eHealth and mHealth in Antimicrobial Stewardship to Reduce Mortality in Empirical Antimicrobial Therapy and a Systematic Review with a Meta-Analysis of Adequate Therapy
Abstract
:1. Introduction
2. The Importance of Antimicrobial Stewardship
3. Artificial Intelligence and Antimicrobial Stewardship
4. Diagnostic Stewardship
5. The Choice of ATMs
6. Systematic Review with Meta-Analysis
6.1. Search Strategy
6.2. Data Collection Process
6.3. Data Items
6.4. Subgroup Evaluation
6.5. Outcomes Assessment
6.6. Statistical Analysis
7. Results of the Meta-Analysis
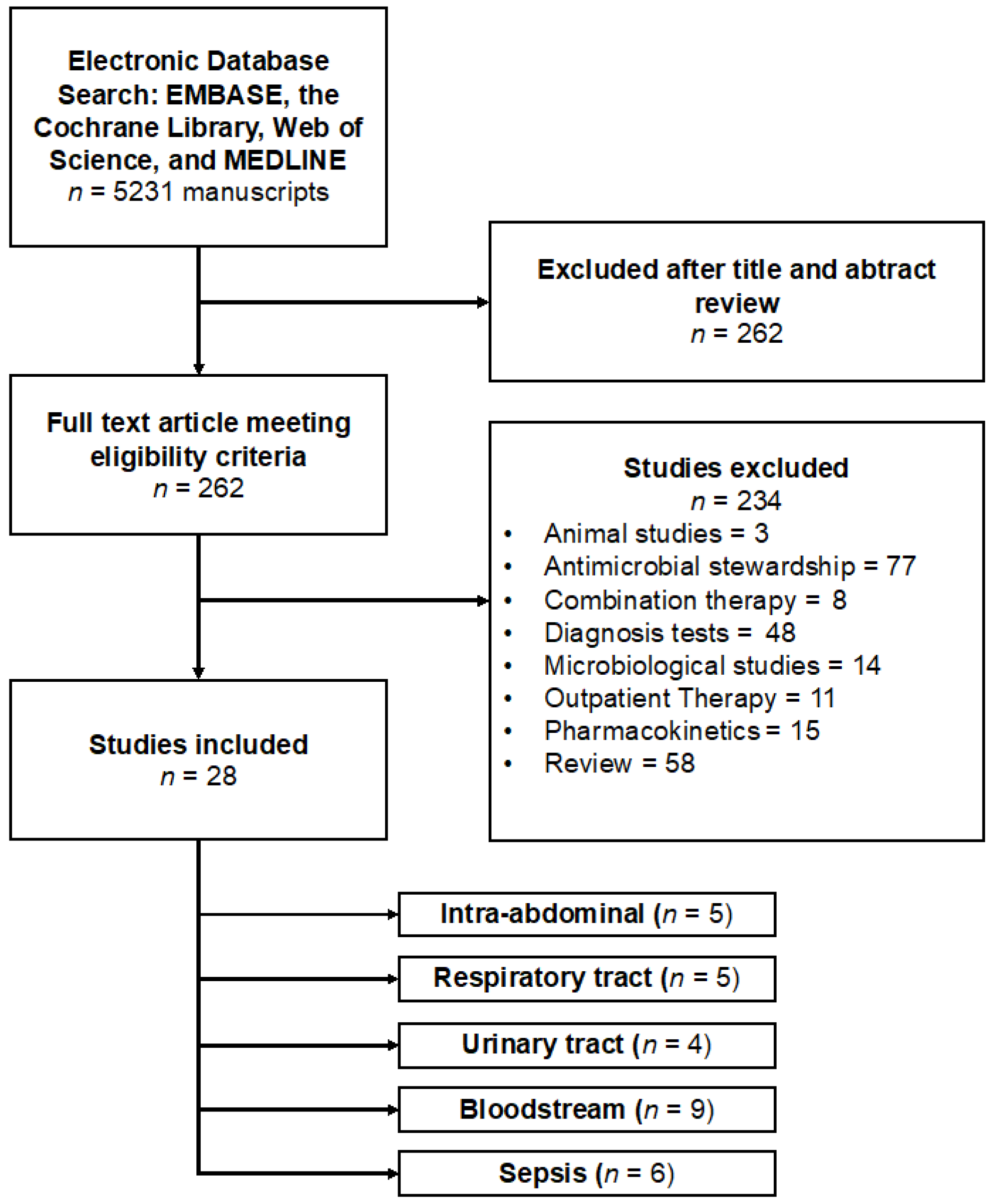
7.1. Study Selection
7.2. Study Characteristics
7.3. Results of All Infection Sites
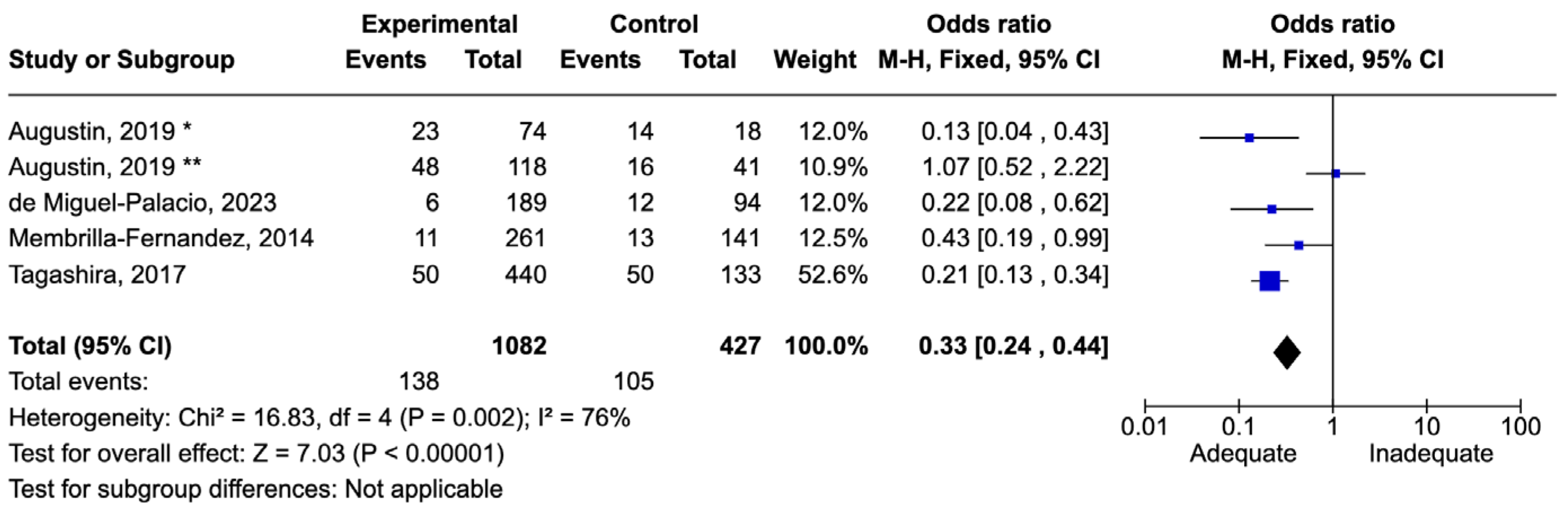
7.4. Abdominal Infections
7.5. Bloodstream Infections
7.6. Respiratory Tract Infections
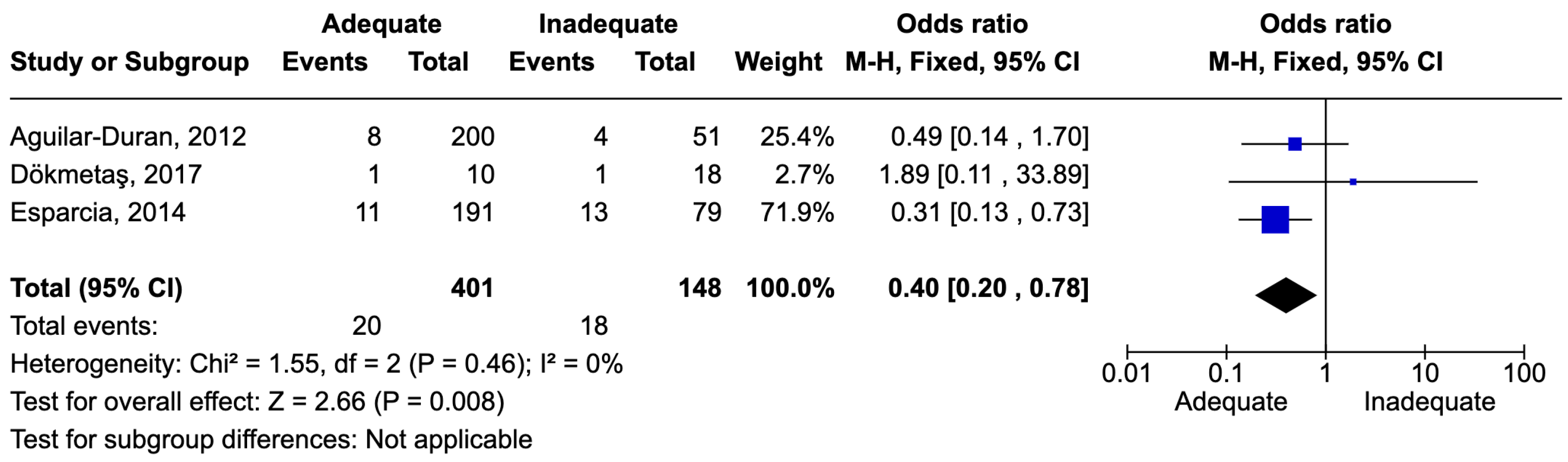
7.7. Urinary Tract Infections
7.8. Sepsis and Septic Shock
7.9. Results of Syntheses
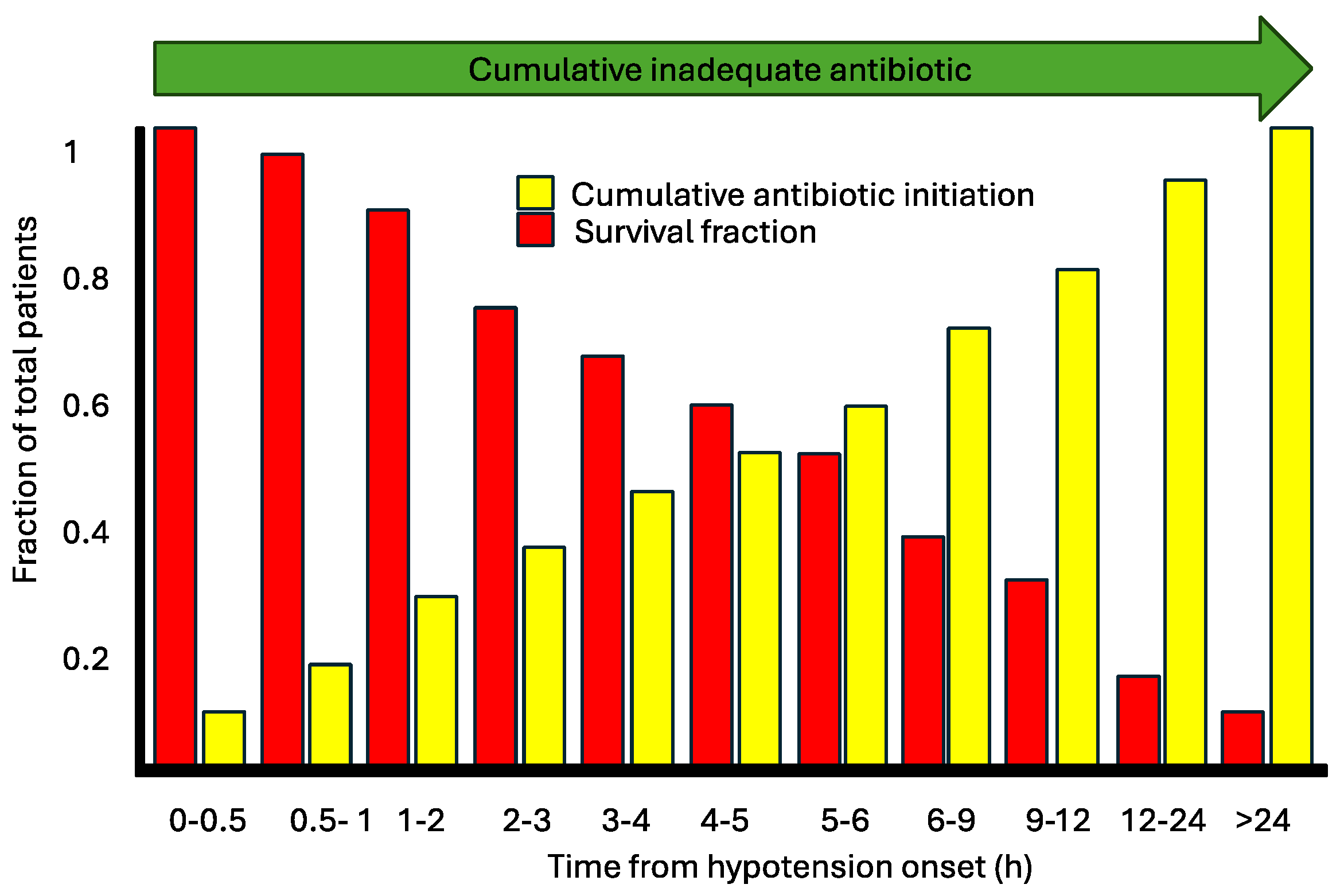
8. Discussion
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmes, A.H.; Moore, L.S.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Wenzler, E.; Maximos, M.; Asempa, T.E.; Biehle, L.; Schuetz, A.N.; Hirsch, E.B. Antimicrobial susceptibility testing: An updated primer for clinicians in the era of antimicrobial resistance: Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2023, 43, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Liu, Y.; Wang, R.; Wang, Q.; Jin, L.; Wang, H. The transferability and evolution of NDM-1 and KPC-2 co-producing Klebsiella pneumoniae from clinical settings. EBioMedicine 2020, 51, 102599. [Google Scholar] [CrossRef]
- Oteo, J.; Ortega, A.; Bartolome, R.; Bou, G.; Conejo, C.; Fernandez-Martinez, M.; Gonzalez-Lopez, J.J.; Martinez-Garcia, L.; Martinez-Martinez, L.; Merino, M.; et al. Prospective multicenter study of carbapenemase-producing Enterobacteriaceae from 83 hospitals in Spain reveals high in vitro susceptibility to colistin and meropenem. Antimicrob. Agents Chemother. 2015, 59, 3406–3412. [Google Scholar] [CrossRef] [PubMed]
