The Paradigm Shift of Using Natural Molecules Extracted from Northern Canada to Combat Malaria
Abstract
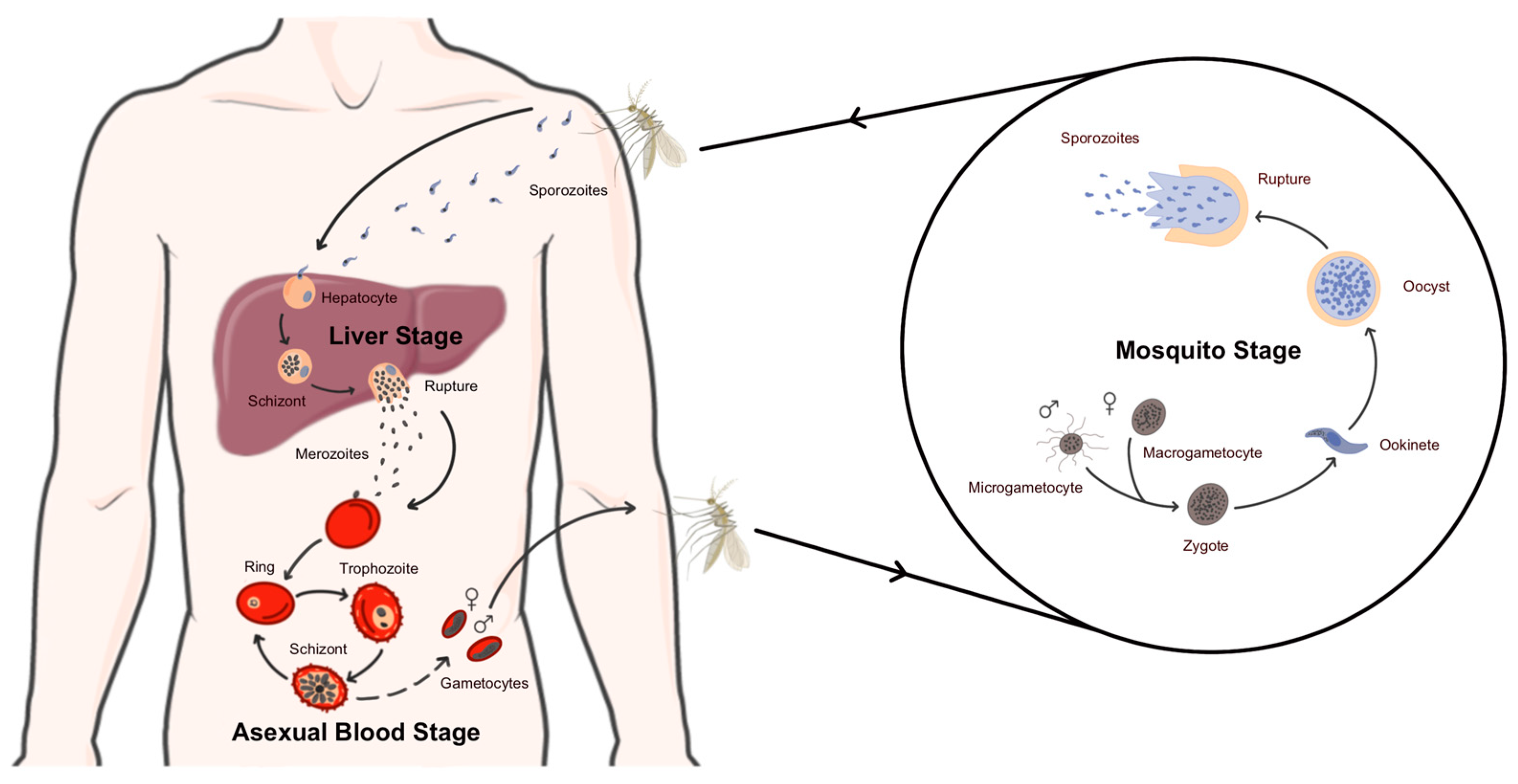
1. Introduction
2. From Natural Remedies to Synthetic Molecules and Back Again
3. Extreme Environments and Their Potential to Fight Disease
4. The Boreal Forest: An Unexplored Potential Treasure Trove of Natural Remedies
5. The Paradigm of Using Molecules Extracted from Northern Canada to Treat Malaria
6. Conclusions
7. Challenges
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wink, M. Medicinal plants: A source of anti-parasitic secondary metabolites. Molecules 2012, 17, 12771–12791. [Google Scholar] [CrossRef] [PubMed]
- Dziduch, K.; Greniuk, D.; Wujec, M. The Current Directions of Searching for Antiparasitic Drugs. Molecules 2022, 27, 1534. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Autino, B.; Noris, A.; Russo, R.; Castelli, F. Epidemiology of malaria in endemic areas. Mediterr. J. Hematol. Infect. Dis. 2012, 4, e2012060. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Sahi, P.K. Malaria: An Update. Indian J. Pediatr. 2017, 84, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Frischknecht, F.; Matuschewski, K. Plasmodium Sporozoite Biology. Cold Spring Harb. Perspect. Med. 2017, 7, a025478. [Google Scholar] [CrossRef] [PubMed]
- Amino, R.; Thiberge, S.; Martin, B.; Celli, S.; Shorte, S.; Frischknecht, F.; Ménard, R. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat. Med. 2006, 12, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Prudêncio, M.; Rodriguez, A.; Mota, M.M. The silent path to thousands of merozoites: The Plasmodium liver stage. Nat. Rev. Microbiol. 2006, 4, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Amino, R.; van de Sand, C.; Regen, T.; Retzlaff, S.; Rennenberg, A.; Krueger, A.; Pollok, J.-M.; Menard, R.; Heussler, V.T. Manipulation of Host Hepatocytes by the Malaria Parasite for Delivery into Liver Sinusoids. Science 2006, 313, 1287–1290. [Google Scholar] [CrossRef] [PubMed]
- Gilson, P.R.; Crabb, B.S. Morphology and kinetics of the three distinct phases of red blood cell invasion by Plasmodium falciparum merozoites. Int. J. Parasitol. 2009, 39, 91–96. [Google Scholar] [CrossRef]
- Al Meslamani, A.Z. How climate change influences pathogen transmission. Pathog. Glob. Health 2023, 1–3. [Google Scholar] [CrossRef]
- Yin, J.H.; Zhang, L.; Yi, B.Y.; Zhou, S.S.; Xia, Z.G. Imported malaria from land bordering countries in China: A challenge in preventing the reestablishment of malaria transmission. Travel Med. Infect. Dis. 2023, 53, 102575. [Google Scholar] [CrossRef]
- Özbilgin, A.; Tunalı, V.; Şenol Akar, Ş.; Çavuş, İ.; Zorbozan, O.; Yıldırım, A.; Turgay, N. Unpleasant Souvenir: Imported Plasmodium falciparum Malaria in Türkiye. Turk. Parazitol. Derg. 2023, 47, 204–208. [Google Scholar] [CrossRef]
- Lu, G.; Zhang, D.; Chen, J.; Cao, Y.; Chai, L.; Liu, K.; Chong, Z.; Zhang, Y.; Lu, Y.; Heuschen, A.-K.; et al. Predicting the risk of malaria re-introduction in countries certified malaria-free: A systematic review. Malar. J. 2023, 22, 175. [Google Scholar] [CrossRef]
- Bansal, V.; Munjal, J.; Lakhanpal, S.; Gupta, V.; Garg, A.; Munjal, R.S.; Jain, R. Epidemiological shifts: The emergence of malaria in America. Bayl. Univ. Med. Cent. Proc. 2023, 36, 745–750. [Google Scholar] [CrossRef]
- Carsley, J.; MacLean, J.D. Malaria in Canada. Can. Med. Assoc. J. 1997, 156, 57–58. [Google Scholar]
- Bagcchi, S. Locally acquired malaria cases in the USA. Lancet Infect. Dis. 2023, 23, e401. [Google Scholar] [CrossRef]
- Blackburn, D.; Drennon, M.; Broussard, K.; Morrison, A.M.; Stanek, D.; Sarney, E.; Ferracci, C.; Huard, S.; Brennan, W.; Eaton, J.; et al. Outbreak of Locally Acquired Mosquito-Transmitted (Autochthonous) Malaria—Florida and Texas, May–July 2023. Morb. Mortal. Wkly. Rep. 2023, 72, 973–978. [Google Scholar] [CrossRef]
