Inadequate Cerebrospinal Fluid Concentrations of Available Salvage Agents Further Impedes the Optimal Treatment of Multidrug-Resistant Enterococcus faecium Meningitis and Bacteremia
Abstract
:1. Introduction
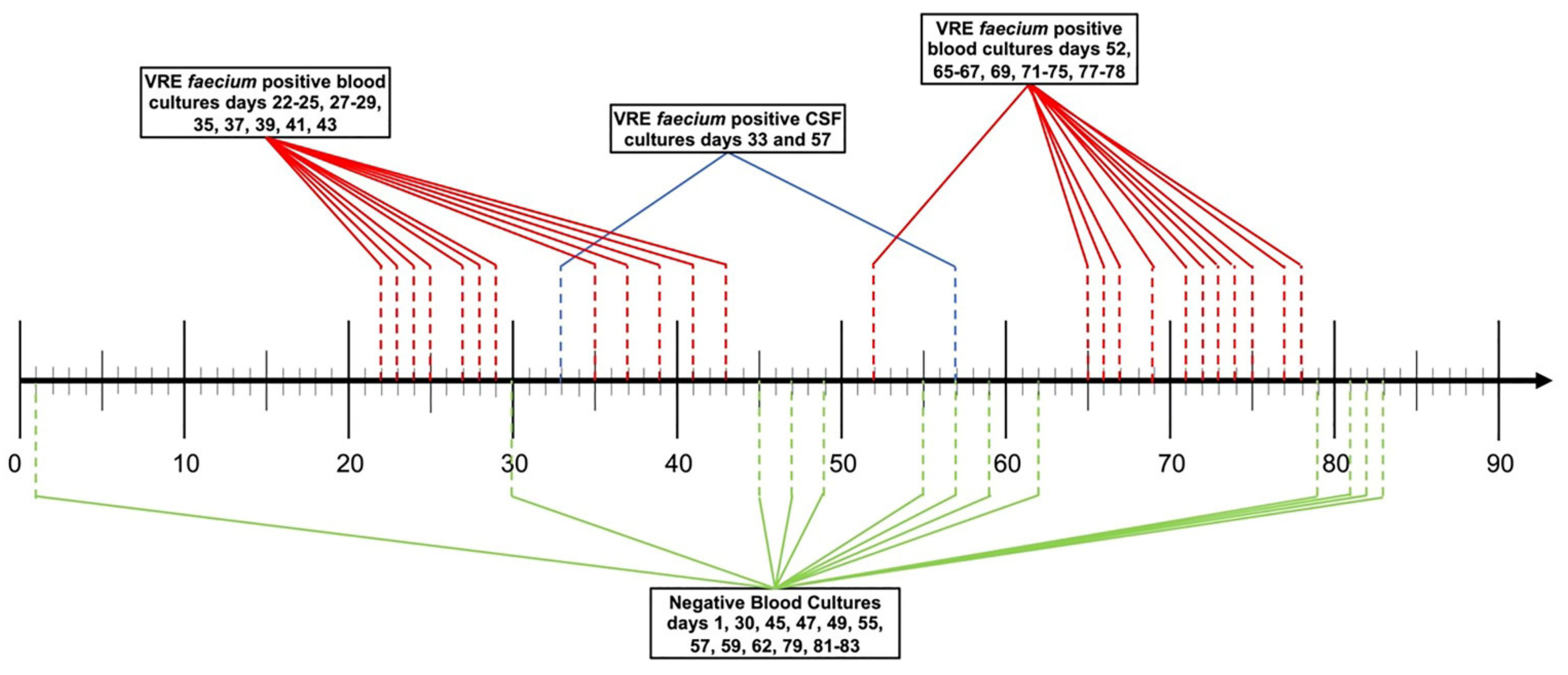
Case Presentation
2. Materials and Methods
2.1. Bacteria and Susceptibility Testing
2.2. Pharmacokinetic Samples and Analyses
2.3. Time-Kill Experiments
3. Results
3.1. Susceptibility
3.2. Pharmacokinetics and CSF Penetration
3.3. Time-Kill Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 20 February 2020).
- Mercuro, N.J.; Davis, S.L.; Zervos, M.J.; Herc, E.S. Combatting resistant enterococcal infections: A pharmacotherapy review. Expert Opin. Pharmacother. 2018, 19, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Yim, J.; Smith, J.R.; Rybak, M.J. Role of Combination Antimicrobial Therapy for Vancomycin-Resistant Enterococcus faecium Infections: Review of the Current Evidence. Pharmacotherapy 2017, 37, 579–592. [Google Scholar] [CrossRef]
- Wenzler, E.; Santarossa, M.; Meyer, K.A.; Harrington, A.T.; Reid, G.E.; Clark, N.M.; Albarillo, F.S.; Bulman, Z.P. In Vitro Pharmacodynamic Analyses Help Guide the Treatment of Multidrug-Resistant Enterococcus faecium and Carbapenem-Resistant Enterobacter cloacae Bacteremia in a Liver Transplant Patient. Open Forum Infect. Dis. 2020, 7, ofz545. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Vu, B.N.; Seddon, A.N.; Hodgson, H.A.; Wang, S.K. Treatment Considerations for CNS Infections Caused by Vancomycin-Resistant Enterococcus faecium: A Focused Review of Linezolid and Daptomycin. Ann. Pharmacother. 2020, 54, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Muzevich, K.; Edmond, M.B.; Bearman, G.; Stevens, M.P. Central nervous system infections due to vancomycin-resistant enterococci: Case series and review of the literature. Int. J. Infect. Dis. 2014, 25, 26–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasmin, M.; Fouts, D.E.; Jacobs, M.R.; Haydar, H.; Marshall, S.H.; White, R.; Dsouza, R.; Lodise, T.P.; Rhoads, D.D.; Hujer, A.M.; et