SARS-CoV-2 and the Immune Response in Pregnancy with Delta Variant Considerations
Abstract
:1. Introduction
2. Implications of Immune Modulation in Pregnancy and SARS-CoV-2
2.1. Overall Immune Attenuation in Pregnant Physiology
- (1)
- The CD4+ T cell population has been shown to shift from T helper type 1 (Th1) to Th2 predominant during pregnancy. Th1 CD4+ T cells help boost cellular immune response through activating macrophages, cytotoxic T lymphocytes and NK cells through IL-2 and IF-γ signaling, whereas Th2 CD4+ T cells coordinate the humoral response through activating eosinophils, basophils, and mast cells via IL-4 and IL-6 signaling. Usually, in non-pregnant individuals, a robust Th1 response is associated with good prognosis for viral infections [6,12,13,14]. With the shift from Th1 to Th2 CD4+ T cells in pregnant women, this carries unfavorable implications. For further evidence, Pavel et al. discussed how the Th2/Th1 cytokine imbalance was found to be significantly higher in patients with known COVID-19 risk factors such as age (>40), sex (male), active smoking, as well as ACE2 expression and patients with asthma, leading to higher mortality risk [15].
- (2)
- NK cells that play a critical role in the innate immune system are shown to decrease in circulation during pregnancy, further impeding the internal battle against COVID-19 exposure [6]. Hsieh et al. demonstrated that the cytolytic effects of NK cell function play an important role in SARS-CoV-2 clearance. In particular, NK cells that expressed receptor DNAM1 are linked to more rapid recovery [16].
- (3)
- Lampe et al. found a significant decrease in the phagocytic index of neutrophil granulocytes and monocytes in both healthy and pre-eclamptic pregnancies, which may have implications for hindering maternal clearance of viral infection [17,18]. However, there is currently too limited data to be able to make solid conclusions.
- (4)
- Progesterone is a steroid with immunomodulatory properties that are increased in maternal circulation [19]. In a mouse model of influenza A infection, this has shown to decrease virus-specific antibody levels as well as virus-specific CD8+ T cells. Upon re-challenging with influenza A, this resulted in a more severe disease course [20].
- (5)
- Plasmacytoid dendritic cells (pDCs) are key for type 1 interferon production against viruses and are also decreased in the maternal circulation [6,21,22]. Additionally, pDCs from pregnant women were reported to have attenuated the inflammatory response to the H1N1/09 virus, which is thought to be one of the reasons why pregnant women were more severely affected [23]. However, it has been recently shown that a robust type 1 interferon response is associated with hyperinflammation and severe COVID-19 infection, as opposed to a more delayed and possibly suppressed interferon response in early infection. This speaks to the importance of understanding the different roles of type 1 interferon at each stage of infection for therapeutic decisionmaking [24]. In previous studies on SARS-CoV and MERS-CoV, type 1 interferons are known to decrease the expression of IFN receptors, leading to a systemic inflammatory response [25]. In the context of pregnancy, what has been discussed thus far suggests that decreased pDCs in maternal circulation are unfavorable for earlier stages of infection when viral clearance is key, however it may play a protective role against the development of a cytokine storm in the later stages of infection.
2.2. Immune Changes in Pregnancy Leading to Hyperinflammation in SARS-CoV-2
- (1)
- Previous studies have shown an increase in maternal serum levels of C3a, C4a, C5a, C4d, C3, C9, and the Serum Complement Membrane Attack Complex SC5b9 when compared to non-pregnant women [27,28]. This increase in complement activation is linked to greater lung injury and disease severity in SARS-CoV-2 infection [29]. Elevation of C3 activation products was observed in the lung as early as 1 day postinfection. In C3 deficient mice (C3−/−), SARS-CoV-2 infection demonstrated reduced weight loss and respiratory dysfunction with an equivalent viral load, as well as significantly less neutrophils and inflammatory monocytes [30]. Gralinski et al. further proposed the attenuation of the complement system as a possible effective treatment option for SARS-CoV-2 [30].
- (2)
- Young et al. from the American Journal of Obstetrics and Gynecology previously showed an increase in IL-6, IL-12, IFN-α, and TNF-α in the maternal sera in uncomplicated pregnancies when compared to nonpregnant controls, with IL-12 remaining elevated into the postpartum period [31]. As previously discussed, an increase in IL-6 is especially correlated with higher mortality in SARS-CoV-2 infection, suggesting an already vulnerable physiological state for expecting mothers.