- Boggild, A.K.; McCarthy, A.E.; Libman, M.D.; Freedman, D.O.; Kain, K.C. Underestimate of annual malaria imports to Canada. Lancet Infect. Dis. 2017, 17, 141–142. [Google Scholar] [CrossRef][Green Version]
- Tian, Y.; Li, Y.L.; Zhao, F.C. Secondary Metabolites from Polar Organisms. Mar. Drugs 2017, 15, 28. [Google Scholar] [CrossRef]
- Uprety, Y.; Asselin, H.; Dhakal, A.; Julien, N. Traditional use of medicinal plants in the boreal forest of Canada: Review and perspectives. J. Ethnobiol. Ethnomed. 2012, 8, 7. [Google Scholar] [CrossRef]
- Kariuki, S.N.; Williams, T.N. Human genetics and malaria resistance. Hum. Genet. 2020, 139, 801–811. [Google Scholar] [CrossRef]
- Neghina, R.; Neghina, A.; Marincu, I.; Iacobiciu, I. Malaria, a Journey in Time: In Search of the Lost Myths and Forgotten Stories. Am. J. Med. Sci. 2010, 340, 492–498. [Google Scholar] [CrossRef]
- Cai, S.; Risinger, A.L.; Nair, S.; Peng, J.; Anderson, T.J.; Du, L.; Powell, D.R.; Mooberry, S.L.; Cichewicz, R.H. Identification of Compounds with Efficacy against Malaria Parasites from Common North American Plants. J. Nat. Prod. 2016, 79, 490–498. [Google Scholar] [CrossRef]
- Hempelmann, E.; Krafts, K. Bad air, amulets and mosquitoes: 2000 years of changing perspectives on malaria. Malar. J. 2013, 12, 232. [Google Scholar] [CrossRef]
- Miller, L.H.; Rojas-Jaimes, J.; Low, L.M.; Corbellini, G. What Historical Records Teach Us about the Discovery of Quinine. Am. J. Trop. Med. Hyg. 2023, 108, 7–11. [Google Scholar] [CrossRef]
- Barcia, J.J. The Giemsa stain: Its history and applications. Int. J. Surg. Pathol. 2007, 15, 292–296. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, Z.; Liao, F.; Jiang, T.; Tu, Y. The birth of artemisinin. Pharmacol. Ther. 2020, 216, 107658. [Google Scholar] [CrossRef]
- Vennerstrom, J.L.; Arbe-Barnes, S.; Brun, R.; Charman, S.A.; Chiu, F.C.K.; Chollet, J.; Dong, Y.; Dorn, A.; Hunziker, D.; Matile, H.; et al. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature 2004, 430, 900–904. [Google Scholar] [CrossRef]
- Challis, M.P.; Devine, S.M.; Creek, D.J. Current and emerging target identification methods for novel antimalarials. Int. J. Parasitol. Drugs Drug Resist. 2022, 20, 135–144. [Google Scholar] [CrossRef]
- Heller, L.E.; Roepe, P.D. Artemisinin-Based Antimalarial Drug Therapy: Molecular Pharmacology and Evolving Resistance. Trop. Med. Infect. Dis. 2019, 4, 89. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, U.; Buttiëns, H. History and importance of antimalarial drug resistance. Trop. Med. Int. Health 2001, 6, 845–848. [Google Scholar] [CrossRef]
- Lyu, H.-N.; Ma, N.; Meng, Y.; Zhang, X.; Wong, Y.-K.; Xu, C.; Liao, F.; Jiang, T.; Tu, Y.; Wang, J. Study towards improving artemisinin-based combination therapies. Nat. Prod. Rep. 2021, 38, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Wells, T.N. Natural products as starting points for future anti-malarial therapies: Going back to our roots? Malar. J. 2011, 10 (Suppl. S1), S3. [Google Scholar] [CrossRef] [PubMed]
- Imwong, M.; Suwannasin, K.; Kunasol, C.; Sutawong, K.; Mayxay, M.; Rekol, H.; Smithuis, F.M.; Hlaing, T.M.; Tun, K.M.; van der Pluijm, R.W.; et al. The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong subregion: A molecular epidemiology observational study. Lancet Infect. Dis. 2017, 17, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Balikagala, B.; Fukuda, N.; Ikeda, M.; Katuro, O.T.; Tachibana, S.-I.; Yamauchi, M.; Opio, W.; Emoto, S.; Anywar, D.A.; Kimura, E.; et al. Evidence of Artemisinin-Resistant Malaria in Africa. N. Engl. J. Med. 2021, 385, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Maciuk, A.; Mazier, D.; Duval, R. Future antimalarials from Artemisia? A rationale for natural product mining against drug-refractory Plasmodium stages. Nat. Prod. Rep. 2023, 40, 1130–1144. [Google Scholar] [CrossRef] [PubMed]
