Inducible Clindamycin Resistance and Biofilm Production among Staphylococci Isolated from Tertiary Care Hospitals in Nepal
Abstract
:1. Introduction
2. Methods
2.1. Bacterial Isolates
2.2. Antibiotic Susceptibility Test
2.3. Detection of Inducible Clindamycin-Resistant Strains
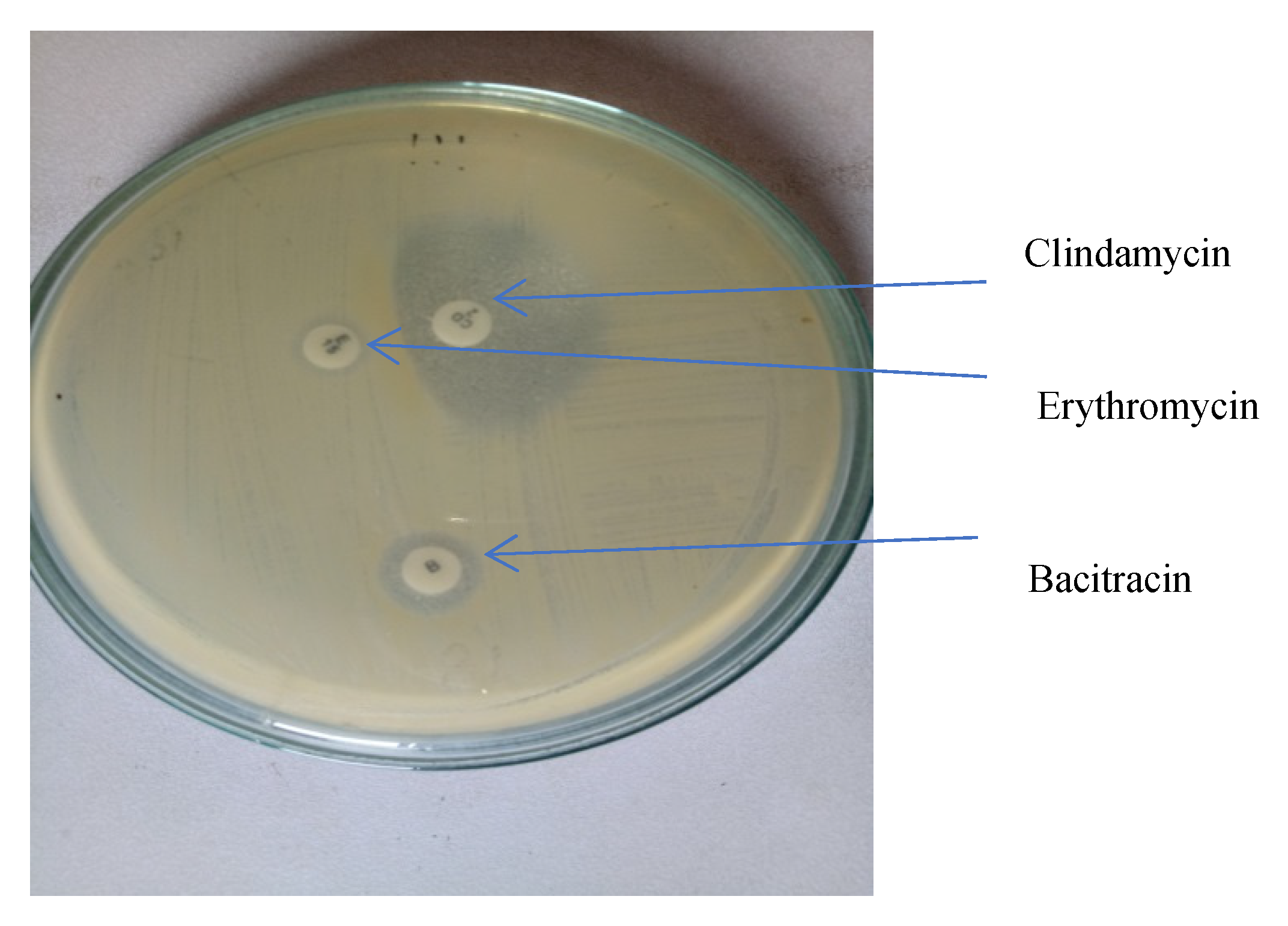
- Inducible MLSB (iMLSB) phenotype—those isolates resistant to erythromycin but sensitive to clindamycin, showing a D-shaped zone of inhibition around clindamycin with flattening towards erythromycin disc.
- Constitutive MLSB (cMLSB) phenotype—those isolates resistant to both erythromycin and clindamycin.