- Quiles, M.G.; Rocchetti, T.T.; Fehlberg, L.C.; Kusano, E.J.; Chebabo, A.; Pereira, R.M.; Gales, A.C.; Pignatari, A.C. Unusual association of NDM-1 with KPC-2 and armA among Brazilian Enterobacteriaceae isolates. Braz. J. Med. Biol. Res. 2015, 48, 174–177. [Google Scholar] [CrossRef]
- Vazquez-Ucha, J.C.; Seoane-Estevez, A.; Rodino-Janeiro, B.K.; Gonzalez-Bardanca, M.; Conde-Perez, K.; Martinez-Guitian, M.; Alvarez-Fraga, L.; Arca-Suarez, J.; Lasarte-Monterrubio, C.; Gut, M.; et al. Activity of imipenem/relebactam against a Spanish nationwide collection of carbapenemase-producing Enterobacterales. J. Antimicrob. Chemother. 2021, 76, 1498–1510. [Google Scholar] [CrossRef]
- Lasarte-Monterrubio, C.; Guijarro-Sanchez, P.; Vazquez-Ucha, J.C.; Alonso-Garcia, I.; Alvarez-Fraga, L.; Outeda, M.; Martinez-Guitian, M.; Pena-Escolano, A.; Maceiras, R.; Lence, E.; et al. Antimicrobial Activity of Cefiderocol against the Carbapenemase-Producing Enterobacter cloacae Complex and Characterization of Reduced Susceptibility Associated with Metallo-beta-Lactamase VIM-1. Antimicrob. Agents Chemother. 2023, 67, e0150522. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Evans, L.E.; Rhodes, A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018, 44, 925–928. [Google Scholar] [CrossRef]
- Bonine, N.G.; Berger, A.; Altincatal, A.; Wang, R.; Bhagnani, T.; Gillard, P.; Lodise, T. Impact of Delayed Appropriate Antibiotic Therapy on Patient Outcomes by Antibiotic Resistance Status From Serious Gram-negative Bacterial Infections. Am. J. Med. Sci. 2019, 357, 103–110. [Google Scholar] [CrossRef]
- Huang, A.M.; Newton, D.; Kunapuli, A.; Gandhi, T.N.; Washer, L.L.; Isip, J.; Collins, C.D.; Nagel, J.L. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin. Infect. Dis. 2013, 57, 1237–1245. [Google Scholar] [CrossRef]
- Kock, R.; Wullenweber, J.; Horn, D.; Lanckohr, C.; Becker, K.; Idelevich, E.A. Implementation of short incubation MALDI-TOF MS identification from positive blood cultures in routine diagnostics and effects on empiric antimicrobial therapy. Antimicrob. Resist. Infect. Control. 2017, 6, 12. [Google Scholar] [CrossRef]