- Carbonara, T.; Pascale, R.; Argentieri, M.P.; Papadia, P.; Fanizzi, F.P.; Villanova, L.; Avato, P. Phytochemical analysis of a herbal tea from Artemisia annua L. J. Pharm. Biomed. Anal. 2012, 62, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Agüero, F.; Al-Lazikani, B.; Aslett, M.; Berriman, M.; Buckner, F.S.; Campbell, R.K.; Carmona, S.; Carruthers, I.M.; Chan, A.W.; Chen, F.; et al. Genomic-scale prioritization of drug targets: The TDR Targets database. Nat. Rev. Drug Discov. 2008, 7, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Plouffe, D.; Brinker, A.; McNamara, C.; Henson, K.; Kato, N.; Kuhen, K.; Nagle, A.; Adrián, F.; Matzen, J.T.; Anderson, P.; et al. In silico activity profiling reveals the mechanism of action of antimalarials discovered in a high-throughput screen. Proc. Natl. Acad. Sci. USA 2008, 105, 9059–9064. [Google Scholar] [CrossRef]
- Gamo, F.-J.; Sanz, L.M.; Vidal, J.; de Cozar, C.; Alvarez, E.; Lavandera, J.-L.; Vanderwall, D.E.; Green, D.V.S.; Kumar, V.; Hasan, S.; et al. Thousands of chemical starting points for antimalarial lead identification. Nature 2010, 465, 305–310. [Google Scholar] [CrossRef]
- Hebert, P.D.; Gregory, T.R. The promise of DNA barcoding for taxonomy. Syst. Biol. 2005, 54, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wu, X.; Liu, C.; Newmaster, S.; Ragupathy, S.; Kress, W.J. Progress in the use of DNA barcodes in the identification and classification of medicinal plants. Ecotoxicol. Environ. Saf. 2021, 208, 111691. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, S.; Armson, A.; Lymbery, A.J.; Zahedi, A.; Ash, A. Medicinal plants as a source of antiparasitics: An overview of experimental studies. Pathog. Glob. Health 2023, 117, 535–553. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.H.; Tounge, B.A.; Bembenek, S.D. Ligand Binding Efficiency: Trends, Physical Basis, and Implications. J. Med. Chem. 2008, 51, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, K.R.; Jancey, J.; Clements, A.C.A.; Rai, R.; Leavy, J.E. Community engagement approaches for malaria prevention, control and elimination: A scoping review. BMJ Open 2024, 14, e081982. [Google Scholar] [CrossRef] [PubMed]
- de Pascale, D.; De Santi, C.; Fu, J.; Landfald, B. The microbial diversity of Polar environments is a fertile ground for bioprospecting. Mar. Genom. 2012, 8, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Frémand, A.C.; Bodart, J.A.; Jordan, T.A.; Ferraccioli, F.; Robinson, C.; Corr, H.F.J.; Peat, H.J.; Bingham, R.G.; Vaughan, D.G. British Antarctic Survey’s aerogeophysical data: Releasing 25 years of airborne gravity, magnetic, and radar datasets over Antarctica. Earth Syst. Sci. Data 2022, 14, 3379–3410. [Google Scholar] [CrossRef]
- Malard, L.A.; Avila-Jimenez, M.-L.; Schmale, J.; Cuthbertson, L.; Cockerton, L.; Pearce, D.A. Aerobiology over the Southern Ocean—Implications for bacterial colonization of Antarctica. Environ. Int. 2022, 169, 107492. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kato, C. Sampling, Isolation, Cultivation, and Characterization of Piezophilic Microbes. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin, Heidelberg, 2010; pp. 3869–3881. [Google Scholar]
- Talalay, P.G. Hot-Water Ice Drills. In Thermal Ice Drilling Technology; Talalay, P.G., Ed.; Springer: Singapore, 2020; pp. 145–250. [Google Scholar]
- Coker, J.A. ‘All about’ Extremophiles. Fac. Rev. 2023, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Convey, P.; Coulson, S.; Worland, M.; Sjöblom, A. The importance of understanding annual and shorter-term temperature patterns and variation in the surface levels of polar soils for terrestrial biota. Polar Biol. 2018, 41, 1587–1605. [Google Scholar] [CrossRef]
