Frequency of SARS-CoV-2 Infections among Healthcare Workers in Germany: 3-Year Follow-Up Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. ELISA
2.3. Neutralization Assay
2.4. In-House ELISpot Assay
2.5. Ethics
2.6. Statistics
3. Results
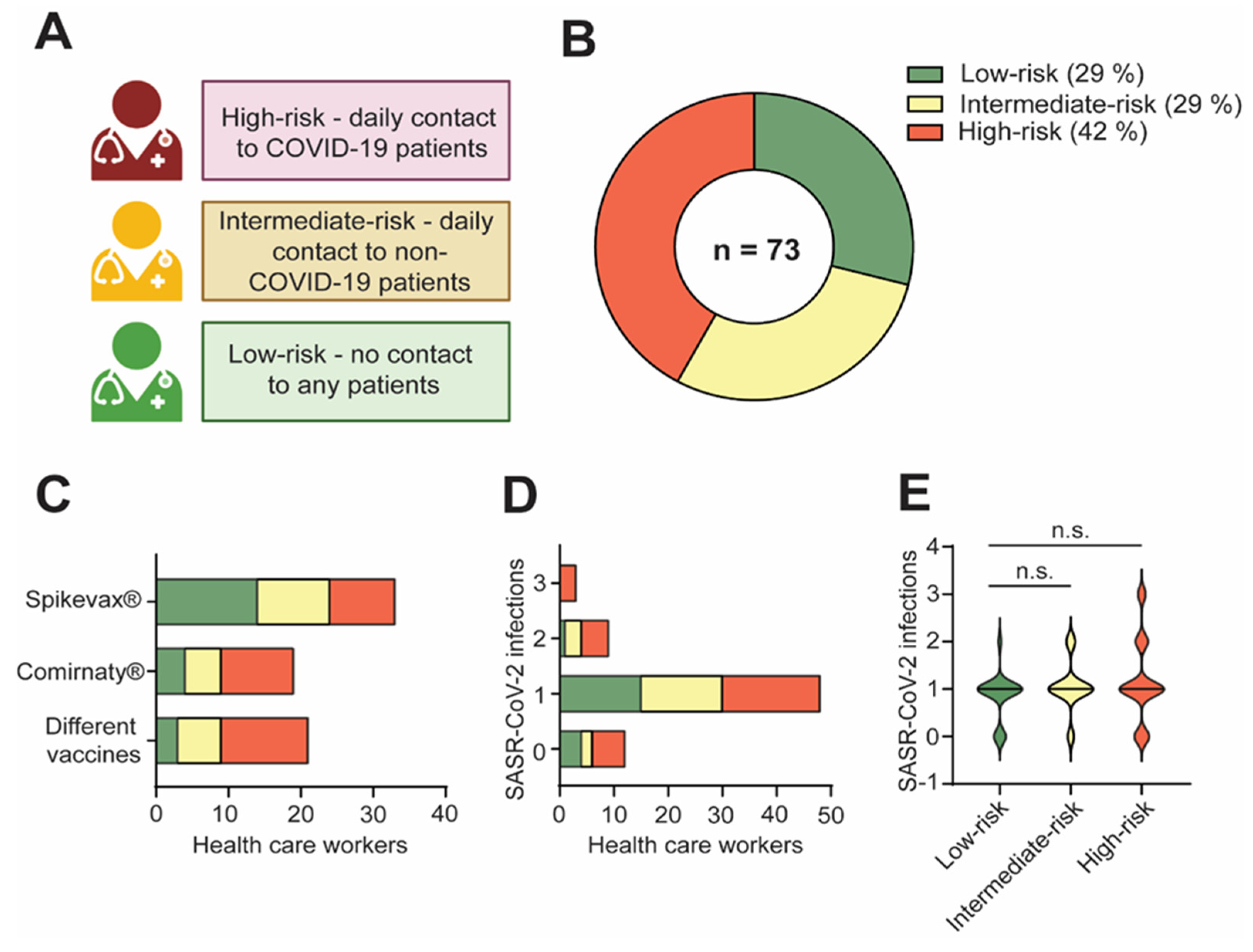
3.1. Study Overview
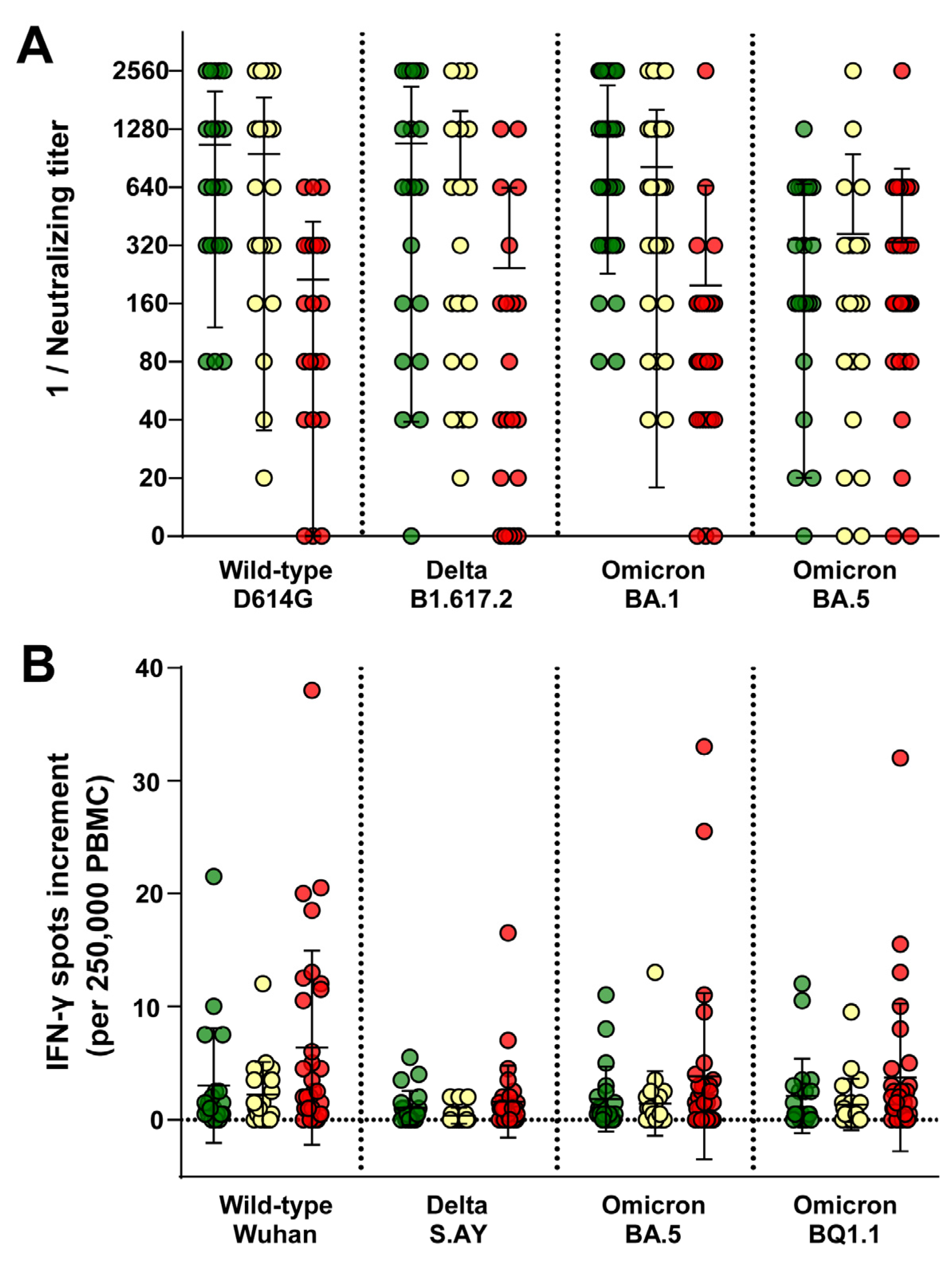
3.2. HCW Had Comparable Immune Responses Regardless of Contact with COVID-19 or Non-COVID-19 Patients
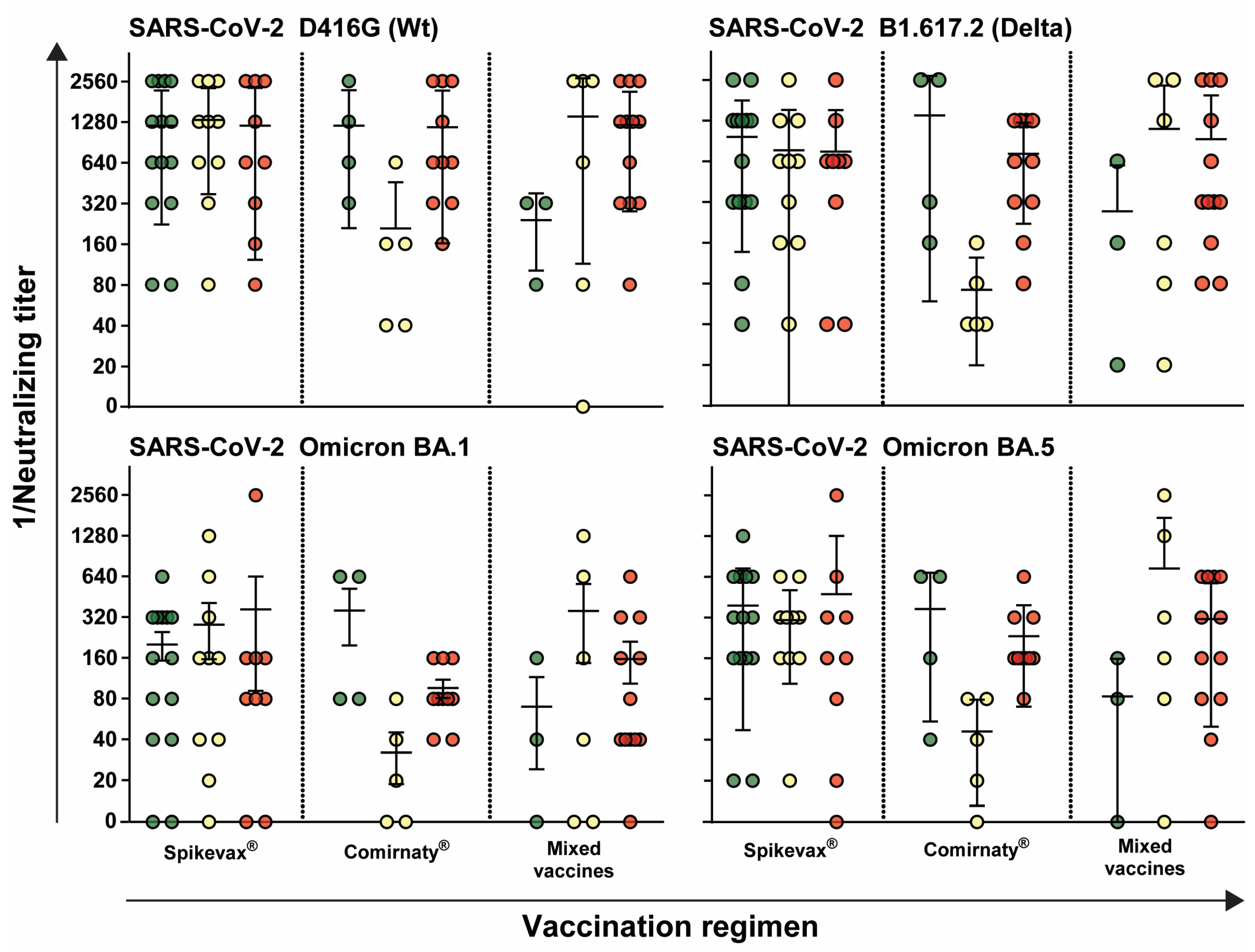
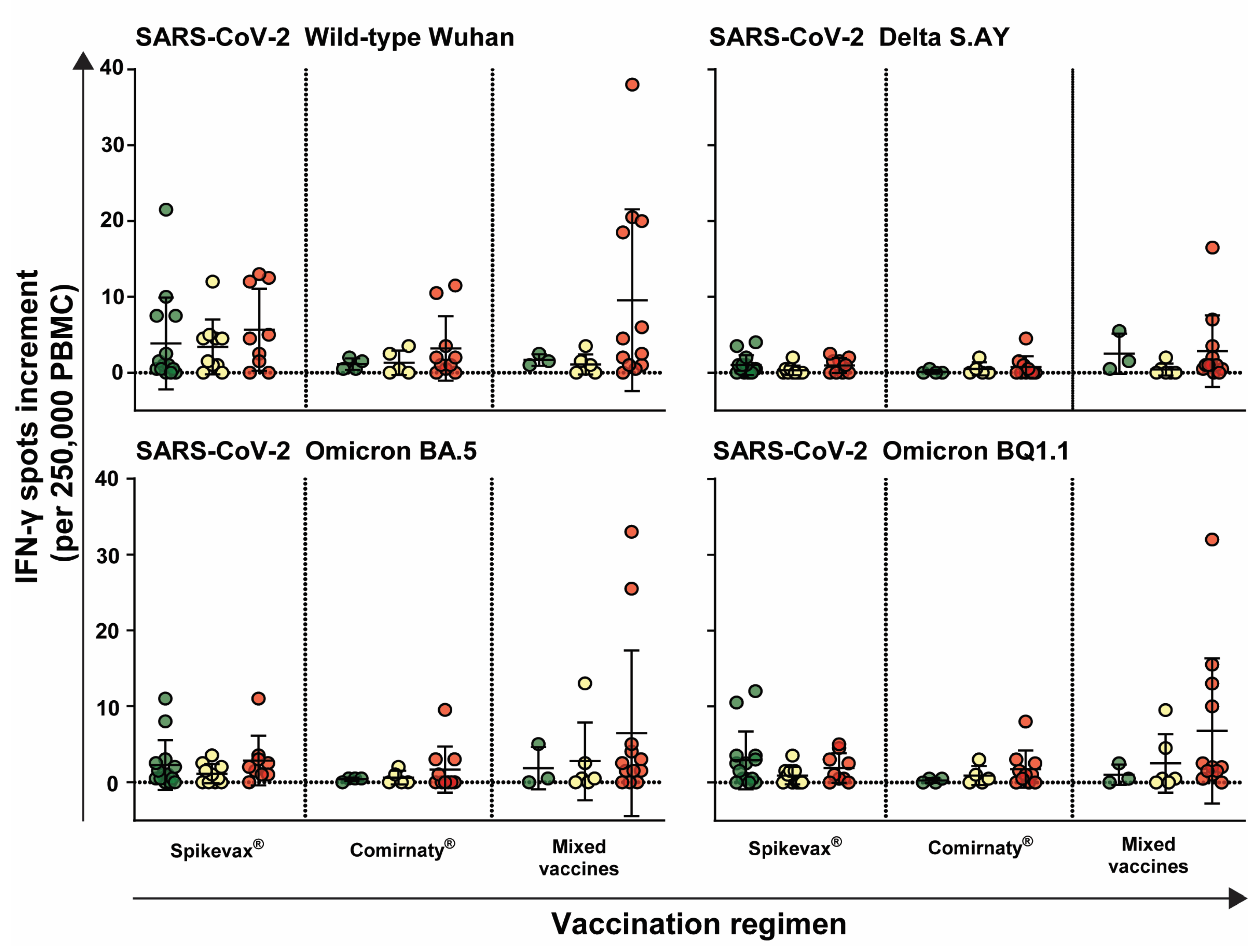
3.3. Immune Response Is Independent of Vaccination Regimen
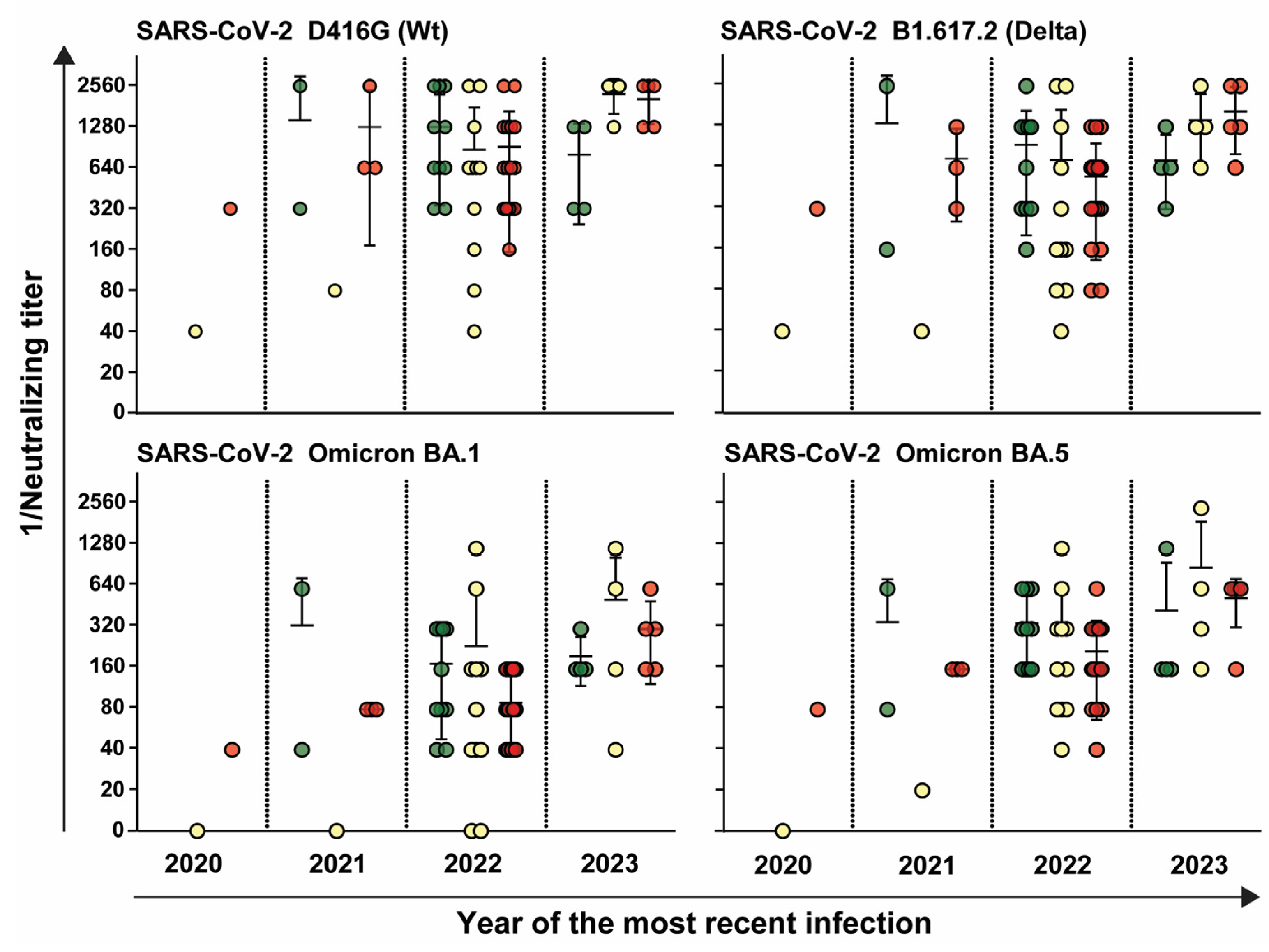
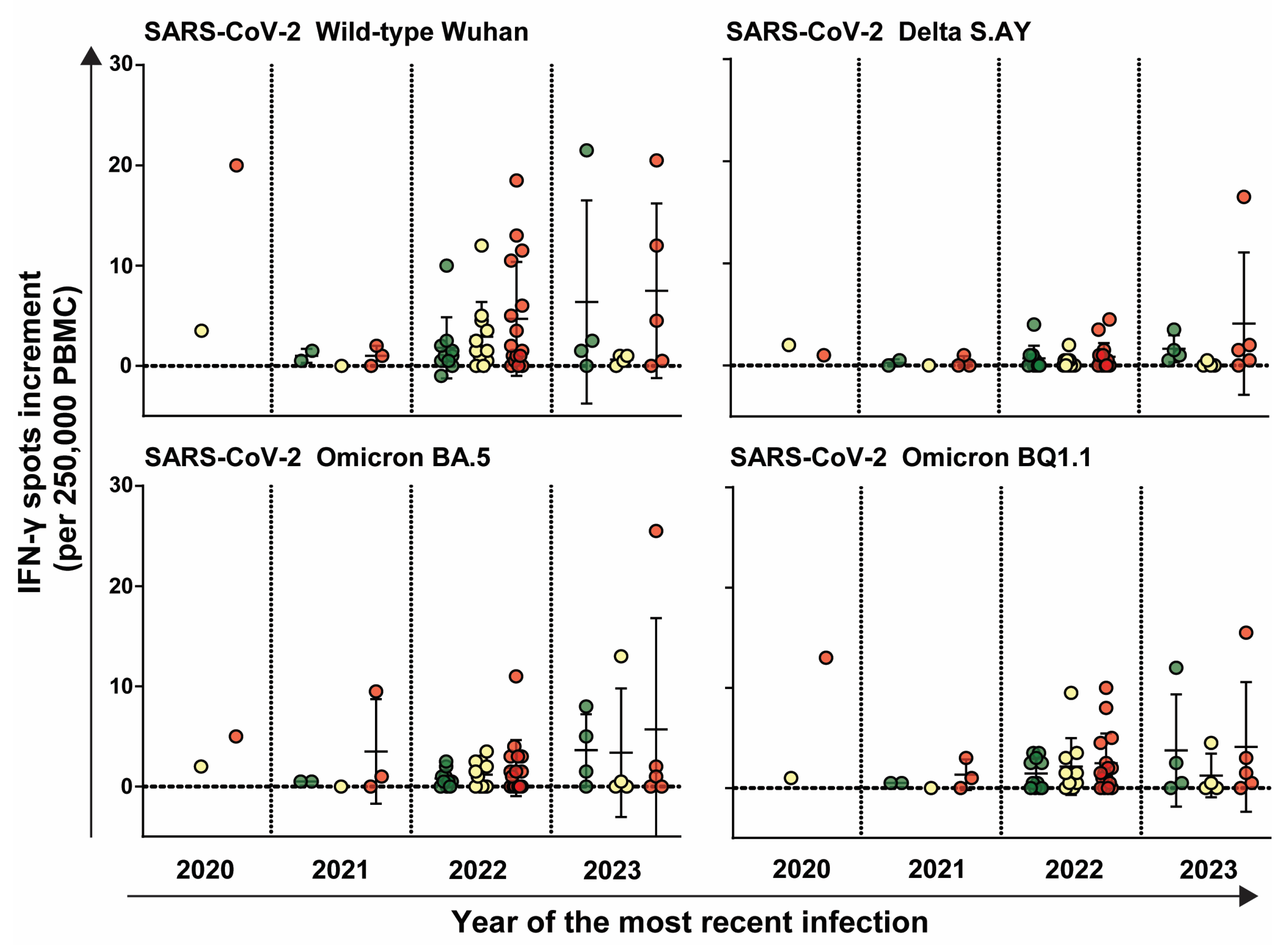