- Lockwood, A.M.; Perez, K.K.; Musick, W.L.; Ikwuagwu, J.O.; Attia, E.; Fasoranti, O.O.; Cernoch, P.L.; Olsen, R.J.; Musser, J.M. Integrating Rapid Diagnostics and Antimicrobial Stewardship in Two Community Hospitals Improved Process Measures and Antibiotic Adjustment Time. Infect. Control Hosp. Epidemiol. 2016, 37, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Timbrook, T.T.; Morton, J.B.; McConeghy, K.W.; Caffrey, A.R.; Mylonakis, E.; LaPlante, K.L. The Effect of Molecular Rapid Diagnostic Testing on Clinical Outcomes in Bloodstream Infections: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2017, 64, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Moungui, H.C.; Nana-Djeunga, H.C.; Anyiang, C.F.; Cano, M.; Ruiz Postigo, J.A.; Carrion, C. Dissemination Strategies for mHealth Apps: Systematic Review. JMIR Mhealth Uhealth 2024, 12, e50293. [Google Scholar] [CrossRef] [PubMed]
- Reno, B.; Oliveira, E.M.; Souza, A.D. A Systematic Literature Review on Trustworthiness for Applications Used in eHealth Environments. J. Multidiscip. Healthc. 2023, 16, 3393–3403. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, D.; Lawson, W.; Dryden, M.; Stephens, J.; Corman, S.; Solem, C.; Li, J.; Charbonneau, C.; Baillon-Plot, N.; Haider, S.; et al. Implementing criteria-based early switch/early discharge programmes: A European perspective. Clin. Microbiol. Infect. 2015, 21 (Suppl. 2), S47–S55. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, D. Developments in outpatient parenteral antimicrobial therapy (OPAT) for Gram-positive infections in Europe, and the potential impact of daptomycin. J. Antimicrob. Chemother. 2009, 64, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Karanika, S.; Paudel, S.; Grigoras, C.; Kalbasi, A.; Mylonakis, E. Systematic Review and Meta-analysis of Clinical and Economic Outcomes from the Implementation of Hospital-Based Antimicrobial Stewardship Programs. Antimicrob. Agents Chemother. 2016, 60, 4840–4852. [Google Scholar] [CrossRef]
