Oral Fosfomycin Formulation in Bacterial Prostatitis: New Role for an Old Molecule-Brief Literature Review and Clinical Considerations
Abstract
:1. Introduction
2. Fosfomycin Pharmacology
2.1. Mechanism of Action
2.2. Antimicrobial Spectrum
2.3. Resistance Mechanisms
2.4. Pharmacokinetic Properties
2.5. Adverse Drug Reaction
3. Rationale for Oral Fosfomycin Administration in Patients with Bacterial Prostatitis
4. Oral Fosfomycin in Chronic Bacterial Prostatitis
5. Oral Fosfomycin in Acute Bacterial Prostatitis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABP | Acute bacterial prostatitis |
| ATP | Adenosine triphosphate |
| AUC | Area under the concentration time curve |
| CIP | Category I prostatitis |
| CIIP | Category II prostatitis |
| CBP | Chronic bacterial prostatitis |
| CLSI | Clinical and laboratory standards institute |
| cAMP | Cyclic adenosine monophosphate |
| ECOFF | Epidemiological cut-off |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| ESBL | Extended spectrum beta-lactamases |
| G6P | Glucose-6-phosphate |
| UhpT | Hexose-6-phosphate transport systems |
| IV | Intra-venous |
| GlpT | L-alpha-glycerophosphate transport system |
| MRSA | Methicillin resistant Staphylococcus aureus |
| MIC | Minimum inhibitory concentration |
| MDR | Multidrug resistant |
| NIH | National Institutes of Health |
| PD | Pharmacodynamic |
| PK | Pharmacokinetic |
| PTS | Sugar phosphotransferase system |
| TURP | Transurethral resection of the prostate |
| UDPMurNAc | UDP N-acetylmuramic acid |
| UNAG | UDP-N-acetylglucosamine |
| UTI | Urinary tract infections |
| Vd | Volume of distribution |
References
- Bouiller, K.; Zayet, S.; Lalloz, P.E.; Potron, A.; Gendrin, V.; Chirouze, C.; Klopfenstein, T. Efficacy and Safety of Oral Fosfomycin-Trometamol in Male Urinary Tract Infections with Multidrug-Resistant Enterobacterales. Antibiotics 2022, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- NIH Consensus Definition and Classification of Prostatitis|JAMA|JAMA Network. Available online: https://jamanetwork.com/journals/jama/article-abstract/1030245 (accessed on 4 July 2022).
- Kwan, A.C.F.; Beahm, N.P. Fosfomycin for bacterial prostatitis: A review. Int. J. Antimicrob. Agents 2020, 56, 106106. [Google Scholar] [CrossRef] [PubMed]
- Erdem, H.; Hargreaves, S.; Ankarali, H.; Caskurlu, H.; Ceviker, S.A.; Bahar-Kacmaz, A.; Meric-Koc, M.; Altindis, M.; Yildiz-Kirazaldi, Y.; Kizilates, F.; et al. Managing adult patients with infectious diseases in emergency departments: International ID-IRI study. J. Chemother. 2021, 33, 302–318. [Google Scholar] [CrossRef] [PubMed]
- El-Sokkary, R.; Uysal, S.; Erdem, H.; Kullar, R.; Pekok, A.U.; Amer, F.; Grgić, S.; Carevic, B.; El-Kholy, A.; Liskova, A.; et al. Profiles of multidrug-resistant organisms among patients with bacteremia in intensive care units: An international ID-IRI survey. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2323–2334. [Google Scholar] [CrossRef]
- Marino, A.; Munafò, A.; Zagami, A.; Ceccarelli, M.; Di Mauro, R.; Cantarella, G.; Bernardini, R.; Nunnari, G.; Cacopardo, B. Ampicillin plus ceftriaxone regimen against enterococcus faecalis endocarditis: A literature review. J. Clin. Med. 2021, 10, 4594. [Google Scholar] [CrossRef]
- Gajdács, M.; Ábrók, M.; Lázár, A.; Burián, K. Epidemiology and antibiotic resistance profile of bacterial uropathogens in male patients: A 10-year retrospective study. Farmacia 2021, 69, 530–539. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Paterson, D.L.; Bonomo, R.A. Extended-spectrum β-lactamases: A clinical update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef]
- Gaviria, L.P.; Montsant, L.; Azuaje, C.; González-Díaz, A.; Horcajada, J.P.; Limón, E.; Viñas, M.; Espinal, P.; Fusté, E. A Descriptive Analysis of Urinary ESBL-Producing-Escherichia coli in Cerdanya Hospital. Microorganisms 2022, 10, 488. [Google Scholar] [CrossRef]
- Aris, P.; Boroumand, M.A.; Rahbar, M.; Douraghi, M. The Activity of Fosfomycin Against Extended-Spectrum Beta-Lactamase-Producing Isolates of Enterobacteriaceae Recovered from Urinary Tract Infections: A Single-Center Study over a Period of 12 Years. Microb. Drug Resist. 2018, 24, 607–612. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kastoris, A.C.; Kapaskelis, A.M.; Karageorgopoulos, D.E. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum β-lactamase producing, Enterobacteriaceae infections: A systematic review. Lancet Infect. Dis. 2010, 10, 43–50. [Google Scholar] [CrossRef]