- (3)
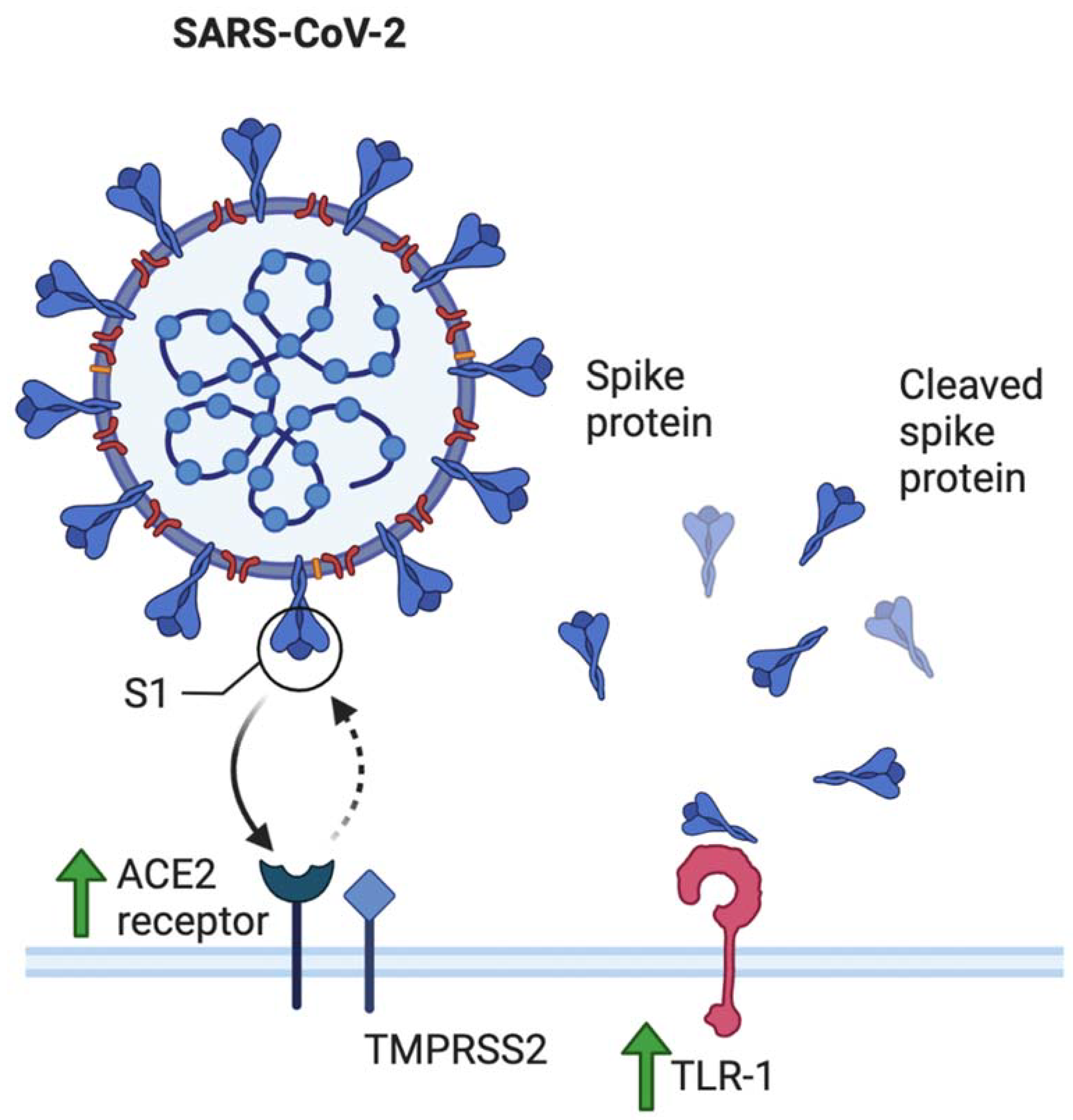
- The role of toll-like receptors is also crucial in this discussion. Young et al. additionally reported elevated levels of TLR-1, TLR-7, and TLR-9 when compared to nonpregnant values in women [31]. This has several implications. Firstly, SARS-CoV-2 spike protein has been shown to bind to TLR-1, as well as TLR-4 and TLR-6, suggesting another mechanism that may increase pregnant women’s susceptibility to COVID-19 infection [32]. TLR-7 is expressed on monocyte-macrophages and dendritic cells, and are important in the recognition of ssRNA viruses such as SARS-CoV-2 [33]. Whole genome sequencing showed that TLR-7 has more ssRNA motifs that can bind to SARS-CoV-2 when compared to SARS-CoV and MERS-CoV [1]. Binding of the S glycoprotein on the surface of the viral envelope to ACE2 may be recognized by TLR-7, leading to an increased production of IL-1, IL-6, monocyte chemoattractant protein-1 (MCP-1), MIP-1A, TNF-α, and type 1 interferons [34], which could lead to a hyperinflammatory state and acute lung injury [25].