- D’Amico, S.; Collins, T.; Marx, J.C.; Feller, G.; Gerday, C. Psychrophilic microorganisms: Challenges for life. EMBO Rep. 2006, 7, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Tytgat, B.; Verleyen, E.; Sweetlove, M.; D’Hondt, S.; Clercx, P.; Van Ranst, E.; Peeters, K.; Roberts, S.; Namsaraev, Z.; Wilmotte, A.; et al. Bacterial community composition in relation to bedrock type and macrobiota in soils from the Sør Rondane Mountains, East Antarctica. FEMS Microbiol. Ecol. 2016, 92, fiw126. [Google Scholar] [CrossRef]
- Tripathi, V.C.; Satish, S.; Horam, S.; Raj, S.; Lal, A.; Arockiaraj, J.; Pasupuleti, M.; Dikshit, D.K. Natural products from polar organisms: Structural diversity, bioactivities and potential pharmaceutical applications. Polar Sci. 2018, 18, 147–166. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Crisafi, F.; La Cono, V.; Yakimov, M.M.; Molinaro, A.; Silipo, A. The Structure of the Lipid A of Gram-Negative Cold-Adapted Bacteria Isolated from Antarctic Environments. Mar. Drugs 2020, 18, 592. [Google Scholar] [CrossRef] [PubMed]
- Mojib, N.; Philpott, R.; Huang, J.P.; Niederweis, M.; Bej, A.K. Antimycobacterial activity in vitro of pigments isolated from Antarctic bacteria. Antonie Leeuwenhoek 2010, 98, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, D.; Luan, Y.; Gu, Q.; Zhu, T. Cytotoxic metabolites from the antarctic psychrophilic fungus Oidiodendron truncatum. J. Nat. Prod. 2012, 75, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Zmitrovich, I.V.; Arefyev, S.P.; Bondartseva, M.A.; Belova, N.V.; Khimich, Y.R.; Isaeva, L.G.; Kapitonov, V.I.; Vlasenko, V.A.; Volobuev, S.V.; Ezhov, O.N.; et al. Profiles of Little-Known Medicinal Polypores: Haploporus odorus (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, C.; Queiroz, E.F.; Marcourt, L.; Wolfender, J.L.; Azelmat, J.; Grenier, D.; Boudreau, S.; Voyer, N. Dibenzofurans and Pseudodepsidones from the Lichen Stereocaulon paschale Collected in Northern Quebec. J. Nat. Prod. 2017, 80, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Nweze, J.A.; Mbaoji, F.N.; Li, Y.-M.; Yang, L.-Y.; Huang, S.-S.; Chigor, V.N.; Eze, E.A.; Pan, L.-X.; Zhang, T.; Yang, D.-F. Potentials of marine natural products against malaria, leishmaniasis, and trypanosomiasis parasites: A review of recent articles. Infect. Dis. Poverty 2021, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Shilling, A.J.; Witowski, C.G.; Maschek, J.A.; Azhari, A.; Vesely, B.A.; Kyle, D.E.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Spongian Diterpenoids Derived from the Antarctic Sponge Dendrilla antarctica Are Potent Inhibitors of the Leishmania Parasite. J. Nat. Prod. 2020, 83, 1553–1562. [Google Scholar] [CrossRef]
- Ma, W.S.; Mutka, T.; Vesley, B.; Amsler, M.O.; McClintock, J.B.; Amsler, C.D.; Perman, J.A.; Singh, M.P.; Maiese, W.M.; Zaworotko, M.J.; et al. Norselic Acids A−E, Highly Oxidized Anti-Infective Steroids that Deter Mesograzer Predation, from the Antarctic Sponge Crella sp. J. Nat. Prod. 2009, 72, 1842–1846. [Google Scholar] [CrossRef] [PubMed]
- von Salm, J.L.; Wilson, N.G.; Vesely, B.A.; Kyle, D.E.; Cuce, J.; Baker, B.J. Shagenes A and B, new tricyclic sesquiterpenes produced by an undescribed Antarctic octocoral. Org. Lett. 2014, 16, 2630–2633. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.; Demain, A.L. 1.10—Secondary Metabolites. In Comprehensive Biotechnology, 3rd ed.; Moo-Young, M., Ed.; Pergamon: Oxford, UK, 2011; pp. 131–143. [Google Scholar]
- Gokulan, K.; Khare, S.; Cerniglia, C. Metabolic Pathways: Production of Secondary Metabolites of Bacteria. In Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 561–569. [Google Scholar]
- Morita, R.Y. Psychrophilic bacteria. Bacteriol. Rev. 1975, 39, 144–167. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.K.; Virjamo, V.; Ghimire, R.P.; Blande, J.D.; Julkunen-Tiitto, R.; Kivimäenpää, M. Climate Change Effects on Secondary Compounds of Forest Trees in the Northern Hemisphere. Front. Plant Sci. 2018, 9, 1445. [Google Scholar] [CrossRef] [PubMed]