- Telles, J.P.; Morales, R., Jr.; Yamada, C.H.; Marins, T.A.; D’Amaro Juodinis, V.; Sztajnbok, J.; Silva, M., Jr.; Bassetti, B.R.; Albiero, J.; Tuon, F.F. Optimization of Antimicrobial Stewardship Programs Using Therapeutic Drug Monitoring and Pharmacokinetics-Pharmacodynamics Protocols: A Cost-Benefit Review. Ther. Drug Monit. 2023, 45, 200–208. [Google Scholar] [CrossRef]
- Cruz, J.A.W.; da Cunha, M.; de Moraes, T.P.; Marques, S.; Tuon, F.F.; Gomide, A.L.; de Paula Linhares, G. Brazilian private health system: History, scenarios, and trends. BMC Health Serv. Res. 2022, 22, 49. [Google Scholar] [CrossRef]
- Loesch, G.H.; Cruz, J.A.W.; Gasparetto, J.; Oliveira, D.D.S.; Telles, J.P.; Tuon, F.F. Cost minimization analysis of outpatient parenteral/oral antibiotic therapy at a trauma hospital: Public health system. Infect. Control Hosp. Epidemiol. 2021, 42, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Van Dijck, C.; Vlieghe, E.; Cox, J.A. Antibiotic stewardship interventions in hospitals in low-and middle-income countries: A systematic review. Bull. World Health Organ. 2018, 96, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Davey, P.; Marwick, C.A.; Scott, C.L.; Charani, E.; McNeil, K.; Brown, E.; Gould, I.M.; Ramsay, C.R.; Michie, S. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2017, 2, CD003543. [Google Scholar] [CrossRef]
- Zequinao, T.; Telles, J.P.; Gasparetto, J.; Tuon, F.F. Carbapenem stewardship with ertapenem and antimicrobial resistance-a scoping review. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200413. [Google Scholar] [CrossRef] [PubMed]
- Zequinao, T.; Gasparetto, J.; Oliveira, D.D.S.; Silva, G.T.; Telles, J.P.; Tuon, F.F. A broad-spectrum beta-lactam-sparing stewardship program in a middle-income country public hospital: Antibiotic use and expenditure outcomes and antimicrobial susceptibility profiles. Braz. J. Infect. Dis. 2020, 24, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Tuon, F.F.; Telles, J.P.; Gasparetto, J.; Zequinao, T. Antibiotic price rise and antibiotic stewardship programs-Stimulus or discouragement? Infect. Control Hosp. Epidemiol. 2020, 41, 994–995. [Google Scholar] [CrossRef] [PubMed]