- Hendlin, D.; Stapley, E.O.; Jackson, M.; Wallick, H.; Miller, A.K.; Wolf, F.J.; Miller, T.W.; Chaiet, L.; Kahan, F.M.; Foltz, E.L.; et al. Phosphonomycin, a new antibiotic produced by strains of streptomyces. Science 1969, 166, 122–123. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, A.S.; Livaditis, I.G.; Gougoutas, V. The revival of fosfomycin. Int. J. Infect. Dis. 2011, 15, e732–e739. [Google Scholar] [CrossRef] [PubMed]
- Eschenburg, S.; Priestman, M.; Schönbrunn, E. Evidence that the fosfomycin target Cys115 in UDP-N-acetylglucosamine Enolpyruvyl Transferase (MurA) is essential for product release. J. Biol. Chem. 2005, 280, 3757–3763. [Google Scholar] [CrossRef]
- Petek, M.; Baebler, S.; Kuzman, D.; Rotter, A.; Podlesek, Z.; Gruden, K.; Ravnikar, M.; Urleb, U. Revealing fosfomycin primary effect on Staphylococcus aureus transcriptome: Modulation of cell envelope biosynthesis and phosphoenolpyruvate induced starvation. BMC Microbiol. 2010, 10, 159. [Google Scholar] [CrossRef]
- Falagas, M.E.; Vouloumanou, E.K.; Samonis, G.; Vardakasa, K.Z. Fosfomycin. Clin. Microbiol. Rev. 2016, 29, 321–347. [Google Scholar] [CrossRef]
- Raz, R. Fosfomycin: An old-new antibiotic. Clin. Microbiol. Infect. 2012, 18, 4–7. [Google Scholar] [CrossRef]
- Liu, H.Y.; Lin, H.C.; Lin, Y.C.; Yu, S.H.; Wu, W.H.; Lee, Y.J. Antimicrobial susceptibilities of urinary extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae to fosfomycin and nitrofurantoin in a teaching hospital in Taiwan. J. Microbiol. Immunol. Infect. 2011, 44, 364–368. [Google Scholar] [CrossRef]
- Bavaro, D.F.; Belati, A.; Diella, L.; Stufano, M.; Romanelli, F.; Scalone, L.; Stolfa, S.; Ronga, L.; Maurmo, L.; Dell’aera, M.; et al. Cefiderocol-based combination therapy for “difficult-to-treat” gram-negative severe infections: Real-life case series and future perspectives. Antibiotics 2021, 10, 652. [Google Scholar] [CrossRef]
- Butcu, M.; Akcay, S.S.; Inan, A.S.; Aksaray, S.; Engin, D.O.; Calisici, G. In vitro susceptibility of enterococci strains isolated from urine samples to fosfomycin and other antibiotics. J. Infect. Chemother. 2011, 17, 575–578. [Google Scholar] [CrossRef]
- Stracquadanio, S.; Musso, N.; Costantino, A.; Lazzaro, L.M.; Stefani, S.; Bongiorno, D. Staphylococcus aureus internalization in osteoblast cells: Mechanisms, interactions and biochemical processes. what did we learn from experimental models? Pathogens 2021, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Reeves, D.S. Fosfomycin trometamol. J. Antimicrob. Chemother. 1994, 34, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Arca, P.; Hardisson, C.; Suarez, J.E. Purification of a glutathione S-transferase that mediates fosfomycin resistance in bacteria. Antimicrob. Agents Chemother. 1990, 34, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Shrestha Nabin; Tomford JW Fosfomycin: A review. Infect. Dis. Clin. Pract. 2001, 10, 255–260. [CrossRef]
- Kadner, R.J.; Winkler, H.H. Isolation and characterization of mutations affecting the transport of hexose phosphates in Escherichia coli. J. Bacteriol. 1973, 113, 895–900. [Google Scholar] [CrossRef]
- Kahan, F.M.; Kahan, J.S.; Cassidy, P.J.; Kropp, H. The Mechanism of Action of Fosfomycin (Phosphonomycin). Ann. N. Y. Acad. Sci. 1974, 235, 364–386. [Google Scholar] [CrossRef]
- Tsuruoka, T.; Miyata, A.; Yamada, Y. Two Kinds of Mutants Defective in Multiple Carbohydrate Utilization Isolated from in Vitro Fosfomycin-Resistant Strains of Escherichia Coli K-12. J. Antibiot. 1978, 31, 192–201. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Furukawa, S.; Ogihara, H.; Yamasaki, M. Fosmidomycin resistance in adenylate cyclase deficient (cya) mutants of Escherichia coli. Biosci. Biotechnol. Biochem. 2003, 67, 2030–2033. [Google Scholar] [CrossRef]
- Venkateswaran, P.S.; Wu, H.C. Isolation and characterization of a phosphonomycin-resistant mutant of Escherichia coli K-12. J. Bacteriol. 1972, 110, 935–944. [Google Scholar] [CrossRef]
- Karageorgopoulos, D.E.; Wang, R.; Yu, X.H.; Falagas, M.E. Fosfomycin: Evaluation of the published evidence on the emergence of antimicrobial resistance in gram-negative pathogens. J. Antimicrob. Chemother. 2012, 67, 255–268. [Google Scholar] [CrossRef]
- Rigsby, R.E.; Fillgrove, K.L.; Beihoffer, L.A.; Armstrong, R.N. Fosfomycin resistance proteins: A nexus of glutathione transferases and epoxide hydrolases in a metalloenzyme superfamily. Methods Enzymol. 2005, 401, 367–379. [Google Scholar] [PubMed]
- Garcia, P.; Arca, P.; Suarez, J.E. Product of fosC, a gene from Pseudomonas syringae, mediates fosfomycin resistance by using ATP as cosubstrate. Antimicrob. Agents Chemother. 1995, 39, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Walkty, A.J.; Karlowsky, J.A. Fosfomycin: A First-Line Oral Therapy for Acute Uncomplicated Cystitis. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 2082693. [Google Scholar] [CrossRef]