- MS phenotype—those isolates resistant to erythromycin and sensitive to clindamycin.
2.4. Detection of Biofilm Formation
2.5. DNA Extraction and Detection of ica Genes
2.6. Statistical Analysis
3. Results
3.1. Antibiotic Susceptibilities of Staphylococcal Isolates
3.2. Inducible Clindamycin Resistance among Staphylococci
3.3. Detection of Biofilm Formation by Phenotypic and Genotypic Methods
3.4. Inducible Resistant Phenotype among Biofilm Producing Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yilmaz, G.; Ayden, K.; Iskender, S.; Caylan, R.; Koksal, I. Detection and prevalence of inducible Clindamycin resistance in staphylococci. J. Med. Microbiol. 2007, 56, 342–345. [Google Scholar] [CrossRef] [Green Version]
- Appelbaum, P.C. Microbiol of antibiotic resistance in Staphylococcus aureus. Clin. Infect. Dis. 2007, 45, S165–S170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, A.L.; Bayer, A.S.; Zhang, G.; Gresham, H.; Xiong, Y.Q. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 2004, 40, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Otto, M. Staphylococcal Biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 207–228. [Google Scholar]
- Rohde, H.; Burandt, E.C.; Siemssen, N.; Frommelt, L.; Burdelski, C.; Wurster, S.; Scherpe, S.; Davies, A.P.; Harris, L.G.; Horstkotte, M.A.; et al. Polysaccharide intercellular adhesin or protein factors in biofilm accumulation of Staphylococcus epidermidis and Staphylococcus aureus isolated from prosthetic hip and knee joint infections. Biomaterials 2007, 28, 1711–1720. [Google Scholar] [CrossRef]
- Deotale, V.; Mendiratta, D.K.; Raut, U.; Narang, P. Inducible clindamycin resistance in Staphylococcus aureus isolated from clinical samples. Indian J. Med. Microbiol. 2010, 28, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Lee, H.; Roh, K.H.; Yum, J.H.; Yong, D.; Lee, K.; Chong, Y. Prevalence of inducible clindamycin resistance in staphylococcal isolates at Korean Tertiary Care Hospital. Yonsei Med. J. 2006, 47, 480–484. [Google Scholar] [CrossRef]
- Schreckenberger, P.C.; Ilendo, E.; Ristow, K.L. Incidence of constitutive and inducible clindamycin resistance in Staphylococcus aureus and coagulase negative staphylococci in a community and a tertiary care hospital. J. Clin. Microbiol. 2004, 42, 2777–2779. [Google Scholar] [CrossRef] [Green Version]
- Leclercq, R. Mechanism of resistance to macrolides and lincosamides: Nature of the resistance elements and their clinical implications. Clin. Infect. Diseases 2002, 34, 482–492. [Google Scholar] [CrossRef] [Green Version]
- Fiebelkorn, K.R.; Crawford, S.A.; McElmeel, M.L.; Jorgensen, J.H. Practical disk diffusion method for detection of inducible clindamycin resistance in Staphylococcus aureus and coagulase-negative staphylococci. J. Clin. Microbiol. 2003, 41, 4740–4744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 25th ed.; Informational Supplement M100-S25; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Forbes, B.; Sahm, D.; Weissfeld, A. Bailey & Scott’s Diagnostic Microbiology; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Christensen, G.; Simpson, W.; Younger, J.; Baddour, L.; Barrett, F.; Melton, D.; Beachey, E. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef] [Green Version]
- Mathur, T.S.S.; Khan, S.; Upadhyay, D.J.; Fatma, T.; Rattan, A. Detection of biofilm formation among the clinical isolates of staphylococci: An evaluation of three different screening methods. Indian J. Med. Microbiol. 2006, 24, 19–25. [Google Scholar] [CrossRef]