- Gasparetto, J.; Tuon, F.F.; Dos Santos Oliveira, D.; Zequinao, T.; Pipolo, G.R.; Ribeiro, G.V.; Beninca, P.D.; Cruz, J.A.W.; Moraes, T.P. Intravenous-to-oral antibiotic switch therapy: A cross-sectional study in critical care units. BMC Infect. Dis. 2019, 19, 650. [Google Scholar] [CrossRef]
- Shortliffe, E.H.; Davis, R.; Axline, S.G.; Buchanan, B.G.; Green, C.C.; Cohen, S.N. Computer-based consultations in clinical therapeutics: Explanation and rule acquisition capabilities of the MYCIN system. Comput. Biomed. Res. 1975, 8, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Yu, V.L.; Fagan, L.M.; Wraith, S.M.; Clancey, W.J.; Scott, A.C.; Hannigan, J.; Blum, R.L.; Buchanan, B.G.; Cohen, S.N. Antimicrobial selection by a computer. A blinded evaluation by infectious diseases experts. JAMA 1979, 242, 1279–1282. [Google Scholar] [CrossRef]
- Beam, A.L.; Kohane, I.S. Big Data and Machine Learning in Health Care. JAMA 2018, 319, 1317–1318. [Google Scholar] [CrossRef]
- Peiffer-Smadja, N.; Rawson, T.M.; Ahmad, R.; Buchard, A.; Georgiou, P.; Lescure, F.X.; Birgand, G.; Holmes, A.H. Machine learning for clinical decision support in infectious diseases: A narrative review of current applications. Clin. Microbiol. Infect. 2020, 26, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA 2016, 316, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, D.W.; Barton, C.W.; Feldman, M.D.; Mataraso, S.J.; Das, R. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: A randomised clinical trial. BMJ Open Respir. Res. 2017, 4, e000234. [Google Scholar] [CrossRef]
- Bucheeri, M.; Elligsen, M.; Lam, P.W.; Daneman, N.; MacFadden, D. A sepsis treatment algorithm to improve early antibiotic de-escalation while maintaining adequacy of coverage (Early-IDEAS): A prospective observational study. PLoS ONE 2023, 18, e0295908. [Google Scholar] [CrossRef] [PubMed]