3. Implications of Other Pregnancy-Specific Physiological Changes and SARS-CoV-2
3.1. Angiotensin Converting Enzyme
3.2. Human Leukocyte Antigen (HLA)
3.3. Pre-Eclampsia in SARS-CoV-2
3.4. Coagulation in SARS-CoV-2
4. COVID-19 Epidemiological Data and Clinical Concerns in Pregnancy Thus Far
4.1. Overview
4.2. Vertical Transmission
4.3. Treatment and Delivery Protocol
4.4. Breastfeeding
5. Delta (B.1617.2) and Other Variant Considerations
5.1. Concerning Data on the Impact of the Delta Variant on Pregnancies
5.2. Possible Pathophysiologic Explanations for the Increased Morbidity of Delta Variant on Pregnant Patients
5.3. Anticipation of Future Variants
6. Vaccine Considerations for Pregnancy
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| (SARS-CoV-2) | Severe acute respiratory syndrome coronavirus 2 |
| (MERS-CoV) | Middle East respiratory syndrome |
| (COVID-19) | Coronavirus disease 2019 |
| (ARDS) | Acute respiratory distress syndrome |
| (ICU) | Intensive care unit |
| (NICU) | Neonatal intensive care unit |
| (ECMO) | Extracorporeal membrane oxygenation |
| (ssRNA) | Single-stranded RNA |
| (NK cell) | Natural killer cell |
| (pDC) | Plasmatoid dendritic cell |
| (Th) | T helper |
| (Treg) | Regulatory T cell |
| (TLR) | Toll-like receptor |
| (IFN) | Interferon |
| (IL) | Interleukin |
| (MCP-1) | Monocyte chemoattractant protein-1 |
| (MIP-1α) | Macrophage inflammatory protein-1 alpha |
| (TNF-α) | Tumor necrosis factor alpha |
| (ACE2) | Angiotensin converting enzyme 2 |
| (ACOG) | American College of Obstetrics and Gynecology |
| (AJOG) | American Journal of Obstetrics and Gynecology |
| (CDC) | Centers for Disease Control and Prevention |
| (FDA) | Food and Drug Administration |
| (NIH) | National Institutes of Health |
References
- Coronavirus World Map: Tracking the Global Outbreak. Available online: https://www.nytimes.com/interactive/2021/world/covid-cases.html (accessed on 28 November 2021).