- Surowiak, A.K.; Balcerzak, L.; Lochyński, S.; Strub, D.J. Biological Activity of Selected Natural and Synthetic Terpenoid Lactones. Int. J. Mol. Sci. 2021, 22, 5036. [Google Scholar] [CrossRef] [PubMed]
- Teoh, E.-S. Medicinal Orchids of Asia; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Burton, P.; Messier, C.; Weetman, G.; Prepas, E.E.; Adamowicz, L.; Tittler, R. The current state of boreal forestry and the drive for change. In Towards Sustainable Management of the Boreal Forest; NRC Research Press: Ottawa, ON, Canada, 2003; pp. 1–40. [Google Scholar]
- Gauthier, S.; Bernier, P.; Kuuluvainen, T.; Shvidenko, A.Z.; Schepaschenko, D.G. Boreal forest health and global change. Science 2015, 349, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Osawa, A.; Matsuura, Y.; Kajimoto, T. Characteristics of Permafrost Forests in Siberia and Potential Responses to Warming Climate. In Permafrost Ecosystems: Siberian Larch Forests; Osawa, A., Zyryanova, O.A., Matsuura, Y., Kajimoto, T., Wein, R.W., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 459–481. [Google Scholar]
- Moerman, D.E. An analysis of the food plants and drug plants of native North America. J. Ethnopharmacol. 1996, 52, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Black, P.; Saleem, A.; Dunford, A.; Guerrero-Analco, J.; Walshe-Roussel, B.; Haddad, P.; Cuerrier, A.; Arnason, J.T. Seasonal variation of phenolic constituents and medicinal activities of Northern Labrador tea, Rhododendron tomentosum ssp. subarcticum, an Inuit and cree First Nations traditional medicine. Planta Med. 2011, 77, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.A.; Johnson, J.A.; Webster, D. Canadian traditionally used medicinal plants: Can they play a role in antituberculosis drug development? Future Med. Chem. 2013, 5, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Dampc, A.; Luczkiewicz, M. Rhododendron tomentosum (Ledum palustre). A review of traditional use based on current research. Fitoterapia 2013, 85, 130–143. [Google Scholar] [CrossRef]
- McCune, L.M.; Johns, T. Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the North American boreal forest. J. Ethnopharmacol. 2002, 82, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Séguin, J.-C.; Gagnon, D.; Bélanger, S.; Richard, D.; Fernandez, X.; Boudreau, S.; Voyer, N. Chemical Composition and Antiplasmodial Activity of the Essential Oil of Rhododendron subarcticum Leaves from Nunavik, Québec, Canada. ACS Omega 2023, 8, 16729–16737. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Pastor, J.; Scull, R.; Gille, L. Antileishmanial activity of essential oil from Chenopodium ambrosioides and its main components against experimental cutaneous leishmaniasis in BALB/c mice. Phytomedicine 2014, 21, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Blanco, M.E.; Rodríguez, M.G.; Moreno Duque, J.L.; Muñoz-Ortega, M.; Ventura-Juárez, J. Amoebicidal Activity of Essential Oil of Dysphania ambrosioides (L.) Mosyakin & Clemants in an Amoebic Liver Abscess Hamster Model. Evid.-Based Complement. Altern. Med. 2014, 2014, 930208. [Google Scholar] [CrossRef]
- Kiuchi, F.; Itano, Y.; Uchiyama, N.; Honda, G.; Tsubouchi, A.; Nakajima-Shimada, J.; Aoki, T. Monoterpene Hydroperoxides with Trypanocidal Activity from Chenopodium ambrosioides. J. Nat. Prod. 2002, 65, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Cysne, D.N.; Fortes, T.S.; Reis, A.S.; de Paulo Ribeiro, B.; dos Santos Ferreira, A.; do Amaral, F.M.M.; Guerra, R.N.M.; Marinho, C.R.F.; Nicolete, R.; Nascimento, F.R.F. Antimalarial potential of leaves of Chenopodium ambrosioides L. Parasitol. Res. 2016, 115, 4327–4334. [Google Scholar] [CrossRef] [PubMed]