- Falagas, M.E.; Athanasaki, F.; Voulgaris, G.L.; Triarides, N.A.; Vardakas, K.Z. Resistance to fosfomycin: Mechanisms, Frequency and Clinical Consequences. Int. J. Antimicrob. Agents 2019, 53, 22–28. [Google Scholar] [CrossRef]
- Borsa, F.; Leroy, A.; Fillastre, J.P.; Godin, M.; Moulin, B. Comparative pharmacokinetics of tromethamine fosfomycin and calcium fosfomycin in young and elderly adults. Antimicrob. Agents Chemother. 1988, 32, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Bergan, T. Degree of absorption, pharmacokinetics of fosfomycin trometamol and duration of urinary antibacterial activity. Infection 1990, 18, S65–S69. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Sugiyama, M.; Nakajima, S.; Yamashina, H. Fosfomycin kinetics after intravenous and oral administration to human volunteers. Antimicrob. Agents Chemother. 1981, 20, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Roussos, N.; Karageorgopoulos, D.E.; Samonis, G.; Falagas, M.E. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int. J. Antimicrob. Agents 2009, 34, 506–515. [Google Scholar] [CrossRef]
- Joukhadar, C.; Klein, N.; Dittrich, P.; Zeitlinger, M.; Geppert, A.; Skhirtladze, K.; Frossard, M.; Heinz, G.; Müller, M. Target site penetration of fosfomycin in critically ill patients. J. Antimicrob. Chemother. 2003, 51, 1247–1252. [Google Scholar] [CrossRef]
- Patel, S.S.; Balfour, J.A.; Bryson, H.M. Fosfomycin Tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs 1997, 53, 637–656. [Google Scholar] [CrossRef]
- Keating, G.M. Fosfomycin trometamol: A review of its use as a single-dose oral treatment for patients with acute lower urinary tract infections and pregnant women with asymptomatic bacteriuria. Drugs 2013, 73, 1951–1966. [Google Scholar] [CrossRef] [PubMed]
- FDA. Cder Monurol (Fosfomycin Tromethamine) Oral Suspension. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050717s005lbl.pdf (accessed on 22 July 2022).
- Dijkmans, A.C.; Zacarías, N.V.O.; Burggraaf, J.; Mouton, J.W.; Wilms, E.B.; van Nieuwkoop, C.; Touw, D.J.; Stevens, J.; Kamerling, I.M.C. Fosfomycin: Pharmacological, clinical and future perspectives. Antibiotics 2017, 6, 24. [Google Scholar] [CrossRef]
- Fan, L.; Shang, X.; Zhu, J.; Ma, B.; Zhang, Q. Pharmacodynamic and pharmacokinetic studies and prostatic tissue distribution of fosfomycin tromethamine in bacterial prostatitis or normal rats. Andrologia 2018, 50, e13021. [Google Scholar] [CrossRef]
- EUCAST. MIC EUCAST. Available online: https://mic.eucast.org/search/?search%5Bmethod%5D=mic&search%5Bantibiotic%5D=100&search%5Bspecies%5D=-1&search%5Bdisk_content%5D=-1&search%5Blimit%5D=50 (accessed on 9 May 2022).
- Tosto, F.; Marino, A.; Moscatt, V.; Cosentino, F.; Campanella, E.; Micali, C.; Russotto, Y.; Caci, G.; Rullo, E.V.; Nunnari, G.; et al. Methicillin-sensitive Staphylococcus aureus prosthetic vascular graft infection after a Fontan procedure in an adult patient: A case report. World Acad. Sci. J. 2022, 4, 19. [Google Scholar] [CrossRef]
- Castañeda-García, A.; Blázquez, J.; Rodríguez-Rojas, A. Molecular mechanisms and clinical impact of acquired and intrinsic fosfomycin resistance. Antibiotics 2013, 2, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Mihailescu, R.; Tafin, U.F.; Corvec, S.; Oliva, A.; Betrisey, B.; Borens, O.; Trampuza, A. High activity of fosfomycin and rifampin against methicillin-resistant staphylococcus aureus biofilm in vitro and in an experimental foreign-body infection model. Antimicrob. Agents Chemother. 2014, 58, 2547–2553. [Google Scholar] [CrossRef] [PubMed]
- Chai, D.; Liu, X.; Wang, R.; Bai, Y.; Cai, Y. Efficacy of Linezolid and Fosfomycin in Catheter-Related Biofilm Infection Caused by Methicillin-Resistant Staphylococcus aureus. Biomed Res. Int. 2016, 2016, 6413982. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Curtolo, A.; Volpicelli, L.; Cogliati Dezza, F.; De Angelis, M.; Cairoli, S.; Dell’utri, D.; Goffredo, B.M.; Raponi, G.; Venditti, M. Synergistic meropenem/vaborbactam plus fosfomycin treatment of kpc producing k. Pneumoniae septic thrombosis unresponsive to ceftazidime/avibactam: From the bench to the bedside. Antibiotics 2021, 10, 781. [Google Scholar] [CrossRef]
- Flamm, R.K.; Rhomberg, P.R.; Lindley, J.M.; Sweeney, K.; Ellis-Grosse, E.J.; Shortridge, D. Evaluation of the bactericidal activity of fosfomycin in combination with selected antimicrobial comparison agents tested against Gram-negative bacterial strains by using time-kill curves. Antimicrob. Agents Chemother. 2019, 63, e02549-18. [Google Scholar] [CrossRef]