- Seasely, A.R.; Blanchard, C.T.; Arora, N.; Battarbee, A.N.; Casey, B.M.; Dionne-Odom, J.; Leal, S.M.; Moates, D.B.; Sinkey, R.G.; Szychowski, J.M.; et al. Maternal and Perinatal Outcomes Associated with the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Delta (B.1.617.2) Variant. Obstet. Gynecol. 2021, 138, 842–844. [Google Scholar] [CrossRef]
- Melenotte, C.; Silvin, A.; Goubet, A.-G.; Lahmar, I.; Dubuisson, A.; Zumla, A.; Raoult, D.; Merad, M.; Gachot, B.; Hénon, C.; et al. Immune responses during COVID-19 infection. OncoImmunology 2020, 9, 1807836. [Google Scholar] [CrossRef] [PubMed]
- Benton, D.J.; Wrobel, A.G.; Xu, P.; Roustan, C.; Martin, S.R.; Rosenthal, P.B.; Skehel, J.J.; Gamblin, S.J. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nat. Cell Biol. 2020, 588, 327–330. [Google Scholar] [CrossRef]
- Hoffmann, M.; Hofmann-Winkler, H.; Pöhlmann, S. Priming Time: How Cellular Proteases Arm Coronavirus Spike Proteins; Springer: Berlin/Heidelberg, Germany, 2018; pp. 71–98. [Google Scholar] [CrossRef] [Green Version]
- Wastnedge, E.A.N.; Reynolds, R.M.; Van Boeckel, S.R.; Stock, S.J.; Denison, F.C.; Maybin, J.A.; Critchley, H.O.D. Pregnancy and COVID-19. Physiol. Rev. 2021, 101, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Nile, A.; Qiu, J.; Li, L.; Jia, X.; Kai, G. COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020, 53, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Ragab, D.; Eldin, H.S.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Darif, D.; Hammi, I.; Kihel, A.; Saik, I.E.I.; Guessous, F.; Akarid, K. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? Microb. Pathog. 2021, 153, 104799. [Google Scholar] [CrossRef]
- Rahimzadeh, M.; Naderi, N. Toward an understanding of regulatory T cells in COVID-19: A systematic review. J. Med Virol. 2021, 93, 4167–4181. [Google Scholar] [CrossRef]
- Updates on COVID-19 and Pregnancy. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-09-22/11-COVID-Meaney-Delman-508.pdf (accessed on 28 November 2021).
- Gil-Etayo, F.J.; Suàrez-Fernández, P.; Cabrera-Marante, O.; Arroyo, D.; Garcinuño, S.; Naranjo, L.; Pleguezuelo, D.E.; Allende, L.M.; Mancebo, E.; Lalueza, A.; et al. T-Helper Cell Subset Response Is a Determining Factor in COVID-19 Progression. Front. Cell. Infect. Microbiol. 2021, 11, 624483. [Google Scholar] [CrossRef]
- Chen, Z.; Wherry, E.J. T cell responses in patients with COVID-19. Nat. Rev. Immunol. 2020, 20, 529–536. [Google Scholar] [CrossRef]
- Alberca, R.W.; Pereira, N.Z.; Oliveira, L.; Gozzi-Silva, S.C.; Sato, M.N. Pregnancy, Viral Infection, and COVID-19. Front. Immunol. 2020, 11, 1672. [Google Scholar] [CrossRef]
- Pavel, A.B.; Glickman, J.W.; Michels, J.R.; Kim-Schulze, S.; Miller, R.L.; Guttman-Yassky, E. Th2/Th1 Cytokine Imbalance Is Associated with Higher COVID-19 Risk Mortality. Front. Genet. 2021, 12, 706902. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.-C.; Lai, E.-Y.; Liu, Y.-T.; Wang, Y.-F.; Tzeng, Y.-S.; Cui, L.; Lai, Y.-J.; Huang, H.-C.; Huang, J.-H.; Ni, H.-C.; et al. NK cell receptor and ligand composition influences the clearance of SARS-CoV-19. J. Clin. Investig. 2021, 131, e146408. [Google Scholar] [CrossRef]