- Manandhar, S.; Singh, A.; Varma, A.; Pandey, S.; Shrivastava, N. Biofilm producing clinical Staphylococcus aureus isolates augmented prevalence of antibiotic resistant cases in tertiary care hospitals of Nepal. Front. Microbiol. 2018, 9, 2749. [Google Scholar] [CrossRef]
- Martin-Lopez, J.V.; Perez-Roth, E.; Claverie-Martin, F.; Diez, O.; Ninive, B.; Morales, M.; Mendez-Alvarez, S. Detection of Staphylococcus aureus clinical isolates harboring the ica gene cluster needed for biofilm establishment. J. Clin. Microbiol. 2002, 40, 1569–1570. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatta, D.R.; Cavaco, L.M.; Nath, G.; Gaur, A.; Gokhale, S.; Bhatta, D.R. Threat of multidrug resistant Staphylococcus aureus in Western Nepal. Asian Pac. J. Trop. Dis. 2015, 5, 617–621. [Google Scholar] [CrossRef]
- Ansari, S.; Nepal, H.P.; Gautam, R.; Rayamajhi, N.; Shrestha, S.; Upadhyay, G.; Acharya, A.; Chapagain, M.L. Threat of drug resistant Staphylococcus aureus to health in Nepal. BMC Infect. Dis. 2014, 14, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhiya, R.K.; Shrestha, A.; Rai, S.K.; Panta, K. Prevalence of Methicillin-Resistant Staphylococcus aureus in Hospitals of Kathmandu Valley. Nepal J. Sci. Technol. 2012, 13, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Diekema, D.J.; Pfaller, M.A.; Schmitz, F.J.; Smayevsky, J.; Bell, J.; Jones, R.N.; Beach, M. Survey of infections due to Staphylococcus species: Frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect. Dis. 2001, 33, S114–S132. [Google Scholar]
- Yousefi, M.; Pourmand, M.R.; Fallah, F.; Hashemi, A.; Mashhadi, R.; Nazari-Alam, R. Characterization of Staphylococcus aureus biofilm formation in urinary tract infection. Iran J. Public Health 2016, 45, 485–493. [Google Scholar]
- Pandey, S.; Raza, M.S.; Bhatta, C.P. Prevalence and antibiotic sensitivity pattern of methicillin resistant Staphylococcus aureus in Kathmandu Medical College Teaching Hospital. J. Inst. Med. 2012, 34, 13–17. [Google Scholar] [CrossRef]
- Belbase, A.; Pant, N.D.; Nepal, K.; Neupane, B. Antibiotic resistance and biofilm production among the strains of Staphylococcus aureus isolated from pus/wound swab samples in a tertiary care hospital in Nepal. Ann. Clin. Microbiol. Antimicrob. 2016, 16, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Ghasemian, A.; Peerayeh, S.N.; Bakhshi, B.; Mirzaee, M. Comparison of biofilm formation between methicillin-resistant and methicillin-susceptible isolates of Staphylococcus aureus. Iran. Biomed. J. 2016, 20, 175–181. [Google Scholar]
- Perez, L.R.R.; Caierão, J.; Antunes, A.L.S.; d’Azevedo, P.A. Use of the D test method to detect inducible clindamycin resistance in coagulase negative staphylococci (CoNS). Braz. J. Infect. Dis. 2007, 11, 186–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knobloch, J.K.M.; Horstkotte, M.A.; Rohde, H.; Mack, D. Evaluation of different detection methods of biofilm formation in Staphylococcus aureus. Med. Microbiol. Immunol. 2002, 191, 101–106. [Google Scholar] [CrossRef]
- Zhou, S.; Chao, X.; Fei, M.; Dai, Y.; Liu, B. Analysis of Staphylococcus epidermidis icaA and icaD genes by polymerase chain reaction and slime production: A case control study. BMC Infect. Dis. 2013, 13, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cafiso, V.; Bertuccio, T.; Santagati, M.; Campanile, F.; Amicosante, G.; Perilli, M.G.; Selan, L.; Artini, M.; Nicoletti, G.; Stefani, S. Presence of the ica operon in clinical isolates of Staphylococcus epidermidis and its role in biofilm production. Clin. Microbiol. Infect. 2004, 10, 1081–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, S.; Nayak, N.; Satpathy, G.; Nag, H.L.; Venkatesh, P.; Ramakrishnan, S.; Ghose, S.; Nag, T.C. Molecular & phenotypic characterization of Staphylococcus epidermidis in implant related infections. Indian J. Med. Res. 2012, 136, 483–490. [Google Scholar]