- Bérubé, C.; Gagnon, D.; Borgia, A.; Richard, D.; Voyer, N. Total synthesis and antimalarial activity of mortiamides A–D. Chem. Commun. 2019, 55, 7434–7437. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, A.L.; Berrue, F.; Robertson, A.W.; Overy, D.P.; Kerr, R.G. Mortiamides A–D, Cyclic Heptapeptides from a Novel Mortierella sp. Obtained from Frobisher Bay. J. Nat. Prod. 2017, 80, 2677–2683. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, T.; Bergeron, C.; Gagnon, D.; Bérubé, C.; Voyer, N.; Richard, D.; Giguère, D. Squaramide Tethered Clindamycin, Chloroquine, and Mortiamide Hybrids: Design, Synthesis, and Antimalarial Activity. ACS Med. Chem. Lett. 2023, 14, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Limon, A.-C.D.; Patabendige, H.M.L.W.; Azhari, A.; Sun, X.; Kyle, D.E.; Wilson, N.G.; Baker, B.J. Chemistry and Bioactivity of the Deep-Water Antarctic Octocoral Alcyonium sp. Mar. Drugs 2022, 20, 576. [Google Scholar] [CrossRef]
- Sak, K.; Jürisoo, K.; Raal, A. Estonian folk traditional experiences on natural anticancer remedies: From past to the future. Pharm. Biol. 2014, 52, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Giordano, D. Bioactive Molecules from Extreme Environments. Mar. Drugs 2020, 18, 640. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.; Chandel, A.K.; Singh, O.V. Challenges in Advancing Extremophiles for Therapeutic Applications. In Extremophiles and Their Applications in Medical Processes; Babu, P., Chandel, A.K., Singh, O.V., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 37–41. [Google Scholar]
- Karjala, M.K.; Sherry, E.E.; Dewhurst, S.M. Criteria and indicators for sustainable forest planning: A framework for recording Aboriginal resource and social values. For. Policy Econ. 2004, 6, 95–110. [Google Scholar] [CrossRef]
- Karst, A. Conservation Value of the North. American Boreal Forest from an Ethnobotanical Perspective; Canadian Boreal Initiative: Ottawa, ON, Canada; David Suzuki Foundation: Vancouver, BC, Canada; Boreal Songbird Initiative: Seattle, WA, USA, 2010. [Google Scholar]
- Redvers, N.; Blondin, B. Traditional Indigenous Medicine in North America: A Scoping Review. PLoS ONE 2020, 15, e0237531. [Google Scholar] [CrossRef]
| Species/Location | Molecule | Properties | Ref |
|---|---|---|---|
| Gram-negative bacteria Ex. Psychrobacter cryohalolentis Pseudoalteromonas Bacteria found in Antarctica | Lipid A Lipopolysaccharide | Immunomodulatory molecule Immunostimulatory (TLR4/ MD2 pathway) Activates TNF production | [57] |
| Janthinobacterium sp. Ant5-2 J-PVP Bacteria isolated from Antarctic lakes | Violacein Pigment | Antimycobacterial (ex. Tuberculosis) Mycobacterium tuberculosis mc26230 Minimal inhibitory concentration (MIC) 5 μg/mL M. tuberculosis H37Rv MIC 34.4 μg/mL | [58] |
| Flavobacterium sp. Ant342 F-YOP Bacteria isolated from Antarctic lakes | Flexirubin Pigment | Antimycobacterial (ex. Tuberculosis) M. tuberculosis H37Rv MIC 10.8 μg/mL | [58] |
| Oidiodendon tuncatum (GW3-13) Fungus isolated from Antarctic soil | Epipolythiodioxopiperazines Diketopiperazines | Cytotoxic Immunomodulatory Antiviral Antimicrobial Antiproliferative Cytotoxic activity against 5 cancer cell lines HCT-8, Bel-7402, BCG-823, A-549, A-2780 Various IC50 ranging from 0.003 μg to 1.83 μg/mL Cytotoxic effect depending on the presence of a sulfide bridge within the molecule | [59] |
| Haploporus odorus Agaricomycetes Northern Hemisphere | Haploporic acid A | Cancer therapy Proliferation pathways are affected coordinating the arrest of the cell cycle often resulting in the apoptosis of cancerous cells | [60] |
| Stereoculon paschale Lichen Northern Canada | Pseudodepsidone-type metabolites Lobaric acid | Antimicrobial activity against selected oral pathogens Porphyromonas gingivalis Sterptococcus mutans MIC ranging from 20 to 80 μM | [61] |
| Plumarella delicatissima Cnidaria Antarctica | Keikipukaldes Pukalide Aldehyde Norditerpenoid ineleganolide | Antiparasitic activity against Leishmania donovani IC50 against L. donovani 1.9 to 12 μM | [62] |