- Tamanna, S.; Clifton, V.L.; Rae, K.; Van Helden, D.F.; Lumbers, E.R.; Pringle, K.G. Angiotensin Converting Enzyme 2 (ACE2) in Pregnancy: Preeclampsia and Small for Gestational Age. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Lampé, R.; Kövér, Á.; Szűcs, S.; Pál, L.; Árnyas, E.; Ádány, R.; Póka, R. Phagocytic index of neutrophil granulocytes and monocytes in healthy and preeclamptic pregnancy. J. Reprod. Immunol. 2015, 107, 26–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siiteri, P.K.; Febres, F.; Clemens, L.E.; Chang, R.J.; Gondos, B.; Stites, D. Progesterone and maintenance of pregnancy: Is progesterone nature’s immunosuppressant? Ann. N. Y. Acad. Sci. 1977, 286, 384–397. [Google Scholar] [CrossRef]
- Hall, O.J.; Nachbagauer, R.; Vermillion, M.S.; Fink, A.L.; Phuong, V.; Krammer, F.; Klein, S.L. Progesterone-Based Contraceptives Reduce Adaptive Immune Responses and Protection against Sequential Influenza A Virus Infections. J. Virol. 2017, 91, e02160-16. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Yang, L.; Wang, X.; Wang, Y.; Wei, Y.; Zhao, Y. Decline of Plasmacytoid Dendritic Cells and Their Subsets in Normal Pregnancy Are Related with Hormones. J. Reprod. Med. 2015, 60, 423–429. [Google Scholar]
- Reizis, B. Plasmacytoid Dendritic Cells: Development, Regulation, and Function. Immunity 2019, 50, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Vanders, R.L.; Gibson, P.G.; Murphy, V.E.; Wark, P.A.B. Plasmacytoid Dendritic Cells and CD8 T Cells From Pregnant Women Show Altered Phenotype and Function Following H1N1/09 Infection. J. Infect. Dis. 2013, 208, 1062–1070. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Shin, E.-C. The type I interferon response in COVID-19: Implications for treatment. Nat. Rev. Immunol. 2020, 20, 585–586. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, S.; Rezaei, N. Role of Toll-like receptors in the pathogenesis of COVID-19. J. Med. Virol. 2021, 93, 2735–2739. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef]
- Richani, K.; Soto, E.; Romero, R.; Espinoza, J.; Chaiworapongsa, T.; Nien, J.K.; Edwin, S.; Kim, Y.M.; Hong, J.-S.; Mazor, M. Normal pregnancy is characterized by systemic activation of the complement system. J. Matern. Neonatal Med. 2005, 17, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Derzsy, Z.; Prohaszka, Z., Jr.; Füst, G.; Molvarec, A. Activation of the complement system in normal pregnancy and preeclampsia. Mol. Immunol. 2010, 47, 1500–1506. [Google Scholar] [CrossRef]
- Java, A.; Apicelli, A.J.; Liszewski, M.K.; Coler-Reilly, A.; Atkinson, J.P.; Kim, A.H.; Kulkarni, H.S. The complement system in COVID-19: Friend and foe? JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Gralinski, L.E.; Sheahan, T.P.; Morrison, T.E.; Menachery, V.; Jensen, K.; Leist, S.R.; Whitmore, A.; Heise, M.T.; Baric, R.S. Complement Activation Contributes to Severe Acute Respiratory Syndrome Coronavirus Pathogenesis. mBio 2018, 9, e01753-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, B.C.; Stanic, A.K.; Panda, B.; Rueda, B.R.; Panda, A. Longitudinal expression of Toll-like receptors on dendritic cells in uncomplicated pregnancy and postpartum. Am. J. Obstet. Gynecol. 2013, 210, 445.e1–445.e6. [Google Scholar] [CrossRef] [Green Version]
- Patra, R.; Das, N.C.; Mukherjee, S. Targeting human TLRs to combat COVID-19: A solution? J. Med. Virol. 2020, 93, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Petes, C.; Odoardi, N.; Gee, K.; Petes, C.; Odoardi, N.; Gee, K. The Toll for Trafficking: Toll-Like Receptor 7 Delivery to the Endosome. Front. Immunol. 2017, 8, 1075. [Google Scholar] [CrossRef] [Green Version]
- Yazdanpanah, F.; Hamblin, M.R.; Rezaei, N. The immune system and COVID-19: Friend or foe? Life Sci. 2020, 256, 117900. [Google Scholar] [CrossRef]
