treatment option in pediatric IBD? Still a long way to go. Expert Rev. Clin. Pharmacol. 2019, 12, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Hoffenberg, E.J.; Mcwilliams, S.K.; Mikulich-Gilbertson, S.K.; Murphy, B.V.; Lagueux, M.; Robbins, K.; Hoffenberg, A.S.; de Zoeten, E.; Hopfer, C.J. Marijuana Use by Adolescents and Young Adults with Inflammatory Bowel Disease. J. Pediatr. 2018, 199, 99–105. [Google Scholar] [CrossRef]
- Hoffenberg, E.J.; Mcwilliams, S.; Mikulich-Gilbertson, S.; Murphy, B.; Hoffenberg, A.; Hopfer, C.J. Cannabis Oil Use by Adolescents and Young Adults With Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Phatak, U.P.; Rojas-Velasquez, D.; Porto, A.; Pashankar, D.S. Prevalence and Patterns of Marijuana Use in Young Adults with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Committee on Substance Abuse, Committee on Adolescence; Committee on Substance Abuse Committee on Adolescence. The impact of marijuana policies on youth: Clinical, research, and legal update. Pediatrics 2015, 135, 584–587. [Google Scholar] [CrossRef]
- Miele, E.; Pascarella, F.; Giannetti, E.; Quaglietta, L.; Baldassano, R.N.; Staiano, A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am. J. Gastroenterol. 2009, 104, 437–443. [Google Scholar] [PubMed]
- Oliva, S.; Di Nardo, G.; Ferrari, F.; Mallardo, S.; Rossi, P.; Patrizi, G.; Cucchiara, S.; Stronati, L. Randomised clinical trial: The effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment. Pharmacol. Ther. 2012, 35, 327–334. [Google Scholar] [CrossRef]
- Bousvaros, A.; Guandalini, S.; Baldassano, R.N.; Botelho, C.; Evans, J.; Ferry, G.D.; Goldin, B.; Hartigan, L.; Kugathasan, S.; Levy, J.; et al. A randomized, double-blind trial of Lactobacillus GG versus placebo in addition to standard maintenance therapy for children with Crohn’s disease. Inflamm. Bowel Dis. 2005, 11, 833–839. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).