3.4. HCW Who Were Infected More Recently Exhibited an Increasedimmune Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- da Rosa Mesquita, R.; Francelino Silva Junior, L.C.; Santos Santana, F.M.; Farias de Oliveira, T.; Campos Alcantara, R.; Monteiro Arnozo, G.; Rodrigues da Silva Filho, E.; Galdino Dos Santos, A.G.; Oliveira da Cunha, E.J.; Salgueiro de Aquino, S.H.; et al. Clinical manifestations of COVID-19 in the general population: Systematic review. Wien. Klin. Wochenschr. 2021, 133, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Min, P.; Lee, S.; Kim, S.W. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J. Korean Med. Sci. 2020, 35, e174. [Google Scholar] [CrossRef] [PubMed]
- Cegolon, L.; Mauro, M.; Sansone, D.; Tassinari, A.; Gobba, F.M.; Modenese, A.; Casolari, L.; Liviero, F.; Pavanello, S.; Scapellato, M.L.; et al. A Multi-Center Study Investigating Long COVID-19 in Healthcare Workers from North-Eastern Italy: Prevalence, Risk Factors and the Impact of Pre-Existing Humoral Immunity-ORCHESTRA Project. Vaccines 2023, 11, 1759. [Google Scholar] [CrossRef] [PubMed]
- Butowt, R.; Bilinska, K.; von Bartheld, C. Why Does the Omicron Variant Largely Spare Olfactory Function? Implications for the Pathogenesis of Anosmia in Coronavirus Disease 2019. J. Infect. Dis. 2022, 226, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Bigdelou, B.; Sepand, M.R.; Najafikhoshnoo, S.; Negrete, J.A.T.; Sharaf, M.; Ho, J.Q.; Sullivan, I.; Chauhan, P.; Etter, M.; Shekarian, T.; et al. COVID-19 and Preexisting Comorbidities: Risks, Synergies, and Clinical Outcomes. Front. Immunol. 2022, 13, 890517. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, A.; Ghisetti, V.; Emanuele, T.; Trunfio, M.; Faraoni, S.; Boglione, L.; Burdino, E.; Audagnotto, S.; Lipani, F.; Nigra, M.; et al. Risk for SARS-CoV-2 Infection in Healthcare Workers, Turin, Italy. Emerg. Infect. Dis. 2021, 27, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Korth, J.; Wilde, B.; Dolff, S.; Anastasiou, O.E.; Krawczyk, A.; Jahn, M.; Cordes, S.; Ross, B.; Esser, S.; Lindemann, M.; et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J. Clin. Virol. 2020, 128, 104437. [Google Scholar] [CrossRef]
- Korth, J.; Wilde, B.; Dolff, S.; Frisch, J.; Jahn, M.; Krawczyk, A.; Trilling, M.; Schipper, L.; Cordes, S.; Ross, B.; et al. SARS-CoV-2 Seroprevalence in Healthcare Workers in Germany: A Follow-Up Study. Int. J. Environ. Res. Public Health 2021, 18, 4540. [Google Scholar] [CrossRef] [PubMed]
- Cegolon, L.; Ronchese, F.; Ricci, F.; Negro, C.; Larese-Filon, F. SARS-CoV-2 Infection in Health Care Workers of Trieste (North-Eastern Italy), 1 October 2020-7 February 2022: Occupational Risk and the Impact of the Omicron Variant. Viruses 2022, 14, 1663. [Google Scholar] [CrossRef]
- Alishaq, M.; Nafady-Hego, H.; Jeremijenko, A.; Al Ajmi, J.A.; Elgendy, M.; Vinoy, S.; Fareh, S.B.; Veronica Plaatjies, J.; Nooh, M.; Alanzi, N.; et al. Risk factors for breakthrough SARS-CoV-2 infection in vaccinated healthcare workers. PLoS ONE 2021, 16, e0258820. [Google Scholar] [CrossRef]
- Bormann, M.; Brochhagen, L.; Alt, M.; Otte, M.; Thummler, L.; van de Sand, L.; Kraiselburd, I.; Thomas, A.; Gosch, J.; Brass, P.; et al. Immune responses in COVID-19 patients during breakthrough infection with SARS-CoV-2 variants Delta, Omicron-BA.1 and Omicron-BA.5. Front. Immunol. 2023, 14, 1150667. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 6 March 2024).