al. Monitoring Ceftazidime-Avibactam and Aztreonam Concentrations in the Treatment of a Bloodstream Infection Caused by a Multidrug-Resistant Enterobacter sp. Carrying Both Klebsiella pneumoniae Carbapenemase–4 and New Delhi Metallo-β-Lactamase–1. Clin. Infect. Dis. 2020, 71, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Perez, F.; Bonomo, R.A. Precision Medicine and Mysteries in Clinical Microbiology: Rationalizing Epidemiology, Genotype, and Phenotype To Guide Therapeutics. Antimicrob. Agents Chemother. 2020, 64, 02264-19. [Google Scholar] [CrossRef] [Green Version]
- Sakoulas, G. Imipenem/Cilistatin and Fosfomycin for Refractory Methicillin-Resistant Staphylococcus aureus Infection: A Novel Combination Therapy. Antimicrob. Agents Chemother. 2020, 65. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, I.; Yamaguchi, T.; Aoki, K.; Miura, Y.; Sato, S.; Fujita, H.; Watanabe, H. Imipenem plus Fosfomycin as Salvage Therapy for Vertebral Osteomyelitis. Antimicrob. Agents Chemother. 2020, 65, 01746-20. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Thirtieth Informational Supplement M100-S30; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Nau, R.; Sörgel, F.; Eiffert, H. Penetration of Drugs through the Blood-Cerebrospinal Fluid/Blood-Brain Barrier for Treatment of Central Nervous System Infections. Clin. Microbiol. Rev. 2010, 23, 858–883. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Karau, M.J.; Patel, R. Evaluation of Non-Tissue Culture- versus Tissue Culture-Treated Microplates for Oritavancin Susceptibility Testing. J. Clin. Microbiol. 2018, 56, e02001-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing: Approved Twenty-Eighth Edition: Document M100-S28; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- SIVEXTRO (Tedizolid); Lexington, M.A. Cubist Pharmaceuticals U.S. Revised June 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205435s000lbl.pdf (accessed on 9 November 2020).