- Papp-Wallace, K.M.; Zeiser, E.T.; Becka, S.A.; Park, S.; Wilson, B.M.; Winkler, M.L.; D’Souza, R.; Singh, I.; Sutton, G.; Fouts, D.E.; et al. Ceftazidime-Avibactam in Combination with Fosfomycin: A Novel Therapeutic Strategy against Multidrug-Resistant Pseudomonas aeruginosa. J. Infect. Dis. 2020, 221, 666–676. [Google Scholar] [CrossRef]
- Cuba, G.T.; Rocha-Santos, G.; Cayô, R.; Streling, A.P.; Nodari, C.S.; Gales, A.C.; Pignatari, A.C.C.; Nicolau, D.P.; Kiffer, C.R.V. In vitro synergy of ceftolozane/tazobactam in combination with fosfomycin or aztreonam against MDR Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2020, 75, 1874–1878. [Google Scholar] [CrossRef]
- Samonis, G.; Maraki, S.; Karageorgopoulos, D.E.; Vouloumanou, E.K.; Falagas, M.E. Synergy of fosfomycin with carbapenems, colistin, netilmicin, and tigecycline against multidrug-resistant Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa clinical isolates. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 695–701. [Google Scholar] [CrossRef]
- Drusano, G.L.; Neely, M.N.; Yamada, W.M.; Duncanson, B.; Brown, D.; Maynard, M.; Vicchiarelli, M.; Louie, A. The Combination of fosfomycin plus meropenem is synergistic for pseudomonas aeruginosa PAO1 in a hollow-fiber infection model. Antimicrob. Agents Chemother. 2018, 62, e01682-18. [Google Scholar] [CrossRef]
- Leelasupasri, S.; Santimaleeworagun, W.; Jitwasinkul, T. Antimicrobial Susceptibility among Colistin, Sulbactam, and Fosfomycin and a Synergism Study of Colistin in Combination with Sulbactam or Fosfomycin against Clinical Isolates of Carbapenem-Resistant Acinetobacter baumannii. J. Pathog. 2018, 2018, 3893492. [Google Scholar] [CrossRef]
- Zhao, M.; Bulman, Z.P.; Lenhard, J.R.; Satlin, M.J.; Kreiswirth, B.N.; Walsh, T.J.; Marrocco, A.; Bergen, P.J.; Nation, R.L.; Li, J.; et al. Pharmacodynamics of colistin and fosfomycin: A “treasure trove” combination combats KPC-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 2017, 72, 1985–1990. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chen, P.Y.; Wang, J.T.; Chang, S.C. Prevalence of fosfomycin resistance and gene mutations in clinical isolates of methicillin-resistant Staphylococcus aureus. Antimicrob. Resist. Infect. Control 2020, 9, 135. [Google Scholar] [CrossRef]
- M100Ed32|Performance Standards for Antimicrobial Susceptibility Testing, 32th Edition. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 4 July 2022).
- Van Mens, S.P.; ten Doesschate, T.; Kluytmans-van den Bergh, M.F.Q.; Mouton, J.W.; Rossen, J.W.A.; Verhulst, C.; Bonten, M.J.M.; Kluytmans, J.A.J.W. Fosfomycin Etest for Enterobacteriaceae: Interobserver and interlaboratory agreement. Int. J. Antimicrob. Agents 2018, 52, 678–681. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Baxter, M.R.; Golden, A.R.; Adam, H.J.; Walkty, A.; Lagacé-Wiens, P.R.S.; Zhanel, G.G. Use of Fosfomycin Etest To Determine In Vitro Susceptibility of Clinical Isolates of Enterobacterales Other than Escherichia coli, Nonfermenting Gram-Negative Bacilli, and Gram-Positive Cocci. J. Clin. Microbiol. 2021, 59, e0163521. [Google Scholar] [CrossRef]
- Van Den Bijllaardt, W.; Schijffelen, M.J.; Bosboom, R.W.; Stuart, J.C.; DIederen, B.; Kampinga, G.; Le, T.N.; Overdevest, I.; Stals, F.; Voorn, P.; et al. Susceptibility of ESBL Escherichia coli and Klebsiella pneumoniae to fosfomycin in the Netherlands and comparison of several testing methods including Etest, MIC test strip, Vitek2, Phoenix and disc diffusion. J. Antimicrob. Chemother. 2018, 73, 2380–2387. [Google Scholar] [CrossRef]
- Aprile, A.; Scalia, G.; Stefani, S.; Mezzatesta, M.L. In vitro fosfomycin study on concordance of susceptibility testing methods against ESBL and carbapenem-resistant Enterobacteriaceae. J. Glob. Antimicrob. Resist. 2020, 23, 286–289. [Google Scholar] [CrossRef]
- Campanile, F.; Wootton, M.; Davies, L.; Aprile, A.; Mirabile, A.; Pomponio, S.; Demetrio, F.; Bongiorno, D.; Walsh, T.R.; Stefani, S.; et al. Gold standard susceptibility testing of fosfomycin in Staphylococcus aureus and Enterobacterales using a new agar dilution panel®. J. Glob. Antimicrob. Resist. 2020, 23, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Parisio, E.M.; Camarlinghi, G.; Coppi, M.; Niccolai, C.; Antonelli, A.; Nardone, M.; Vettori, C.; Giani, T.; Mattei, R.; Rossolini, G.M. Evaluation of the commercial AD fosfomycin test for susceptibility testing of multidrug-resistant Enterobacterales and Pseudomonas aeruginosa. Clin. Microbiol. Infect. 2021, 27, 788.e5. [Google Scholar] [CrossRef] [PubMed]
- European Society of Clinical Microbiology and Infectious Diseases EUCAST: Clinical Breakpoints and Dosing of Antibiotics. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 9 May 2022).