- Chen, Q.; Zhu, K.; Liu, X.; Zhuang, C.; Huang, X.; Huang, Y.; Yao, X.; Quan, J.; Lin, H.; Huang, S.; et al. The Protection of Naturally Acquired Antibodies Against Subsequent SARS-CoV-2 Infection: A Systematic Review and Meta-Analysis. Emerg. Microbes Infect. 2022, 11, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.L.; Francescangeli, F.; Rossi, R.; Giuliani, A.; De Maria, R.; Zeuner, A. Repeated Exposure to Subinfectious Doses of SARS-CoV-2 May Promote T Cell Immunity and Protection against Severe COVID-19. Viruses 2021, 13, 961. [Google Scholar] [CrossRef]
- Heilingloh, C.S.; Aufderhorst, U.W.; Schipper, L.; Dittmer, U.; Witzke, O.; Yang, D.; Zheng, X.; Sutter, K.; Trilling, M.; Alt, M.; et al. Susceptibility of SARS-CoV-2 to UV Irradiation. Am. J. Infect. Control 2020, 48, 1273–1275. [Google Scholar] [CrossRef]
- Thummler, L.; Koldehoff, M.; Fisenkci, N.; Brochhagen, L.; Horn, P.A.; Krawczyk, A.; Lindemann, M. Cellular and Humoral Immunity after the Third Vaccination against SARS-CoV-2 in Hematopoietic Stem-Cell Transplant Recipients. Vaccines 2022, 10, 972. [Google Scholar] [CrossRef]
- Widera, M.; Wilhelm, A.; Toptan, T.; Raffel, J.M.; Kowarz, E.; Roesmann, F.; Grozinger, F.; Siemund, A.L.; Luciano, V.; Kulp, M.; et al. Generation of a Sleeping Beauty Transposon-Based Cellular System for Rapid and Sensitive Screening for Compounds and Cellular Factors Limiting SARS-CoV-2 Replication. Front. Microbiol. 2021, 12, 701198. [Google Scholar] [CrossRef]
- Lindemann, M.; Lenz, V.; Knop, D.; Klump, H.; Alt, M.; Aufderhorst, U.W.; Schipper, L.; Schwarzkopf, S.; Meller, L.; Steckel, N.; et al. Convalescent plasma treatment of critically ill intensive care COVID-19 patients. Transfusion 2021, 61, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Schwarzkopf, S.; Krawczyk, A.; Knop, D.; Klump, H.; Heinold, A.; Heinemann, F.M.; Thummler, L.; Temme, C.; Breyer, M.; Witzke, O.; et al. Cellular Immunity in COVID-19 Convalescents with PCR-Confirmed Infection but with Undetectable SARS-CoV-2-Specific IgG. Emerg. Infect. Dis. 2021, 27, 122–129. [Google Scholar] [CrossRef]
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/region/euro/country/de (accessed on 1 June 2024).
- Siekmann, D.M. Corona-Zahlen für Deutschland. Available online: https://www.corona-in-zahlen.de/weltweit/deutschland/ (accessed on 1 June 2024).
- Gehring, S.; Kowalzik, F.; Okasha, O.; Engelmann, T.; Schreiner, D.; Jensen, C.; Mahringer-Kunz, A.; Hartig-Merkel, W.; Mai Phuong Tran, T.; Oostvogels, C.; et al. A prospective cohort study of SARS-CoV-2 infection-induced seroconversion and disease incidence in German healthcare workers before and during the rollout of COVID-19 vaccines. PLoS ONE 2024, 19, e0294025. [Google Scholar] [CrossRef]
- Reynolds, L.; Dewey, C.; Asfour, G.; Little, M. Vaccine efficacy against SARS-CoV-2 for Pfizer BioNTech, Moderna, and AstraZeneca vaccines: A systematic review. Front. Public. Health 2023, 11, 1229716. [Google Scholar] [CrossRef] [PubMed]
- Tenbusch, M.; Schumacher, S.; Vogel, E.; Priller, A.; Held, J.; Steininger, P.; Beileke, S.; Irrgang, P.; Brockhoff, R.; Salmanton-Garcia, J.; et al. Heterologous prime-boost vaccination with ChAdOx1 nCoV-19 and BNT162b2. Lancet Infect. Dis. 2021, 21, 1212–1213. [Google Scholar] [CrossRef]
- Hachmann, N.P.; Miller, J.; Collier, A.Y.; Ventura, J.D.; Yu, J.; Rowe, M.; Bondzie, E.A.; Powers, O.; Surve, N.; Hall, K.; et al. Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5. N. Engl. J. Med. 2022, 387, 86–88. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Y.; Iketani, S.; Nair, M.S.; Li, Z.; Mohri, H.; Wang, M.; Yu, J.; Bowen, A.D.; Chang, J.Y.; et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature 2022, 608, 603–608. [Google Scholar] [CrossRef] [PubMed]