| Dendrilla antarctica Sea sponge Antarctica | Diterpenoids Tetrahydroaplysulphurin-1 Membranoids B, D, G | Antiparasitic activity against Leishmania donovani Tetrahydroaplysulphurin-1 IC50 3.5 μM Membranoid B IC50 0.8 μM Membranoid D IC50 1.4 μM Membranoid G IC50 1.9 μM | [63] |
| Crella sp. Sea sponge Antarctica | Norselic acid A–E | Antiparasitic activity against Leishmania donovani Norselic acid A IC50 2.5 μM Norselic acid B IC50 2.4 μM Norselic acid C IC50 2.6 μM Norselic acid D IC50 2.0 μM Norselic acid E IC50 3.6 μM | [64] |
| Undescribed Antarctic soft coral Scotia Arc, Antarctica | Tricyclic sesquiterpenoid Shagene A | Antiparasitic activity against Leishmania donovani IC50 5 μM | [65] |
| Species/Common Name | Parts Used | Preparations | Primary Uses |
|---|---|---|---|
| Abies balsamea Balsam fir | Gum Sap Branches Needles Cones Bark Roots Buds | Salve/Ointment Poultice Infusion Dried/Powder Decoction | Application as topical treatments for sores and cuts Arthritis Muscular pain Stomachache, nausea and colic Tuberculosis |
| Achillea millefolium Yarrow | Whole plant Leaves Roots Flowers | Salve/Ointment Dried/Powder Decoction Infusion Poultice Burned/Smoked | Fever Respiratory illnesses Aches and pains Arthritis Migraines Treatments for sores and cuts |
| Acorus calamus Sweet flag | Roots Rhizome | Scalded Infusion Tonic Dried/Powder Decoction Smoked | Cold and flu symptoms Fever Inflammation Topical treatment for sores, cuts and infections Aches and pains Treatment of parasitic intestinal worms |
| Aralia nudicaulis Wild sarsaparilla | Leaves Roots Stalk Rhizomes | Infusion Tonic Decoction Dried/Powder Poultice | Pneumonia Weakness Aches and pains Cold and flu symptoms Stomachache Topical treatment of wounds, infections and sores |
| Betula papyrifera Paper birch | Leaves Stem/Bark Buds Wood Roots Sap | Decoction Dried/Powder Infusion Poultice Salve/Ointment | Topical treatment for stings, cleanser, rashes and infection Stomachache Tonsilitis Cough |
| Cornus sericea Red-osier dogwood | Bark Roots Stems Fruits/Pith Twigs Leaves | Infusion Decoction Smoked | Diarrhea Topical treatment for poison ivy, sores and stings Sore throat Cold and flu symptoms Fever Weakness Stomachache Tuberculosis |
| Heracleum maximum Cow parsnip | Roots Leaves Flowers | Infusion Dried Decoction Paste Poultice | Cholera Topical treatment for sores, boils and infections Cold and flu symptoms Tooth ache Sore throat Inflammation Smallpox Tuberculosis Headache |
| Juniperus communis Juniper | Fruits Roots Leaves Bark Stem Gum | Infusion Juice (berries) Decoction Dried/Powdered Poultice Tonic Smoked | Tuberculosis Cold and flu symptoms Stomachache Aches and pains Topical treatment for skin problems, boils and wounds Fever |
| Larix laricina Tamarack | Branches Bark Needles Gum Leaves Cones Sap Roots Pulp | Infusion Decoction Poultice Inhalation | Topical treatment of boils, wounds, frostbite, infection and burns Stomachache Cold and flu symptoms Anemia Gonorrhea Inflammation Sore throat Ache and pains Jaundice Arthritis |
| Mentha arvensis Wild mint | Whole plant Flowers Leaves Stem | Infusion Tonic | Stomachache Topical treatment of sores and infection Cold and flu symptoms Weakness Diarrhea Fever Toothache |
| Nuphar lutea Yellow water lily | Rhizomes Roots Whole plant Stems | Dried/Powder Infusion Poultice | Arthritis Inflammation Topical treatments of boils, infection and stings Aches and pains Cold and flu symptoms Stomach pain |
| Picea glauca White spruce | Twigs Bark Sap Gum | Infusion Decoction Poultice Salve/Ointment Dried/Powder | Fever Cold and flu symptoms Headaches Joint pains Topical treatment for sores, burns, irritation, infection and wounds Sore throat Toothache Intestinal problems Aches and pains |
| Picea mariana Black spruce | Twigs Sap Bark Charcoal Cones/Young tips Leaves Roots Gum | Infusion Decoction Salve/Ointment Dried/Powder | Cold and flu symptoms Fever Topical treatment for boils, sores, infections and burns Stomachache Toothache Sore throat |