- Tamanna, S.; Lumbers, E.R.; Morosin, S.K.; Delforce, S.J.; Pringle, K.G. ACE2: A key modulator of RAS and pregnancy. Am. J. Physiol. Integr. Comp. Physiol. 2021. [Google Scholar] [CrossRef]
- Irani, R.A.; Xia, Y. Renin Angiotensin Signaling in Normal Pregnancy and Preeclampsia. Semin. Nephrol. 2011, 31, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Ober, C. HLA and Pregnancy: The Paradox of the Fetal Allograft. Am. J. Hum. Genet. 1998, 62, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Hunt, J.S.; Langat, D.K.; McIntire, R.H.; Morales, P.J. The role of HLA-G in human pregnancy. Reprod. Biol. Endocrinol. 2006, 4, S10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McElwain, C.J.; Tuboly, E.; McCarthy, F.P.; McCarthy, C.M. Mechanisms of Endothelial Dysfunction in Pre-eclampsia and Gestational Diabetes Mellitus: Windows Into Future Cardiometabolic Health? Front. Endocrinol. 2020, 11, 655. [Google Scholar] [CrossRef] [PubMed]
- Gathiram, P.; Moodley, J. Pre-eclampsia: Its pathogenesis and pathophysiolgy. Cardiovasc. J. Afr. 2016, 27, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Eutimio, M.A.; Tovar-Rodríguez, J.M.; Vargas-Avila, K.; Nieto-Velázquez, N.G.; Frías-De-León, M.G.; Sierra-Martinez, M.; Acosta-Altamirano, G. Increased Serum Levels of Inflammatory Mediators and Low Frequency of Regulatory T Cells in the Peripheral Blood of Preeclamptic Mexican Women. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nath, M.C.; Cubro, H.; McCormick, D.J.; Milic, N.M.; Garovic, V.D. Preeclamptic Women Have Decreased Circulating IL-10 (Interleukin-10) Values at the Time of Preeclampsia Diagnosis. Hypertension 2020, 76, 1817–1827. [Google Scholar] [CrossRef]
- Di Mascio, D.; Khalil, A.; Saccone, G.; Rizzo, G.; Buca, D.; Liberati, M.; Vecchiet, J.; Nappi, L.; Scambia, G.; Berghella, V.; et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2020, 2, 100107. [Google Scholar] [CrossRef]
- Siennicka, A.; Kłysz, M.; Chełstowski, K.; Tabaczniuk, A.; Marcinowska, Z.; Tarnowska, P.; Kulesza, J.; Torbe, A.; Jastrzębska, M. Reference Values of D-Dimers and Fibrinogen in the Course of Physiological Pregnancy: The Potential Impact of Selected Risk Factors—A Pilot Study. BioMed Res. Int. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Favresse, J.; Lippi, G.; Roy, P.-M.; Chatelain, B.; Jacqmin, H.; Cate, H.T.; Mullier, F. D-dimer: Preanalytical, analytical, postanalytical variables, and clinical applications. Crit. Rev. Clin. Lab. Sci. 2018, 55, 548–577. [Google Scholar] [CrossRef]
- Miesbach, W.; Makris, M. COVID-19: Coagulopathy, Risk of Thrombosis, and the Rationale for Anticoagulation. Clin. Appl. Thromb. 2020, 26, 1076029620938149. [Google Scholar] [CrossRef]
- Barma, P.; Tazrean, S.; Khalil, I. D-Dimer May be a Prognostic Hematological Marker for COVID-19: A Retrospective Case Analysis. Mymensingh Med. J. 2021, 30, 1177. [Google Scholar]
- Coronavirus (COVID-19) Infection and Abortion Care. Available online: https://www.rcog.org.uk/globalassets/documents/guidelines/2020-07-31-coronavirus-covid-19-infection-and-abortion-care.pdf (accessed on 28 November 2021).
- Antithrombotic Therapy in Patients With COVID-19. Available online: https://www.covid19treatmentguidelines.nih.gov/therapies/antithrombotic-therapy/ (accessed on 28 November 2021).
- COVID-19 FAQs for Obstetrician-Gynecologists, Obstetrics. Available online: https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics (accessed on 28 November 2021).