- Martín-Gutiérrez, G.; Docobo-Pérez, F.; Rodríguez-Martínez, J.M.; Pascual, A.; Blázquez, J.; Rodriguez-Beltrán, J. Detection of low-level fosfomycin-resistant variants by decreasing glucose-6-phosphate concentration in fosfomycin susceptibility determination. Antibiotics 2020, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Cattoir, V.; Guérin, F. How is fosfomycin resistance developed in Escherichia coli? Future Microbiol. 2018, 13, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Los-Arcos, I.; Pigrau, C.; Rodríguez-Pardo, D.; Fernández-Hidalgo, N.; Andreu, A.; Larrosa, N.; Almirantea, B. Long-Term Fosfomycin-Tromethamine Oral Therapy for Difficult-To-Treat Chronic Bacterial Prostatitis. Antimicrob. Agents Chemother. 2016, 60, 1854–1858. [Google Scholar] [CrossRef]
- Karaiskos, I.; Galani, L.; Sakka, V.; Gkoufa, A.; Sopilidis, O.; Chalikopoulos, D.; Alivizatos, G.; Giamarellou, E. Oral fosfomycin for the treatment of chronic bacterial prostatitis. J. Antimicrob. Chemother. 2019, 74, 1430–1437. [Google Scholar] [CrossRef]
- Almeida, F.; Santos Silva, A.; Silva Pinto, A.; Sarmento, A. Chronic prostatitis caused by extended-spectrum β-lactamase-producing Escherichia coli managed using oral fosfomycin—A case report. IDCases 2019, 15, e00493. [Google Scholar] [CrossRef]
- Grayson, M.L.; Macesic, N.; Trevillyan, J.; Ellis, A.G.; Zeglinski, P.T.; Hewitt, N.H.; Gardiner, B.J.; Frauman, A.G. Fosfomycin for Treatment of Prostatitis: New Tricks for Old Dogs. Clin. Infect. Dis. 2015, 61, 1141–1143. [Google Scholar] [CrossRef]
- Cunha, B.A.; Gran, A.; Raza, M. Persistent extended-spectrum β-lactamase-positive Escherichia coli chronic prostatitis successfully treated with a combination of fosfomycin and doxycycline. Int. J. Antimicrob. Agents 2015, 45, 427–429. [Google Scholar] [CrossRef]
- Denes, E. Prolonged course of Fosfomycin-Trometamol for chronic prostatitis: An unknown good option. Scand. J. Urol. 2021, 55, 344–345. [Google Scholar] [CrossRef]