- MacFadden, D.R.; Coburn, B.; Shah, N.; Robicsek, A.; Savage, R.; Elligsen, M.; Daneman, N. Decision-support models for empiric antibiotic selection in Gram-negative bloodstream infections. Clin. Microbiol. Infect. 2019, 25, 108.e1–108.e7. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, J.P.; Robicsek, A.; Shah, N.; Smith, B.A.; Singh, K.; Semel, J.; Acree, M.E.; Grant, J.; Ravichandran, U.; Peterson, L.R. A Randomized Controlled Trial of an Electronic Clinical Decision Support Tool for Inpatient Antimicrobial Stewardship. Clin. Infect. Dis. 2021, 72, e265–e271. [Google Scholar] [CrossRef] [PubMed]
- Carreno, J.J.; Eaton, R.; Itro, L.; Babowicz, F.; Falvo, J.; Tobin, E.; Mitchell, C.; George, M. Time to clinical response in sepsis associated with an algorithm for blood-culture pathogen identification using matrix-assisted laser desorption ionization time-of-flight mass spectroscopy. Am. J. Health Syst. Pharm. 2019, 76, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, N.; Asai, K.; Kuroda, M.; Watanabe, R.; Kujiraoka, M.; Sekizuka, T.; Katagiri, M.; Moriyama, H.; Watanabe, M.; Saida, Y. Rapid identification of bacteria using a multiplex polymerase chain reaction system for acute abdominal infections. Front. Microbiol. 2023, 14, 1220651. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.S.T.; Cieslinski, J.; Bertol, J.; Schumacher, A.L.; Telles, J.P.; Tuon, F.F. Detection of Microorganisms in Clinical Sonicated Orthopedic Devices Using Conventional Culture and qPCR. Rev. Bras. Ortop. 2022, 57, 689–696. [Google Scholar] [CrossRef]
- Cieslinski, J.; Ribeiro, V.S.T.; Kraft, L.; Suss, P.H.; Rosa, E.; Morello, L.G.; Pillonetto, M.; Tuon, F.F. Direct detection of microorganisms in sonicated orthopedic devices after in vitro biofilm production and different processing conditions. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 1113–1120. [Google Scholar] [CrossRef]