- Gu, L.; Ma, M.; Zhang, Y.; Zhang, L.; Zhang, S.; Huang, M.; Zhang, M.; Xin, Y.; Zheng, G.; Chen, S.; et al. Comparative pharmacokinetics of tedizolid in rat plasma and cerebrospinal fluid. Regul. Toxicol. Pharmacol. 2019, 107, 104420. [Google Scholar] [CrossRef]
- Myrianthefs, P.; Markantonis, S.L.; Vlachos, K.; Anagnostaki, M.; Boutzouka, E.; Panidis, D.; Baltopoulos, G. Serum and Cerebrospinal Fluid Concentrations of Linezolid in Neurosurgical Patients. Antimicrob. Agents Chemother. 2006, 50, 3971–3976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beer, R.; Engelhardt, K.W.; Pfausler, B.; Broessner, G.; Helbok, R.; Lackner, P.; Brenneis, C.; Kaehler, S.T.; Georgopoulos, A.; Schmutzhard, E. Pharmacokinetics of Intravenous Linezolid in Cerebrospinal Fluid and Plasma in Neurointensive Care Patients with Staphylococcal Ventriculitis Associated with External Ventricular Drains. Antimicrob. Agents Chemother. 2007, 51, 379–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viaggi, B.; Di Paolo, A.; Danesi, R.; Polillo, M.; Ciofi, L.; Del Tacca, M.; Malacarne, P. Linezolid in the central nervous system: Comparison between cerebrospinal fluid and plasma pharmacokinetics. Scand. J. Infect. Dis. 2011, 43, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Luque, S.; Grau, S.; Alvarez-Lerma, F.; Ferrández, O.; Campillo, N.; Horcajada, J.P.; Basas, M.; Lipman, J.; Roberts, J. Plasma and cerebrospinal fluid concentrations of linezolid in neurosurgical critically ill patients with proven or suspected central nervous system infections. Int. J. Antimicrob. Agents 2014, 44, 409–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodvold, K.A.; Gotfried, M.H.; Cwik, M.; Korth-Bradley, J.M.; Dukart, G.; Ellis-Grosse, E.J. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J. Antimicrob. Chemother. 2006, 58, 1221–1229. [Google Scholar] [CrossRef]
- Ray, L.; Levasseur, K.; Nicolau, D.P.; Scheetz, M.H. Cerebral Spinal Fluid Penetration of Tigecycline in a Patient with Acinetobacter baumannii Cerebritis. Ann. Pharmacother. 2010, 44, 582–586. [Google Scholar] [CrossRef]
- Trostdorf, F.; Reinert, R.R.; Schmidt, H.; Nichterlein, T.; Stuertz, K.; Schmitz-Salue, M.; Sadowski, I.; Brück, W.; Nau, R. Quinupristin/dalfopristin attenuates the inflammatory response and reduces the concentration of neuron-specific enolase in the cerebrospinal fluid of rabbits with experimental Streptococcus pneumoniae meningitis. J. Antimicrob. Chemother. 1999, 43, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Bearden, D.T. Clinical Pharmacokinetics of Quinupristin/Dalfopristin. Clin. Pharmacokinet. 2004, 43, 239–252. [Google Scholar] [CrossRef]
- Cottagnoud, P.; Gerber, C.M.; Cottagnoud, M.; Täuber, M.G. Gentamicin Increases the Efficacy of Vancomycin against Penicillin-Resistant Pneumococci in the Rabbit Meningitis Model. Antimicrob. Agents Chemother. 2002, 46, 188–190. [Google Scholar] [CrossRef] [Green Version]
- Gerber, J.; Smirnov, A.; Wellmer, A.; Ragheb, J.; Prange, J.; Schütz, E.; Wettich, K.; Kalich, S.; Nau, R. Activity of LY333328 in Experimental Meningitis Caused by a Streptococcus pneumoniae Strain Susceptible to Penicillin. Antimicrob. Agents Chemother. 2001, 45, 2169–2172. [Google Scholar] [CrossRef] [Green Version]
- Bergeron, M.; Montay, G. The pharmacokinetics of quinupristin/dalfopristin in laboratory animals and in humans. J. Antimicrob. Chemother. 1997, 39 (Suppl. A), 129–138. [Google Scholar] [CrossRef] [Green Version]
- Prematunge, C.; MacDougall, C.; Johnstone, J.; Adomako, K.; Lam, F.; Robertson, J.; Garber, G. VRE and VSE Bacteremia Outcomes in the Era of Effective VRE Therapy: A Systematic Review and Meta-analysis. Infect. Control Hosp. Epidemiol. 2016, 37, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, H.-Y.; Perencevich, E.N.; Nair, R.; Nelson, R.E.; Samore, M.; Khader, K.; Chorazy, M.L.; Herwaldt, L.A.; Blevins, A.; Ward, M.A.; et al. Incidence and Outcomes Associated With Infections Caused by Vancomycin-Resistant Enterococci in the United States: Systematic Literature Review and Meta-Analysis. Infect. Control Hosp. Epidemiol. 2016, 38, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F. Pharmacokinetic/pharmacodynamic (PK/PD) considerations in the management of Gram-positive bacteraemia. Int. J. Antimicrob. Agents 2010, 36 (Suppl. 2), S33–S39. [Google Scholar] [CrossRef] [Green Version]
- Tunkel, A.R.; Hasbun, R.; Bhimraj, A.; Byers, K.; Kaplan, S.L.; Scheld, W.M.; van de Beek, D.; Bleck, T.P.; Garton, H.J.; Zunt, J.R. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin. Infect. Dis. 2017, 64, e34–e65. [Google Scholar] [CrossRef] [PubMed]
- Tunkel, A.R.; Hartman, B.J.; Kaplan, S.L.; Kaufman, B.A.; Roos, K.L.; Scheld, W.M.; Whitley, R.J. Practice Guidelines for the Management of Bacterial Meningitis. Clin. Infect. Dis. 2004, 39, 1267–1284. [Google Scholar] [CrossRef]
- Peppard, W.J.; Johnston, C.J.; Urmanski, A.M. Pharmacologic options for CNS infections caused by resistant Gram-positive organisms. Expert Rev. Anti-Infect. Ther. 2008, 6, 83–99. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 10.0. 2020. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_10.0_Breakpoint_Tables.pdf (accessed on 7 August 2021).