- Esumi, N.; Ikushima, S.; Hibi, S.; Todo, S.; Imashuku, S. High serum ferritin level as a marker of malignant histiocytosis and virus-associated hemophagocytic syndrome. Cancer 1988, 61, 2071–2076. [Google Scholar] [CrossRef]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef]
- Wei, S.Q.; Bilodeau-Bertrand, M.; Liu, S.; Auger, N. The impact of COVID-19 on pregnancy outcomes: A systematic review and meta-analysis. Can. Med Assoc. J. 2021, 193, E540–E548. [Google Scholar] [CrossRef] [PubMed]
- Fenizia, C.; Biasin, M.; Cetin, I.; Vergani, P.; Mileto, D.; Spinillo, A.; Gismondo, M.R.; Perotti, F.; Callegari, C.; Mancon, A.; et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Kotlyar, A.M.; Grechukhina, O.; Chen, A.; Popkhadze, S.; Grimshaw, A.; Tal, O.; Taylor, H.S.; Tal, R. Vertical transmission of coronavirus disease 2019: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2020, 224, 35–53.e3. [Google Scholar] [CrossRef]
- Pique-Regi, R.; Romero, R.; Tarca, A.L.; Luca, F.; Xu, Y.; Alazizi, A.; Leng, Y.; Hsu, C.-D.; Gomez-Lopez, N. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? eLife 2020, 9, e58716. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Tang, M.; Gao, Y.; Zhang, H.; Yang, Y.; Zhang, D.; Wang, H.; Liang, H.; Zhang, R.; Wu, B. Cesarean Section or Vaginal Delivery to Prevent Possible Vertical Transmission from a Pregnant Mother Confirmed With COVID-19 to a Neonate: A Systematic Review. Front. Med. 2021, 8, 634949. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Preliminary report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Davis, M.R.; E Lapinsky, S. A review of remdesivir for COVID-19 in pregnancy and lactation. J. Antimicrob. Chemother. 2021, dkab311. [Google Scholar] [CrossRef]
- Burwick, R.M.; Yawetz, S.; Stephenson, K.E.; Collier, A.-R.Y.; Sen, P.; Blackburn, B.G.; Kojic, E.M.; Hirshberg, A.; Suarez, J.F.; Sobieszczyk, M.E.; et al. Compassionate Use of Remdesivir in Pregnant Women With Severe Coronavirus Disease. Clin. Infect. Dis. 2020, ciaa1466. [Google Scholar] [CrossRef] [PubMed]
- Mammen, M.; Aryal, K.; Alhazzani, W.; Alexander, P.E. Corticosteroids for patients with acute respiratory distress syndrome: A systematic review and meta-analysis of randomized trials. Pol. Arch. Intern Med. 2020, 130, 276–286. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Mullins, E.; Hudak, M.L.; Banerjee, J.; Getzlaff, T.; Townson, J.; Barnette, K.; Playle, R.; Bourne, T.; Lees, C.; PAN-COVID In-vestigators and the National Perinatal COVID-19 Registry Study Group. Pregnancy and neonatal outcomes of COVID-19: Co-reporting of common outcomes from PAN-COVID and AAP SONPM registries. Ultrasound Obs. Gynecol. 2021, 57, 573–581. [Google Scholar] [CrossRef]
- Corticosteroids. Available online: https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/corticosteroids/ (accessed on 28 November 2021).
- Sukarna, N.; Tan, P.C.; Hong, J.G.S.; Sulaiman, S.; Omar, S.Z. Glycemic control following two regimens of antenatal corticosteroids in mild gestational diabetes: A randomized controlled trial. Arch. Gynecol. Obstet. 2021, 304, 345–353. [Google Scholar] [CrossRef]
- Novel Coronavirus 2019 (COVID-19). Available online: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/novel-coronavirus-2019 (accessed on 28 November 2021).
- Raschetti, R.; Vivanti, A.J.; Vauloup-Fellous, C.; Loi, B.; Benachi, A.; De Luca, D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Perl, S.H.; Uzan-Yulzari, A.; Klainer, H.; Asiskovich, L.; Youngster, M.; Rinott, E.; Youngster, I. SARS-CoV-2-Specific Antibodies in Breast Milk After COVID-19 Vaccination of Breastfeeding Women. JAMA 2021, 325, 2013–2014. [Google Scholar] [CrossRef] [PubMed]
- Valcarce, V.; Stafford, L.S.; Neu, J.; Cacho, N.; Parker, L.; Mueller, M.; Burchfield, D.J.; Li, N.; Larkin, J. Detection of SARS-CoV-2-Specific IgA in the Human Milk of COVID-19 Vaccinated Lactating Health Care Workers. Breastfeed. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Delta Variant: What We Know About the Science. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html (accessed on 28 November 2021).
- Fisman, D.N.; Tuite, A.R. Evaluation of the relative virulence of novel SARS-CoV-2 variants: A retrospective cohort study in Ontario, Canada. Can. Med Assoc. J. 2021, 193, E1619–E1625. [Google Scholar] [CrossRef] [PubMed]
- Increasing severity of COVID-19 in Pregnancy with Delta (B.1.617.2) Variant Surge. Available online: https://www.ajog.org/article/S0002-9378(21)01005-X/fulltext#relatedArticles (accessed on 28 November 2021).