- Gian, J.; Cunha, B.A. Raoultella planticola chronic bacterial prostatitis with prostatic calcifications: Successful treatment with prolonged fosfomycin therapy. Int. J. Antimicrob. Agents 2016, 47, 414. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Stracquadanio, S.; Ceccarelli, M.; Zagami, A.; Nunnari, G.; Cacopardo, B. Oral fosfomycin formulation for acute bacterial prostatitis; a new role for an old molecule: A case report and brief literature review. World Acad. Sci. J. 2022, 4, 26. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Amuh, D.; Goldman, M.P.; Riebel, W.J.; Walton Tomford, J. Treatment of a complicated vancomycinresistant enterococcal urinary tract infection with fosfomycin. Infect. Dis. Clin. Pract. 2000, 9, 368–371. [Google Scholar] [CrossRef]
- Gill, B.C.; Shoskes, D.A. Bacterial prostatitis. Curr. Opin. Infect. Dis. 2016, 29, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute Clinical & Laboratory Standards Institute: CLSI Guidelines. Available online: https://clsi.org/ (accessed on 4 July 2022).
- Magri, V.; Boltri, M.; Cai, T.; Colombo, R.; Cuzzocrea, S.; De Visschere, P.; Giuberti, R.; Granatieri, C.M.; Latino, M.A.; Larganà, G.; et al. Multidisciplinary approach to prostatitis. Arch. Ital. Urol. Androl. 2018, 90, 227–248. [Google Scholar] [CrossRef]
- Demonchy, E.; Courjon, J.; Ughetto, E.; Durand, M.; Risso, K.; Garraffo, R.; Roger, P.M. Cefoxitin-based antibiotic therapy for extended-spectrum β-lactamase-producing Enterobacteriaceae prostatitis: A prospective pilot study. Int. J. Antimicrob. Agents 2018, 51, 836–841. [Google Scholar] [CrossRef]
- Marino, A.; Caltabiano, E.; Zagami, A.; Onorante, A.; Zappalà, C.; Locatelli, M.E.; Pampaloni, A.; Scuderi, D.; Bruno, R.; Cacopardo, B. Rapid emergence of cryptococcal fungemia, Mycobacterium chelonae vertebral osteomyelitis and gastro intestinal stromal tumor in a young HIV late presenter: A case report. BMC Infect. Dis. 2018, 18, 693. [Google Scholar] [CrossRef]
- Celesia, B.M.; Marino, A.; Del Vecchio, R.F.; Bruno, R.; Palermo, F.; Gussio, M.; Nunnari, G.; Cacopardo, B. Is it safe and cost saving to defer the CD4+ cell count monitoring in stable patients on ART with more than 350 or 500 cells/µL? Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019063. [Google Scholar] [CrossRef]
