Prevalence of Chlamydia trachomatis, Ureaplasma urealyticum, and Neisseria gonorrhoeae in Asymptomatic Women from Urban-Peripheral and Rural Populations of Cuenca, Ecuador
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Sample Collection
2.3. Sample Processing
2.4. Statistical Analyses
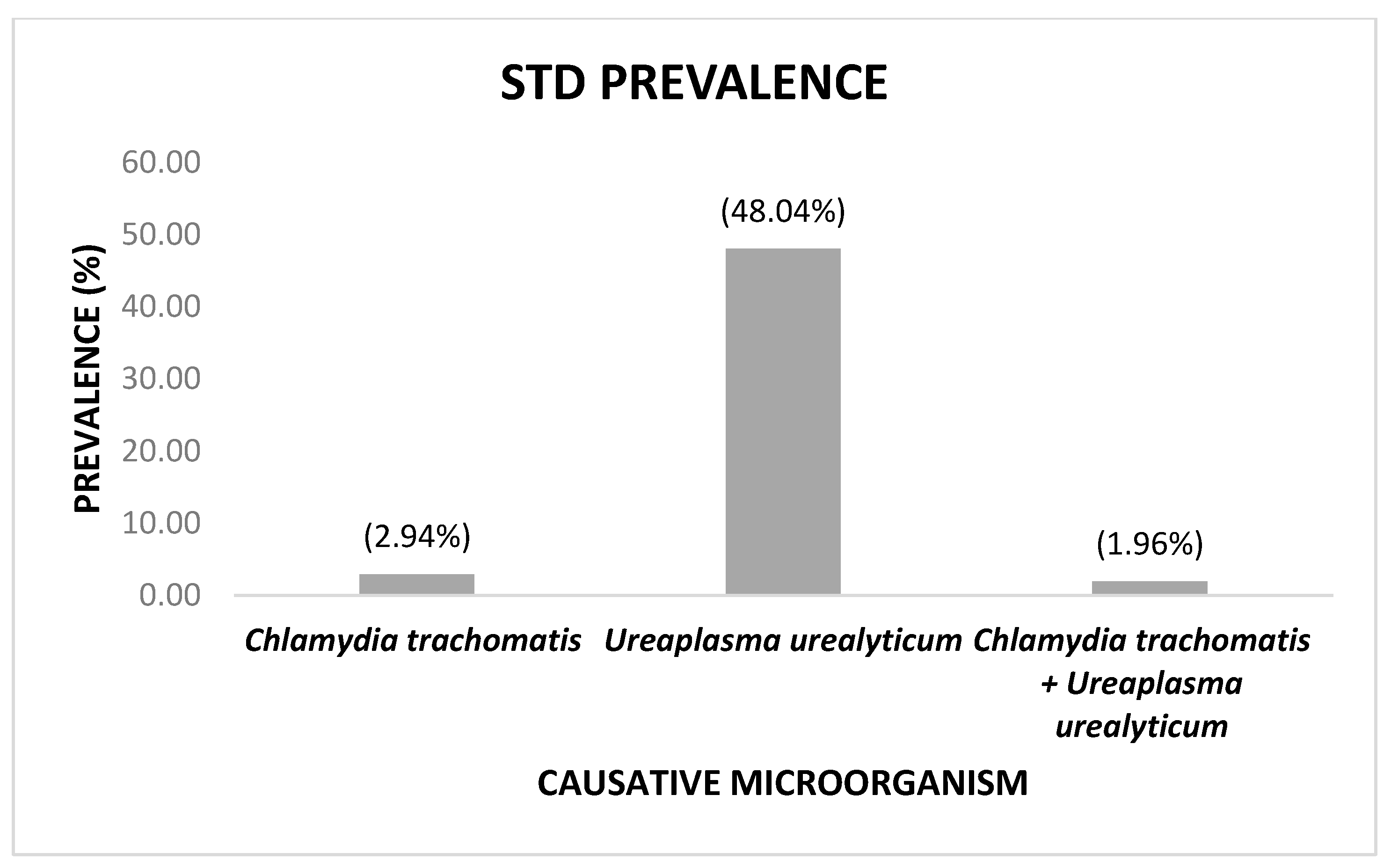
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sexually Transmitted Infections (STIs). Who.int. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed on 15 August 2022).