| Populus balsamifera Balsam poplar | Buds Sap Bark Leaves Roots Catkins Rotten wood | Infusion Salve/Ointment Decoction Poultice | Internal blood diseases Topical treatment for frost bite, sores, infection, skin diseases and stings Toothache Aches and pains Seizures Stomachache Inflammation |
| Populus tremuloides Quaking aspen | Sap Bark Leaves Buds Seeds Roots Rotten wood | Infusion Decoction Poultice Tonic Dried/Powder | Treatment of intestinal parasitic worms Stomachache Cold and flu symptoms Food poisoning Fever Topical treatment of wounds and stings Toothache |
| Rhododendron groenlandicum Labrador tea | Whole plant Leaves Roots | Infusion Decoction Tonic Dried/Powder Salve/Ointment | Cold and flu symptoms Pneumonia Whooping cough Ache and pains Arthritis Topical treatment of wounds, sores, burns Sore throat |
| Salix sp. Willow | Bark Roots Leaves | Dried/Powder Salve/Ointment Infusion Decoction Poultice | Topical treatment for sores, bruises and stings Toothache Arthritis Cold and flu symptoms Aches and pains Stomachache Dysentery |
| Sorbus americana American mountain ash | Leaves Bark Roots Buds Stem | Infusion Decoction Burned Poultice Paste Tonic | Stomachache and colic Sore throat Cholera Aches and pains Topical treatment for boils Cold and flu symptoms Toothache Arthritis Weakness |
| Thuja occidentalis Arborvitae | Wood Leaves Cones Charcoal Bark Gum | Infusion Burned Decoction Salve/Ointment Dried/Powder | Fumigation against disease Fever Toothache Infection Inflammation Cold and flu symptoms Topical treatment for infections, wounds, burns and paralysis Arthritis Colic Convulsions |
| Rhododendron tomentosum spp. Subarticum Northern Labrador tea [76,78] | Whole plant | Infusions | Cold and flu symptoms Toothache Stomachache Cough Tuberculosis Throat aches Aches and pains Headache Eye problems Nasal congestion Wound treatment Arthritis Infections Inflammation Weakness Heart and chest pain |
| Species/Location | Molecule | Disease and Properties | Ref |
|---|---|---|---|
| Rhododendron tomentosum spp. Subarticum Isolated compound from essential oil Northern Canada | Ascaridole | Antiplasmodial (3D7 and Dd2 parasite strains) IC50 against 3D7 147.3 ± 7.3 nM IC50 against Dd2 104.9 ± 11.2 nM | [80] |
| Mortierella Fungus Northern Canada | Mortiamides (A, B, D) Clindamycin (Mortiamide analogue) | Antiplasmodial (3D7 and Dd2 parasite strains) Mortiamide A IC50 against 3D7 7.85 ± 0.97 μM IC50 against Dd2 5.31 ± 0.24 μM Mortiamide B IC50 against 3D7 3.16 ± 0.65 μM IC50 against Dd2 2.10 ± 0.18 μM Mortiamide D IC50 against 3D7 1.31 ± 0.12 μM IC50 against Dd2 0.94 ± 0.07 μM | [85,86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourgeois, A.; Lemos, J.A.S.; Roucheray, S.; Sergerie, A.; Richard, D. The Paradigm Shift of Using Natural Molecules Extracted from Northern Canada to Combat Malaria. Infect. Dis. Rep. 2024, 16, 543-560. https://doi.org/10.3390/idr16040041
Bourgeois A, Lemos JAS, Roucheray S, Sergerie A, Richard D. The Paradigm Shift of Using Natural Molecules Extracted from Northern Canada to Combat Malaria. Infectious Disease Reports. 2024; 16(4):543-560. https://doi.org/10.3390/idr16040041
Chicago/Turabian StyleBourgeois, Alexandra, Juliana Aline Souza Lemos, Stéphanie Roucheray, Audrey Sergerie, and Dave Richard. 2024. "The Paradigm Shift of Using Natural Molecules Extracted from Northern Canada to Combat Malaria" Infectious Disease Reports 16, no. 4: 543-560. https://doi.org/10.3390/idr16040041
APA StyleBourgeois, A., Lemos, J. A. S., Roucheray, S., Sergerie, A., & Richard, D. (2024). The Paradigm Shift of Using Natural Molecules Extracted from Northern Canada to Combat Malaria. Infectious Disease Reports, 16(4), 543-560. https://doi.org/10.3390/idr16040041