- Boukthir, S.; Dejoies, L.; Zouari, A.; Collet, A.; Potrel, S.; Auger, G.; Cattoir, V. In vitro activity of eravacycline and mechanisms of resistance in enterococci. Int. J. Antimicrob. Agents 2020, 56, 106215. [Google Scholar] [CrossRef]
- Heaney, M.; Mahoney, M.V.; Gallagher, J.C. Eravacycline: The Tetracyclines Strike Back. Ann. Pharmacother. 2019, 53, 1124–1135. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Steenbergen, J.; Zhanel, G.G. Microbiology and Preclinical Review of Omadacycline. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 69, S6–S15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veve, M.P.; Wagner, J.L. Lefamulin: Review of a Promising Novel Pleuromutilin Antibiotic. Pharmacotherapy 2018, 38, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Petraitis, V.; Petraitiene, R.; Maung, B.B.W.; Khan, F.; Alisauskaite, I.; Olesky, M.; Newman, J.; Mutlib, A.; Niu, X.; Satlin, M.; et al. Pharmacokinetics and Comprehensive Analysis of the Tissue Distribution of Eravacycline in Rabbits. Antimicrob. Agents Chemother. 2018, 62, e00275-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Source (Hospital Day of Isolation) | CSF (33) | Blood (35) | CSF (57) | Blood (52) | ||||
|---|---|---|---|---|---|---|---|---|
| Antibiotic | MIC (mg/L) | Interpretive Category | MIC (mg/L) | Interpretive Category | MIC (mg/L) | Interpretive Category | MIC (mg/L) | Interpretive Category |
| Ampicillin | ≥16 | R | ≥16 | R | ≥16 | R | ≥16 | R |
| Ceftaroline | ≥128 | NC | ≥128 | NC | ≥128 | NC | ≥128 | NC |
| Ceftriaxone | ≥128 | NC | ≥128 | NC | ≥128 | NC | ≥128 | NC |
| Chloramphenicol | ≥32 | R | ≥32 | R | ≥32 | R | ≥32 | R |
| Ciprofloxacin | ≥4 | R | ≥4 | R | ≥4 | R | ≥4 | R |
| Dalbavancin b | ≥4 | NS | ≥4 | NS | ≥4 | NS | ≥4 | NS |
| Daptomycin | ≥8 | R | ≥8 | R | ≥8 | R | ≥8 | R |
| Eravacycline d | - | - | - | - | 0.12 | S | 0.12 | S |
| Delafloxacin c | >8 | R | >8 | R | >8 | R | >8 | R |
| Erythromycin | ≥8 | R | ≥8 | R | ≥8 | R | ≥8 | R |
| Fosfomycin b | 64 | S | 64 | S | 64 | S | 64 | S |
| Gentamicin synergy | ≤500 | S | ≤500 | S | ≤500 | S | ≤500 | S |
| Lefamulin | 1 | NC | 1 | NC | 2 | NC | 2 | NC |
| Levofloxacin | ≥8 | R | ≥8 | R | ≥8 | R | ≥8 | R |
| Linezolid | 16 | R | 16 | R | 16 | R | 16 | R |
| Moxifloxacin c | ≥8 | R | ≥8 | R | ≥8 | R | ≥8 | R |
| Nitrofurantoin | ≥128 | R | ≥128 | R | ≥128 | R | ≥128 | R |
| Omadacycline c | 1 | R | 1 | R | 1 | R | 1 | R |
| Oritavancin b | 0.25 | NS | 0.25 | NS | 0.25 | NS | 0.25 | NS |
| Quinupristin-dalfopristin | 2 | I | 2 | I | 0.5 | S | 0.5 | S |
| Rifampin | ≥4 | R | ≥4 | R | ≥4 | R | ≥4 | R |
| Tedizolid b | 2 | NS | 2 | NS | 2 | NS | 2 | NS |
| Telavancin | ≥4 | NS | ≥4 | NS | ≥4 | NS | ≥4 | NS |