- Qian, Y.; Ai, J.; Wu, J.; Yu, S.; Cui, P.; Gao, Y.; Jin, J.; Weng, X.; Zhang, W. Rapid detection of respiratory organisms with FilmArray respiratory panel and its impact on clinical decisions in Shanghai, China, 2016–2018. Influenza Other Respir Viruses 2020, 14, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Rule, R.; Paruk, F.; Becker, P.; Neuhoff, M.; Chausse, J.; Said, M. Clinical utility of the BioFire FilmArray Blood Culture Identification panel in the adjustment of empiric antimicrobial therapy in the critically ill septic patient. PLoS ONE 2021, 16, e0254389. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.; Lau, S.K.; Teng, J.L.; Tse, H.; Yuen, K.Y. Then and now: Use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin. Microbiol. Infect. 2008, 14, 908–934. [Google Scholar] [CrossRef] [PubMed]
- Messacar, K.; Parker, S.K.; Todd, J.K.; Dominguez, S.R. Implementation of Rapid Molecular Infectious Disease Diagnostics: The Role of Diagnostic and Antimicrobial Stewardship. J. Clin. Microbiol. 2017, 55, 715–723. [Google Scholar] [CrossRef]
- Claeys, K.C.; Johnson, M.D. Leveraging diagnostic stewardship within antimicrobial stewardship programmes. Drugs Context 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Hindler, J.F.; Stelling, J. Analysis and presentation of cumulative antibiograms: A new consensus guideline from the Clinical and Laboratory Standards Institute. Clin. Infect. Dis. 2007, 44, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Bielec, F.; Wenecka, M.; Brauncajs, M.; Pastuszak-Lewandoska, D. Analysis of Cumulative Antibiogram Reports in Search for Optimal Empirical Urinary Tract Infection Treatment at the Central Teaching Hospital of the Medical University of Lodz, Poland: Results of a 3-Year Surveillance. J. Clin. Med. 2023, 12, 6270. [Google Scholar] [CrossRef] [PubMed]
- Bailey, P.; Antosz, K.; Daniels, R.; Gainey, A.B.; Burch, A.K. Providing value to patients and providers via a pediatric statewide antibiogram in South Carolina. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e78. [Google Scholar] [CrossRef] [PubMed]