- De Maria, L.; Sponselli, S.; Caputi, A.; Stefanizzi, P.; Pipoli, A.; Giannelli, G.; Delvecchio, G.; Tafuri, S.; Inchingolo, F.; Migliore, G.; et al. SARS-CoV-2 Breakthrough Infections in Health Care Workers: An Italian Retrospective Cohort Study on Characteristics, Clinical Course and Outcomes. J. Clin. Med. 2023, 12, 628. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.T.; Kwan, A.T.; Rodriguez-Barraquer, I.; Singer, B.J.; Park, H.J.; Lewnard, J.A.; Sears, D.; Lo, N.C. Infectiousness of SARS-CoV-2 breakthrough infections and reinfections during the Omicron wave. Nat. Med. 2023, 29, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Cegolon, L.; Magnano, G.; Negro, C.; Larese Filon, F.; Group, O.W. SARS-CoV-2 Reinfections in Health-Care Workers, 1 March 2020–31 January 2023. Viruses 2023, 15, 1551. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Hamada, K.; Jubishi, D.; Hashimoto, H.; Okamoto, K.; Hisasue, N.; Sunohara, M.; Saito, M.; Shinohara, T.; Yamashita, M.; et al. Waning cellular immune responses and predictive factors in maintaining cellular immunity against SARS-CoV-2 six months after BNT162b2 mRNA vaccination. Sci. Rep. 2023, 13, 9607. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, H.; Piralla, A.; Zuo, F.L.; Du, L.K.; Cassaniti, I.; Wan, H.; Kumagai-Braesh, M.; Andréll, J.; Percivalle, E.; Sammartino, J.C.; et al. Immunity to SARS-CoV-2 up to 15 months after infection. Iscience 2022, 25, 103743. [Google Scholar] [CrossRef] [PubMed]
- Perreault, J.; Tremblay, T.; Fournier, M.J.; Drouin, M.; Beaudoin-Bussières, G.; Prévost, J.; Lewin, A.; Bégin, P.; Finzi, A.; Bazin, R. Waning of SARS-CoV-2 RBD antibodies in longitudinal convalescent plasma samples within 4 months after symptom onset. Blood 2020, 136, 2588–2591. [Google Scholar] [CrossRef]
| Low-Risk | Intermediate-Risk | High-Risk | Overall | |
|---|---|---|---|---|
| Total number | 21 | 21 | 31 | 73 |
| Gender (m/f) | 5/16 | 2/19 | 12/19 | 19/54 |
| Median age (range) | 34 (22–62) | 34 (23–62) | 39 (23–70) | 37 (22–70) |
| Nurse | 0 | 2 (10%) | 14 (45%) | 16 |
| Physician | 1 (5%) | 4 (19%) | 14 (45%) | 19 |
| Lab assistant | 13 (62%) | 0 | 0 | 13 |
| Other | 7 (33%) | 15 (71%) | 3 (10%) | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stammkötter, C.; Thümmler, L.; Korth, J.; Marenbach, B.; Braß, P.; Horn, P.A.; Lindemann, M.; Dittmer, U.; Witzke, O.; Rohn, H.; et al. Frequency of SARS-CoV-2 Infections among Healthcare Workers in Germany: 3-Year Follow-Up Study. Infect. Dis. Rep. 2024, 16, 615-627. https://doi.org/10.3390/idr16040047
Stammkötter C, Thümmler L, Korth J, Marenbach B, Braß P, Horn PA, Lindemann M, Dittmer U, Witzke O, Rohn H, et al. Frequency of SARS-CoV-2 Infections among Healthcare Workers in Germany: 3-Year Follow-Up Study. Infectious Disease Reports. 2024; 16(4):615-627. https://doi.org/10.3390/idr16040047
Chicago/Turabian StyleStammkötter, Christian, Laura Thümmler, Johannes Korth, Beate Marenbach, Peer Braß, Peter A. Horn, Monika Lindemann, Ulf Dittmer, Oliver Witzke, Hana Rohn, and et al. 2024. "Frequency of SARS-CoV-2 Infections among Healthcare Workers in Germany: 3-Year Follow-Up Study" Infectious Disease Reports 16, no. 4: 615-627. https://doi.org/10.3390/idr16040047
APA StyleStammkötter, C., Thümmler, L., Korth, J., Marenbach, B., Braß, P., Horn, P. A., Lindemann, M., Dittmer, U., Witzke, O., Rohn, H., & Krawczyk, A. (2024). Frequency of SARS-CoV-2 Infections among Healthcare Workers in Germany: 3-Year Follow-Up Study. Infectious Disease Reports, 16(4), 615-627. https://doi.org/10.3390/idr16040047