- World Health Organization. Report on Global Sexually Transmitted Infection Surveillance. 2018. Available online: http://apps.who.int/iris/bitstream/handle/10665/277258/9789241565691-eng.pdf?ua=1 (accessed on 20 October 2021).
- Borrel Martínez, J.; Diaz Franco, A.; Herrera Puente, Á.; Sánchez Bursón, L.; Sanmartín Sánchez, E. Guía de Buena Práctica Clínica en Infecciones de Transmisión Sexual. Organización Médica Colegial de España: Madrid, Spain, 2011; p. 45. [Google Scholar]
- Murray, P.; Rosenthal, K.; Pfaller, M. Medical Microbiology, 8th ed.; Elsevier: Philadelphia, PA, USA, 2016; pp. 235–242, 348–354. [Google Scholar]
- Kohl, K.S.; Markowitz, L.E.; Koumans, E.H. Developments in the screening for Chlamydia trachomatis: A review. Obstet. Gynecol. Clin. N. Am. 2003, 30, 637–658. [Google Scholar] [CrossRef]
- Waites, K.B.; Katz, B.; Schelonka, R.L. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin. Microbiol. Rev. 2005, 18, 757–789. [Google Scholar] [CrossRef] [PubMed]
- Waites, K.B.; Schelonka, R.L.; Xiao, L.; Grigsby, P.L.; Novy, M.J. Congenital and opportunistic infections: Ureaplasma species and Mycoplasma hominis. Semin. Fetal Neonatal Med. 2009, 14, 190–199. [Google Scholar] [CrossRef]
- Hook, E.W.; Reichart, C.A.; Upchurch, D.M.; Ray, P.; Celentano, D.; Quinn, T.C. Comparative behavioral epidemiology of gonococcal and chlamydial infections among patients attending a Baltimore Maryland, sexually transmitted disease clinic. Am. J. Epidemiol. 1992, 136, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.D.; Sternberg, M.; Johnson, R.E.; Berman, S.; Papp, J.R.; McQuillan, G.; Weinstock, H. Gonorrhea and Chlamydia in the United States among persons 14 to 39 years of age, 1999 to 2002. Ann. Intern. Med. 2007, 147, 89–96. [Google Scholar] [CrossRef]
- Niccolai, L.M.; Livingston, K.A.; Laufer, A.S.; Pettigrew, M.M. Behavioural sources of repeat Chlamydia trachomatis infections: Importance of different sex partners. Sex. Transm. Infect. 2011, 87, 248–253. [Google Scholar] [CrossRef][Green Version]
- Kost, K.; Forrest, J.D. American women’s sexual behavior and exposure to risk of sexually transmitted diseases. Fam. Plann. Perspect. 1992, 24, 244–254. [Google Scholar] [CrossRef]
- Brunham, R.C.; Pourbohloul, B.; Mak, S.; White, R.; Rekart, M.L. The unexpected impact of a Chlamydia trachomatis Infection control program on susceptibility to reinfection. J. Infect. Dis. 2005, 192, 1836–1844. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, D.C.; Lee, D.S.; Choe, H.S.; Cho, Y.H. Evaluation of Seeplex® STD6 ACE Detection kit for the diagnosis of six bacterial sexually transmitted infections. J. Infect. Chemother. 2012, 18, 494–500. [Google Scholar] [CrossRef]
- Keane, F.E.; Thomas, B.J.; Gilroy, C.B.; Renton, A.; Taylor-Robinson, D. The association of Mycoplasma hominis, Ureaplasma urealyticum and Mycoplasma genitalium with bacterial vaginosis: Observations on heterosexual women and their male partners. Int. J. STD AIDS 2000, 11, 356–360. [Google Scholar] [CrossRef]
- Verteramo, R.; Patella, A.; Calzolari, E.; Recine, N.; Marcone, V.; Osborn, J.; Chiarini, F.; Degener, A.M. An epidemiological survey of Mycoplasma hominis and Ureaplasma urealyticum in gynaecological outpatients, Rome, Italy. Epidemiol. Infect. 2013, 141, 2650–2657. [Google Scholar] [CrossRef] [PubMed]
- Hunjak, B.; Sabol, I.; Vojnović, G.; Fistonić, I.; Erceg, A.B.; Peršić, Z.; Grce, M. Ureaplasma urealyticum and Ureaplasma parvum in women of reproductive age. Arch. Gynecol. Obstet. 2014, 289, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Tibaldi, C.; Cappello, N.; Latino, M.A.; Masuelli, G.; Marini, S.; Benedetto, C. Vaginal and endocervical microorganisms in symptomatic and asymptomatic non-pregnant females: Risk factors and rates of occurrence. Clin. Microbiol. Infect. 2009, 15, 670–679. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rumyantseva, T.; Khayrullina, G.; Guschin, A.; Donders, G. Prevalence of Ureaplasma spp. and Mycoplasma hominis in healthy women and patients with flora alterations. Diagn. Microbiol. Infect. Dis. 2019, 93, 227–231. [Google Scholar] [CrossRef]