- Arciola, C.R.; Campoccia, D.; Ravaioli, S.; Montanaro, L. Polysaccharide intercellular adhesin in biofilm: Structural and regulatory aspects. Front. Cell. Infect. Microbiol. 2015, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, L.B.; Bhattarai, N.R.; Khanal, B. Antibiotic resistance and biofilm formation among coagulase-negative staphylococci isolated from clinical samples at a tertiary care hospital of eastern Nepal. Antimicrob. Resist. Infect. Control 2017, 6, 89. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.; Cunha, M.D.L.R. Comparison of methods for the detection of biofilm production in coagulase-negative staphylococci. BMC Res. Notes 2010, 3, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Los, R.; Sawicki, R.; Juda, M.; Stankevic, M.; Rybojad, P.; Sawicki, M.; Malm, A.; Ginalska, G. A comparative analysis of phenotypic and genotypic methods for the determination of the biofilm-forming abilities of Staphylococcus epidermidis. FEMS Microbiol. Lett. 2010, 310, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasr, R.A.; AbuShady, H.M.; Hussein, H.S. Biofilm formation and presence of icaAD gene in clinical isolates of staphylococci. Egypt. J. Med. Hum. Genet. 2012, 13, 269–274. [Google Scholar] [CrossRef] [Green Version]
- de Silva, G.D.I.; Kantzanou, M.; Justice, A.; Massey, R.C.; Wilkinson, A.R.; Day, N.P.J.; Peacock, S.J. The ica operon and biofilm production in coagulase-negative staphylococci associated with carriage and disease in a neonatal intensive care unit. J. Clin. Microbiol. 2002, 40, 382–388. [Google Scholar] [CrossRef] [PubMed]
| Antimicrobial Agent | Antimicrobial Class | S. aureus (n = 161) | CNS (n = 214) | Total (n = 375) | |||
|---|---|---|---|---|---|---|---|
| Sensitive n (%) | Resistant n (%) | Sensitive n (%) | Resistant n (%) | Sensitive n (%) | Resistant n (%) | ||
| Penicillin | β lactams | 8 (5%) | 153 (95%) | 18 (8.4%) | 196 (91.6%) | 26 (6.9%) | 349 (93.1%) |
| Ciprofloxacin | Fluroquinolone | 42 (26.1%) | 119 (73.9%) | 138 (64.5%) | 76 (35.5%) | 180 (48%) | 195 (52%) |
| Tetracycline | Tetracycline | 152 (94.4%) | 9 (5.6%) | 187 (87.4%) | 27 (12.6%) | 339 (90.4%) | 36 (9.6%) |
| Clindamycin | Lincosamide | 145 (90.1%) | 16 (9.9%) | 151 (70.6%) | 63 (29.4%) | 296 (78.9%) | 79 (21.1%) |
| Chloramphenicol | Phenicols | 157 (97.5%) | 4 (2.5%) | 195 (91.1%) | 19 (8.9%) | 352 (93.9%) | 23 (6.1%) |
| Cefoxitin | β lactams | 30 (18.6%) | 131 (81.4%) | 71 (33.2%) | 143 (66.8%) | 101 (26.9%) | 274 (73.1%) |
| Erythromycin | Macrolides | 34 (21.1%) | 127 (78.9%) | 59 (27.6%) | 155 (72.4%) | 93 (24.8%) | 282 (75.2%) |
| Cotrimoxazole | Folic acid synthesis inhibitors | 71 (44.1%) | 90 (55.9%) | 134 (62.6%) | 80 (37.4%) | 205 (54.7%) | 170 (45.3%) |
| Gentamicin | Aminoglycosides | 94 (58.4%) | 67 (41.6%) | 169 (79%) | 45 (21%) | 263 (70.1%) | 112 (9.9%) |
| Phenotypes | S. aureus (n, %) | CNS (n, %) | Total (n, %) | p Value | OR | 95% CI |
|---|---|---|---|---|---|---|
| E-S, CD-S (susceptible) | 31 (19.3%) | 53 (24.8%) | 84 (22.4%) | 0.303 | 0.77 | 0.47–1.26 |
| E-R, CD-R (cMLSB) | 17 (10.6%) | 60 (28.0%) | 77 (20.5%) | ≤0.001 | 0.18 | 0.08–0.41 |