| Tetracycline | ≥16 | R | ≥16 | R | ≥16 | R | ≥16 | R |
| Tigecycline d | 0.25 | S | 0.25 | S | 0.25 | S | 0.25 | S |
| Vancomycin | ≥256 | R | ≥256 | R | ≥256 | R | ≥256 | R |
| Antimicrobial | Last Dose Administered (mg) | Time between Last Dose and CSF Collection (h:m) | Mean (±SD) Measured CSF Concentration (mg/L) | Estimated Corresponding Unbound Plasma Concentration Range at Time of CSF Collection (mg/L) | Predicted CSF/Plasma Penetration (%) | Projected Maximum CSF Concentration (mg/L) | Published % CSF/Plasma Penetration (REF) |
|---|---|---|---|---|---|---|---|
| Gentamicin | 400 | 77:30 | 0.410 ± 0.05 | ND | - | 5.74 | 27 [25] |
| Oritavancin | 1200 | 46:54 | 0.013 ± 0.005 | 15.5–25.1 | 0.07 | 0.01–0.02 | 1–5 [26] |
| Tigecycline | 100 | 8:27 | 0.172 ± 0.002 | 0.16–0.46 | 71.7 | 0.27–0.78 | 5–41 [21,22] |
| Tedizolid | 200 | 8:25 | 0.204 ± 0.006 | 0.30–0.49 | 54.8 | 0.25–0.41 | 2.2 [16] |
| Quinupristin | 207 | 2:00 | ND | 0.33–0.43 | 0 | 0 | 0 [23,27] |
| Dalfopristin | 483 | 2:00 | ND | 1.84–3.04 | 0 | 0 | 0 [23,27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wenzler, E.; Adeel, A.; Wu, T.; Jurkovic, M.; Walder, J.; Ramasra, E.; Campion, M.; Cerny, J.; Theodoropoulos, N.M. Inadequate Cerebrospinal Fluid Concentrations of Available Salvage Agents Further Impedes the Optimal Treatment of Multidrug-Resistant Enterococcus faecium Meningitis and Bacteremia. Infect. Dis. Rep. 2021, 13, 843-854. https://doi.org/10.3390/idr13030076
Wenzler E, Adeel A, Wu T, Jurkovic M, Walder J, Ramasra E, Campion M, Cerny J, Theodoropoulos NM. Inadequate Cerebrospinal Fluid Concentrations of Available Salvage Agents Further Impedes the Optimal Treatment of Multidrug-Resistant Enterococcus faecium Meningitis and Bacteremia. Infectious Disease Reports. 2021; 13(3):843-854. https://doi.org/10.3390/idr13030076
Chicago/Turabian StyleWenzler, Eric, Alina Adeel, Tiffany Wu, Michele Jurkovic, Jeremy Walder, Emily Ramasra, Maureen Campion, Jan Cerny, and Nicole M. Theodoropoulos. 2021. "Inadequate Cerebrospinal Fluid Concentrations of Available Salvage Agents Further Impedes the Optimal Treatment of Multidrug-Resistant Enterococcus faecium Meningitis and Bacteremia" Infectious Disease Reports 13, no. 3: 843-854. https://doi.org/10.3390/idr13030076
APA StyleWenzler, E., Adeel, A., Wu, T., Jurkovic, M., Walder, J., Ramasra, E., Campion, M., Cerny, J., & Theodoropoulos, N. M. (2021). Inadequate Cerebrospinal Fluid Concentrations of Available Salvage Agents Further Impedes the Optimal Treatment of Multidrug-Resistant Enterococcus faecium Meningitis and Bacteremia. Infectious Disease Reports, 13(3), 843-854. https://doi.org/10.3390/idr13030076