- Horner, P.; Donders, G.; Cusini, M.; Gomberg, M.; Jensen, J.S.; Unemo, M. Should we be testing for urogenital Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum in men and women?—A position statement from the European STI Guidelines Editorial Board. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1845–1851. [Google Scholar] [CrossRef]
- Moridi, K.; Hemmaty, M.; Azimian, A.; Fallah, M.H.; Khaneghahi Abyaneh, H.; Ghazvini, K. Epidemiology of genital infections caused by Mycoplasma hominis, M. genitalium and Ureaplasma urealyticum in Iran; a systematic review and meta-analysis study (2000–2019). BMC Public Health 2020, 20, 1020. [Google Scholar] [CrossRef]
- Huai, P.; Li, F.; Chu, T.; Liu, D.; Liu, J.; Zhang, F. Prevalence of genital Chlamydia trachomatis infection in the general population: A meta-analysis. BMC Infect. Dis. 2020, 20, 589. [Google Scholar] [CrossRef]
- Owusu-Edusei, K.; Chesson, H.W.; Gift, T.L.; Tao, G.; Mahajan, R.; Ocfemia, M.C.; Kent, C.K. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex. Transm. Dis. 2013, 40, 197–201. [Google Scholar] [CrossRef]
- Christofolini, D.M.; Leuzzi, L.; Mafra, F.A.; Rodart, I.; Kayaki, E.A.; Bianco, B.; Barbosa, C.P. Prevalence of cases of Mycoplasma hominis, Mycoplasma genitalium, Ureaplasma urealyticum and Chlamydia trachomatis in women with no gynecologic complaints. Reprod. Med. Biol. 2012, 11, 201–205. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.; Lee, K.A. Prevalence of sexually transmitted infections among healthy Korean women: Implications of multiplex PCR pathogen detection on antibiotic therapy. J. Infect. Chemother. 2014, 20, 74–76. [Google Scholar] [CrossRef]
- Sanchez, J. Infección por Clamidias: Preguntas Frecuentes (WHO Web Site). Available online: https://www3.paho.org/hq/index.php?option=com_content&view=article&id=15066:infeccion-for-clamidias-preguntas-frecuentes&Itemid=3670&lang=es (accessed on 15 August 2022).
- Unemo, M.; Bradshaw, C.S.; Hocking, J.S.; de Vries, H.J.C.; Francis, S.C.; Mabey, D.; Marrazzo, J.M.; Sonder, G.J.B.; Schwebke, J.R.; Hoornenborg, E.; et al. Sexually transmitted infections: Challenges ahead. Lancet Infect. Dis. 2017, 17, e235–e279. [Google Scholar] [CrossRef]
- Denks, K.; Spaeth, E.L.; Jõers, K.; Randoja, R.; Talpsep, T.; Ustav, M.; Kurg, R. Coinfection of Chlamydia trachomatis, Ureaplasma urealyticum and human papillomavirus among patients attending STD clinics in Estonia. Scand. J. Infect. Dis. 2007, 39, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Embil, J.A.; Pereira, L.H. Prevalence of Chlamydia trachomatis and genital mycoplasmas in asymptomatic women. Can. Med. Assoc. J. 1985, 133, 34–35. [Google Scholar] [PubMed]
- Hogan, R.J.; Mathews, S.A.; Mukhopadhyay, S.; Summersgill, J.T.; Timms, P. Chlamydial persistence: Beyond the biphasic paradigm. Infect. Immun. 2004, 72, 1843–1855. [Google Scholar] [CrossRef]
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef]
- Riera-Monroig, J.; Corbeto, E.L.; Bosch, J.; Fuertes, I. Screening for asymptomatic Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma genitalium in medical students in Barcelona. J. Skin Sex Transm. Dis. 2020, 2, 134–136. [Google Scholar]
- Cole, M.; Pitt, R. Gonococcal Antimicrobial Susceptibility Surveillance in Europe, 2012; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2014; Available online: http://www.ecdc.europa.eu (accessed on 11 July 2022).