- Celesia, B.M.; Marino, A.; Borracino, S.; Arcadipane, A.F.; Pantò, G.; Gussio, M.; Coniglio, S.; Pennisi, A.; Cacopardo, B.; Panarello, G. Successful extracorporeal membrane oxygenation treatment in an acquired immune deficiency syndrome (AIDS) patient with acute respiratory distress syndrome (ARDS) complicating pneumocystis jirovecii pneumonia: A challenging case. Am. J. Case Rep. 2020, 21, e919570-1–e919570-5. [Google Scholar] [CrossRef]
- Marino, A.; Cosentino, F.; Ceccarelli, M.; Moscatt, V.; Pampaloni, A.; Scuderi, D.; D’andrea, F.; Venanzi Rullo, E.; Nunnari, G.; Benanti, F.; et al. Entecavir resistance in a patient with treatment-naïve hbv: A case report. Mol. Clin. Oncol. 2021, 14, 113. [Google Scholar] [CrossRef]
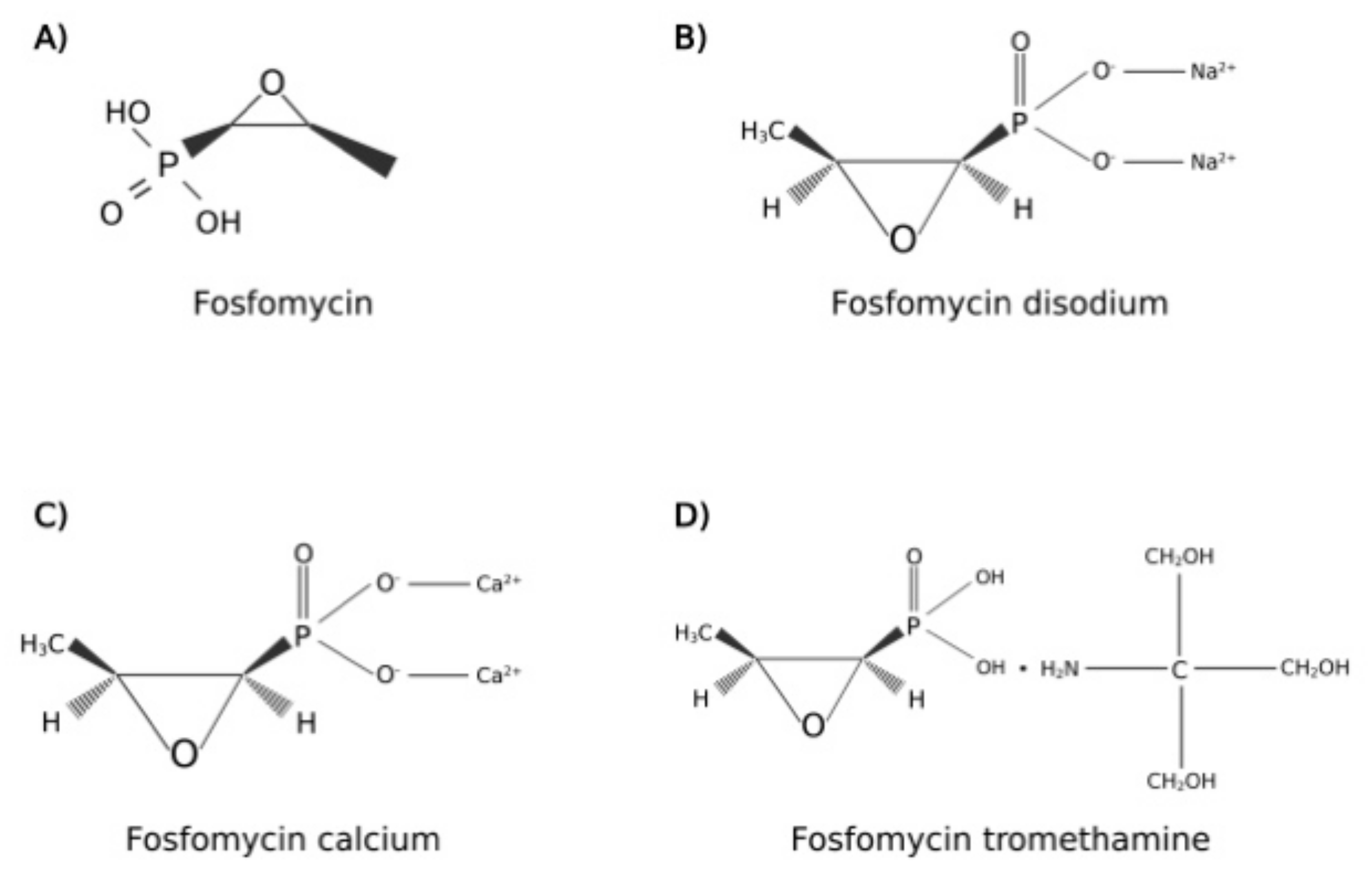
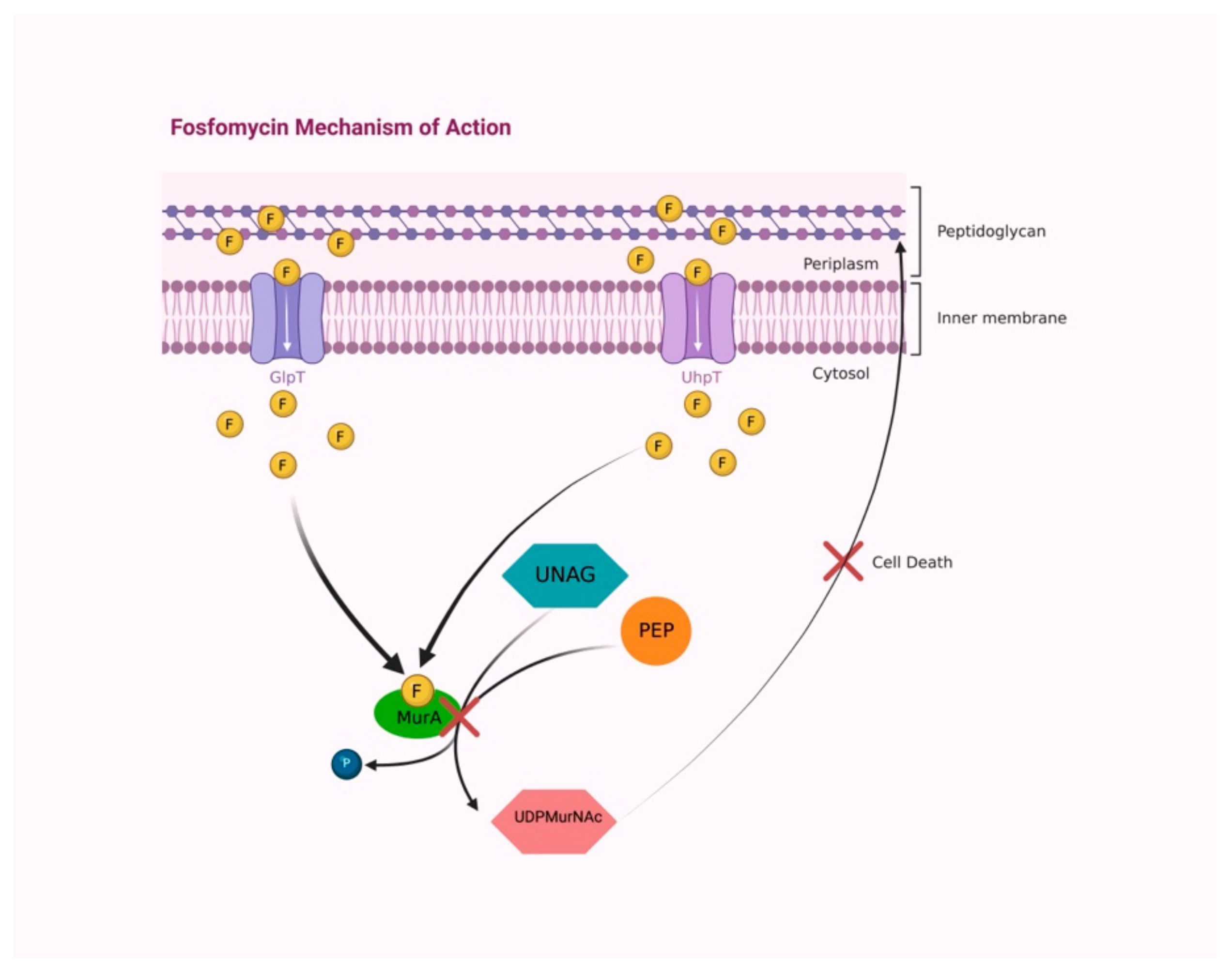
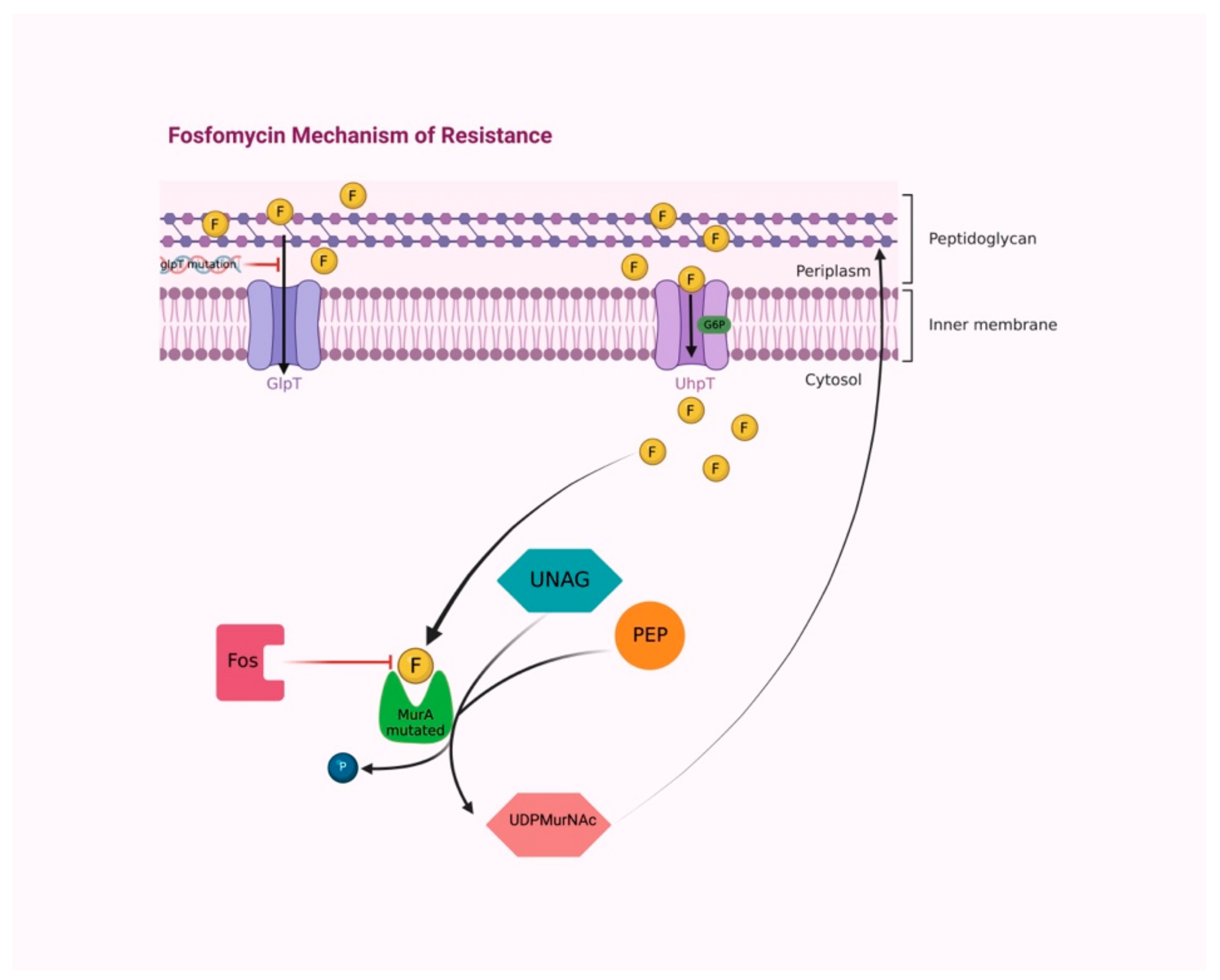
| Prostatitis Type | Pathogen (n° of Isolates) | Combination Therapy | Fosfomycin Dosage | Adverse Effect | Clinical Cure | Microbiological Cure | Reference |
|---|---|---|---|---|---|---|---|
| CBP | E. coli (12) | 3/12 | 3 g/24–48 h for 5.5 weeks (mean duration) | Diarrhea (4/12) | Yes | 8/12 | [1] |
| K. pneumoniae (5) | 1/5 | - | 4/5 | 3/5 | |||
| E. coli (14) | 1/14 | 3 g/48–72 h for 6 weeks | - | 7/14 | 8/14 | [70] | |
| K. oxytoca | No | No | No | ||||
| E. coli (29) | No | 3 g/24 h for the first week, then 3 g/48 h or 3 g/72 h for 6–13 weeks | Diarrhea (4/44) | 23/29 | 23/29 | [71] | |
| K. oxytoca (3) | 3/3 | 3/3 | |||||
| K. pneumoniae (3) | 2/3 | 2/3 | |||||
| P. mirabilis (2) | 2/2 | 1/2 | |||||
| P. aeruginosa | No | No | |||||
| E. faecalis (6) | 5/6 | 6/6 | |||||
| ESBL-E. coli | No | 3 g/24 h for 9 days, then 3 g/48 h for 3 months and 3 g/weekly for 9 months | Diarrhea during the first week | Yes | Yes | [72] | |
| ESBL-E. coli | No | 15 weeks of 3 g once daily; 5 days of 3 g twice daily | Diarrhea with the doubled dose | Yes | Yes | [73] | |