- Teyssou, E.; Delagrèverie, H.; Visseaux, B.; Lambert-Niclot, S.; Brichler, S.; Ferre, V.; Marot, S.; Jary, A.; Todesco, E.; Schnuriger, A.; et al. The Delta SARS-CoV-2 variant has a higher viral load than the Beta and the historical variants in nasopharyngeal samples from newly diagnosed COVID-19 patients. J. Infect. 2021, 83, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Johnson, B.A.; Xia, H.; Ku, Z.; Schindewolf, C.; Widen, S.G.; An, Z.; Weaver, S.C.; Menachery, V.D.; et al. Delta spike P681R mutation enhances SARS-CoV-2 fitness over Alpha variant. bioRxiv Prepr. 2021, 5, 456173. [Google Scholar] [CrossRef]
- SARS-CoV-2 Spike P681R Mutation, a Hallmark of the Delta Variant, Enhances Viral Fusogenicity and Pathogenicity. Available online: https://www.biorxiv.org/content/10.1101/2021.06.17.448820v2 (accessed on 28 November 2021).
- Paudel, S.; Dahal, A.; Bhattarai, H.K. Temporal Analysis of SARS-CoV-2 Variants during the COVID-19 Pandemic in Nepal. COVID 2021, 1, 423–434. [Google Scholar] [CrossRef]
- Vijgen, L.; Keyaerts, E.; Moës, E.; Thoelen, I.; Wollants, E.; Lemey, P.; Vandamme, A.-M.; Van Ranst, M. Complete Genomic Sequence of Human Coronavirus OC43: Molecular Clock Analysis Suggests a Relatively Recent Zoonotic Coronavirus Transmission Event. J. Virol. 2005, 79, 1595–1604. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zeng, F.; Rao, J.; Yuan, S.; Ji, M.; Lei, X.; Xiao, X.; Li, Z.; Li, X.; Du, W.; et al. Comparison of Clinical Features and Outcomes of Medically Attended COVID-19 and Influenza Patients in a Defined Population in the 2020 Respiratory Virus Season. Front. Public Health 2021, 9, 587425. [Google Scholar] [CrossRef]
- Manzanares-Meza, L.D.; Medina-Contreras, O. SARS-CoV-2 and influenza: A comparative overview and treatment implications. Bol. Med. Hosp. Infant Mex. 2020, 77, 262–273. [Google Scholar] [CrossRef]
- Kannan, S.R.; Spratt, A.N.; Cohen, A.R.; Naqvi, S.H.; Chand, H.S.; Quinn, T.P.; Lorson, C.L.; Byrareddy, S.N.; Singh, K. Evolutionary analysis of the Delta and Delta Plus variants of the SARS-CoV-2 viruses. J. Autoimmun. 2021, 124, 102715. [Google Scholar] [CrossRef]
- An Offshoot of the Delta Variant is Rising in the U.K. Available online: https://www.nationalgeographic.com/science/article/an-offshoot-of-the-delta-variant-is-rising-in-the-uk (accessed on 28 November 2021).
- COVID-19 Vaccines. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines (accessed on 28 November 2021).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangchaikul, P.; Venketaraman, V. SARS-CoV-2 and the Immune Response in Pregnancy with Delta Variant Considerations. Infect. Dis. Rep. 2021, 13, 993-1008. https://doi.org/10.3390/idr13040091
Rangchaikul P, Venketaraman V. SARS-CoV-2 and the Immune Response in Pregnancy with Delta Variant Considerations. Infectious Disease Reports. 2021; 13(4):993-1008. https://doi.org/10.3390/idr13040091
Chicago/Turabian StyleRangchaikul, Patrida, and Vishwanath Venketaraman. 2021. "SARS-CoV-2 and the Immune Response in Pregnancy with Delta Variant Considerations" Infectious Disease Reports 13, no. 4: 993-1008. https://doi.org/10.3390/idr13040091
APA StyleRangchaikul, P., & Venketaraman, V. (2021). SARS-CoV-2 and the Immune Response in Pregnancy with Delta Variant Considerations. Infectious Disease Reports, 13(4), 993-1008. https://doi.org/10.3390/idr13040091