- Bingham, A.L.; Kavanagh, A.M.; Fairley, C.K.; Keogh, L.A.; Bentley, R.J.; Hocking, J.S. Income inequality and Neisseria gonorrhoeae notifications in females: A country-level analysis. Sex. Health 2014, 11, 556–560. [Google Scholar] [CrossRef]
- Familias, C.R. A rurales y sus procesos de transformación: Estudio de casos en un escenario de ruralidad en tensión. Psicoperspectivas 2012, 11, 180–203. [Google Scholar]
- World Health Organization. Comité de Expertos de la OMS en Enfermedades Venéreas y Treponematosis, Sexto Informe; Organización Mundial de la Salud: Ginebra, Switzerland, 1986; p. 35. Available online: https://apps.who.int/iris/handle/10665/39332 (accessed on 11 July 2022).
| Sociodemographic Variables | Total (n = 102) | Positive (n = 50) | Negative (n = 52) | p-Value |
|---|---|---|---|---|
| Age (years) M (SD) | 31.54 (6.27) | 31.08 (6.08) | 31.98 (6.47) | 0.584 * |
| Residence n (%) | 0.747 ‡ | |||
| Urban | 74 (72.55) | 37 (74) | 37 (71.15) | |
| Rural | 28(27.45) | 13 (26) | 15 (28.85) | |
| SES n (%) | 0.313 ‡ | |||
| Medium | 74 (72.55) | 34 (68) | 40 (76.92) | |
| Low | 28 (27.45) | 16 (32) | 12 (23.08) | |
| Highest educational level n (%) | 0.879 † | |||
| Primary school | 21 (20.59) | 9 (18) | 12 (23.08) | |
| High school | 37 (36.27) | 19 (38) | 18 (34.62) | |
| University graduate | 41 (40.20) | 21 (42) | 20 (38.46) | |
| Postgraduate | 3 (2.94) | 1 (2) | 2 (3.85) | |
| Marital status n (%) | 0.596 † | |||
| Single | 33 (32.35) | 19 (38) | 14 (26.92) | |
| In a relationship | 12 (11.76) | 5 (10) | 7 (13.46) | |
| Married | 48 (47.06) | 21 (42) | 27 (51.92) | |
| Divorced | 9 (8.82) | 5 (10) | 4 (7.69) | |
| Ethnicity n (%) | 0.485 † | |||
| Mestizo | 99 (97.06) | 48 (96) | 51 (98.08) | |
| White | 3 (2.94) | 2 (4) | 1 (1.92) |
| Risk Variables | Total (n = 102) | Positive (n = 50) | Negative (n = 52) | p Value |
|---|---|---|---|---|
| Sexual partners ¥ M (SD) | 2.55 (2.1) | 2.64 (2.28) | 2.47 (1.91) | 0.626 ‣ |
| Onset of sexual intercourse (age) M (SD) | 17.93 (3.52) | 17.74 (3.12) | 18.12 (3.89) | 0.635 ‣ |
| Miscarriages M (SD) | 0.29 (0.57) | 0.3 (0.50) | 0.29 (0.64) | 0.514 ‣ |
| Difficulties in conception n (%) | 0.893 ‡ | |||
| No difficulty | 77 (75.49) | 38 (76) | 39 (75) | |
| Did not attempt conception | 11 (10.78) | 6 (12) | 5 (9.62) | |
| Has had difficulty | 14 (13.73) | 6 (12) | 8 (15.38) | |
| Miscarriage diagnosis n (%) | 0.422 ‡ | |||
| Yes | 25 (24.51) | 14 (28) | 11 (21.15) | |
| No | 77 (75.49) | 36 (72) | 41 (78.85) | |
| Number of miscarriages n (%) | 0.430 ‡ | |||
| 0 | 77 (75.49) | 36 (72) | 41 (78.85) | |
| 1 | 21 (20.59) | 13 (26) | 8 (15.38) | |
| 2 | 3 (2.94) | 1 (2) | 2 (3.85) | |
| 3 | 1 (0.98) | 0 (0) | 1 (1.92) | |
| Treated for vaginal infection n (%) | 0.907 † | |||