| ESBL-E. coli | Yes | 3 g/72 h | - | Yes | Yes | [74] | |
| E. coli | No | 3 g/24 h for 1 week, then 3 g/48 h for 3 months | - | Yes | Yes | [75] | |
| R. planticola | No | 3 g/48 h for 3 months | - | Yes | Yes | [76] | |
| ABP | ESBL-E. coli | No | 3 g/24 h for 1 week—3 g/48 h for 2 weeks | Diarrhea during the first week | Yes | Yes | [77] |
| ESBL-E. coli | Yes | 3 g/24 h—3 g/twice daily (5 days) for 16 weeks | Diarrhea with the doubled dose | Yes | Yes | [73] | |
| E. faecium | No | 3 g/72 h for 3 weeks | - | Yes | Yes | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, A.; Stracquadanio, S.; Bellanca, C.M.; Augello, E.; Ceccarelli, M.; Cantarella, G.; Bernardini, R.; Nunnari, G.; Cacopardo, B. Oral Fosfomycin Formulation in Bacterial Prostatitis: New Role for an Old Molecule-Brief Literature Review and Clinical Considerations. Infect. Dis. Rep. 2022, 14, 621-634. https://doi.org/10.3390/idr14040067
Marino A, Stracquadanio S, Bellanca CM, Augello E, Ceccarelli M, Cantarella G, Bernardini R, Nunnari G, Cacopardo B. Oral Fosfomycin Formulation in Bacterial Prostatitis: New Role for an Old Molecule-Brief Literature Review and Clinical Considerations. Infectious Disease Reports. 2022; 14(4):621-634. https://doi.org/10.3390/idr14040067
Chicago/Turabian StyleMarino, Andrea, Stefano Stracquadanio, Carlo Maria Bellanca, Egle Augello, Manuela Ceccarelli, Giuseppina Cantarella, Renato Bernardini, Giuseppe Nunnari, and Bruno Cacopardo. 2022. "Oral Fosfomycin Formulation in Bacterial Prostatitis: New Role for an Old Molecule-Brief Literature Review and Clinical Considerations" Infectious Disease Reports 14, no. 4: 621-634. https://doi.org/10.3390/idr14040067
APA StyleMarino, A., Stracquadanio, S., Bellanca, C. M., Augello, E., Ceccarelli, M., Cantarella, G., Bernardini, R., Nunnari, G., & Cacopardo, B. (2022). Oral Fosfomycin Formulation in Bacterial Prostatitis: New Role for an Old Molecule-Brief Literature Review and Clinical Considerations. Infectious Disease Reports, 14(4), 621-634. https://doi.org/10.3390/idr14040067