| E-R, CD-S, D+ (iMLSB) | 56 (34.8%) | 31 (14.5%) | 87 (23.2%) | 0.046 | 0.54 | 0.29–0.99 |
| E-R, CD-S, D− (MS phenotype) | 57 (35.4%) | 70 (32.7%) | 127 (33.9%) | ≤0.001 | 2.70 | 1.60–4.55 |
| Total | 161 (100%) | 214 (100%) | 375 (100%) |
| Phenotype | MRSA n (%) | MSSA n (%) | p Value | OR | 95% CI | MRCNS n (%) | MSCNS n (%) | p Value | OR | 95% CI | Total n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| E-S, CD-S (susceptible) | 20 (15.3%) | 11 (36.7%) | 0.001 | 0.24 | 0.09–0.56 | 18 (12.6%) | 35 (49.3%) | ≤0.001 | 0.15 | 0.08–0.29 | 84 (22.4%) |
| E-R, CD-R (cMLSB) | 15 (11.4%) | 2 (6.7%) | 0.052 | 1.26 | 1.16–1.37 | 51 (35.7%) | 9 (12.7%) | ≤0.001 | 4.11 | 1.82–9.25 | 77 (20.5%) |
| E-R, CD-S, D+ (iMLSB) | 42 (32.1%) | 14 (46.7%) | 0.034 | 3.18 | 1.04–9.68 | 25 (17.5%) | 6 (8.5%) | 0.157 | 1.97 | 0.76–5.10 | 87 (23.2%) |
| E-R, CD-S, D− (MS phenotype) | 54 (41.2%) | 3 (10.0%) | 0.714 | 0.86 | 0.39–1.92 | 49 (34.3%) | 21 (29.6%) | 0.318 | 1.37 | 0.74–2.55 | 127 (33.9%) |
| Total | 131 (34.9%) | 30 (8.0%) | 143 (38.1%) | 71 (18.9%) | 375 |
| Method | Biofilm Formation | MRSA (131) | MSSA (30) | p Value | MRCNS (143) | MSCNS (71) | p Value | Total (375) |
| TCP | Positive | 70 (53.4%) | 14 (46.7%) | 0.681 | 58 (40.6%) | 32 (45.1%) | 0.412 | 174 (46.4%) |
| Negative | 61 (46.6%) | 16 (53.3%) | 85 (59.4%) | 39 (54.9%) | 201 (53.6%) | |||
| ica genes | Positive | 29 (22.1%) | 16 (53.3%) | 0.001 | 28 (19.6%) | 13 (18.3%) | 0.824 | 86 (22.9%) |
| Negative | 102 (77.9%) | 14 (46.7%) | 45 (31.5%) | 58 (81.7%) | 289 (7.1%) |
| Phenotypes | Biofilm Detection | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TCP | Ica | |||||||||
| Positive | Negative | p Value | OR | 95% CI | Positive | Negative | p Value | OR | 95% CI | |
| E-S, CD-S (susceptible) | 43 (24.7%) | 44 (21.9%) | 0.446 | 0.83 | 0.51–1.34 | 31 (36.0%) | 55 (19.0%) | 0.001 | 0.42 | 0.25–0.71 |
| E-R, CD-R (cMLSB) | 35 (20.1%) | 41 (20.4%) | 0.503 | 1.23 | 0.67–2.24 | 11 (12.8%) | 61 (21.1%) | 0.210 | 1.66 | 0.75–3.68 |
| E-R, CD-S, D+ (iMLSB) | 27 (15.5%) | 49 (24.4%) | 0.043 | 1.71 | 1.01–2.88 | 19 (22.1%) | 56 (19.4%) | 0.580 | 0.85 | 0.47–1.52 |
| E-R, CD-S, D− (MS phenotype) | 69 (39.7%) | 67 (33.3%) | 0.66 | 1.14 | 0.64–2.03 | 25 (29.1%) | 117 (40.5%) | 0.575 | 1.22 | 0.60–2.49 |
| Total | 174 (46.4%) | 201 (53.6%) | 86 (22.9%) | 289 (7.1%) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manandhar, S.; Shrestha, R.; Tuladhar, R.S.; Lekhak, S. Inducible Clindamycin Resistance and Biofilm Production among Staphylococci Isolated from Tertiary Care Hospitals in Nepal. Infect. Dis. Rep. 2021, 13, 1043-1052. https://doi.org/10.3390/idr13040095
Manandhar S, Shrestha R, Tuladhar RS, Lekhak S. Inducible Clindamycin Resistance and Biofilm Production among Staphylococci Isolated from Tertiary Care Hospitals in Nepal. Infectious Disease Reports. 2021; 13(4):1043-1052. https://doi.org/10.3390/idr13040095
Chicago/Turabian StyleManandhar, Sarita, Raju Shrestha, Ratna Shova Tuladhar, and Sunil Lekhak. 2021. "Inducible Clindamycin Resistance and Biofilm Production among Staphylococci Isolated from Tertiary Care Hospitals in Nepal" Infectious Disease Reports 13, no. 4: 1043-1052. https://doi.org/10.3390/idr13040095
APA StyleManandhar, S., Shrestha, R., Tuladhar, R. S., & Lekhak, S. (2021). Inducible Clindamycin Resistance and Biofilm Production among Staphylococci Isolated from Tertiary Care Hospitals in Nepal. Infectious Disease Reports, 13(4), 1043-1052. https://doi.org/10.3390/idr13040095