| Yes | 77 (75.49) | 38 (76) | 39 (75) | |
| No | 25 (24.51) | 12 (24) | 13 (25) | |
| Copper T or IUD n (%) | 0.732 † | |||
| Yes | 25 (24.51) | 13 (26) | 12 (23.08) | |
| No | 77 (75.49) | 37 (74) | 40 (76.92) |
| Bivariate Models | |||||
|---|---|---|---|---|---|
| Independent Variables | OR * | SE † | Confidence Interval | p Value | |
| Lower Limit | Upper Limit | ||||
| Age (years) | 0.98 | 0.03 | 0.92 | 1.04 | 0.467 |
| Sexual partners | 1.04 | 0.10 | 0.86 | 1.26 | 0.684 |
| Onset of sexual intercourse | 0.97 | 0.06 | 0.87 | 1.08 | 0.590 |
| Abortion history | 1.45 | 0.67 | 0.58 | 3.59 | 0.423 |
| Residence (rural) | 0.87 | 0.38 | 0.36 | 2.07 | 0.748 |
| SES (low) | 1.57 | 0.70 | 0.65 | 3.77 | 0.314 |
| Education level (Primary school) | |||||
| High school | 1.41 | 0.77 | 0.48 | 4.14 | 0.534 |
| University graduate | 1.4 | 0.76 | 0.49 | 4.04 | 0.534 |
| Postgraduate | 0.67 | 0.87 | 0.05 | 8.55 | 0.755 |
| Marital status (Single) | |||||
| In a relationship | 0.53 | 0.36 | 0.14 | 2.01 | 0.348 |
| Married | 0.57 | 0.26 | 0.23 | 1.40 | 0.223 |
| Divorced | 0.92 | 0.70 | 0.21 | 4.07 | 0.914 |
| Ethnicity (White) | 2.13 | 2.64 | 0.19 | 24.20 | 0.544 |
| Difficulty in conception (Yes) | 0.75 | 0.44 | 0.24 | 2.34 | 0.620 |
| Miscarriage diagnosis (Yes) | 1.45 | 0.67 | 0.58 | 3.59 | 0.423 |
| Vaginal infection (Yes) | 1.06 | 0.49 | 0.43 | 2.60 | 0.907 |
| IUD (Yes) | 1.17 | 0.54 | 0.47 | 2.89 | 0.732 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abad, S.; Neira, E.; Viñansaca, L.; Escandón, S.; Neira, V.A. Prevalence of Chlamydia trachomatis, Ureaplasma urealyticum, and Neisseria gonorrhoeae in Asymptomatic Women from Urban-Peripheral and Rural Populations of Cuenca, Ecuador. Infect. Dis. Rep. 2022, 14, 646-654. https://doi.org/10.3390/idr14050070
Abad S, Neira E, Viñansaca L, Escandón S, Neira VA. Prevalence of Chlamydia trachomatis, Ureaplasma urealyticum, and Neisseria gonorrhoeae in Asymptomatic Women from Urban-Peripheral and Rural Populations of Cuenca, Ecuador. Infectious Disease Reports. 2022; 14(5):646-654. https://doi.org/10.3390/idr14050070
Chicago/Turabian StyleAbad, Sebastián, Elizavet Neira, Lourdes Viñansaca, Samuel Escandón, and Vivian Alejandra Neira. 2022. "Prevalence of Chlamydia trachomatis, Ureaplasma urealyticum, and Neisseria gonorrhoeae in Asymptomatic Women from Urban-Peripheral and Rural Populations of Cuenca, Ecuador" Infectious Disease Reports 14, no. 5: 646-654. https://doi.org/10.3390/idr14050070
APA StyleAbad, S., Neira, E., Viñansaca, L., Escandón, S., & Neira, V. A. (2022). Prevalence of Chlamydia trachomatis, Ureaplasma urealyticum, and Neisseria gonorrhoeae in Asymptomatic Women from Urban-Peripheral and Rural Populations of Cuenca, Ecuador. Infectious Disease Reports, 14(5), 646-654. https://doi.org/10.3390/idr14050070