- Darboe, S.; Mirasol, R.; Adejuyigbe, B.; Muhammad, A.K.; Nadjm, B.; De St Maurice, A.; Dogan, T.L.; Ceesay, B.; Umukoro, S.; Okomo, U.; et al. Using an Antibiogram Profile to Improve Infection Control and Rational Antimicrobial Therapy in an Urban Hospital in The Gambia, Strategies and Lessons for Low- and Middle-Income Countries. Antibiotics 2023, 12, 790. [Google Scholar] [CrossRef]
- Negm, E.M.; Elgharabawy, E.S.; Badran, S.G.; Soliman, A.M.; El Sayed, A.M.; Raafat, A.O.N.; Soliman, S.T.; Mahmoud, H.M.; Tawfik, A.E.; El Hawary, A.T.; et al. Analysis of cumulative antibiogram reports in intensive care units at an Egyptian University Hospital. J. Infect. Public Health 2023, 16, 1220–1229. [Google Scholar] [CrossRef]
- CLSI. Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test. Data, 5th ed.; CLSI: Berwyn, PA, USA, 2022. [Google Scholar]
- Dupuis, C.; Timsit, J.F. Antibiotics in the first hour: Is there new evidence? Expert Rev. Anti Infect. 2021, 19, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Degoricija, V.; Sharma, M.; Legac, A.; Gradiser, M.; Sefer, S.; Vucicevic, Z. Survival analysis of 314 episodes of sepsis in medical intensive care unit in university hospital: Impact of intensive care unit performance and antimicrobial therapy. Croat. Med. J. 2006, 47, 385–397. [Google Scholar] [PubMed]
- Muscedere, J.G.; Shorr, A.F.; Jiang, X.; Day, A.; Heyland, D.K.; Canadian Critical Care Trials, G. The adequacy of timely empiric antibiotic therapy for ventilator-associated pneumonia: An important determinant of outcome. J. Crit. Care 2012, 27, 322.e7–322.e14. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Duran, S.; Horcajada, J.P.; Sorli, L.; Montero, M.; Salvado, M.; Grau, S.; Gomez, J.; Knobel, H. Community-onset healthcare-related urinary tract infections: Comparison with community and hospital-acquired urinary tract infections. J. Infect. 2012, 64, 478–483. [Google Scholar] [CrossRef]
- Tabah, A.; Koulenti, D.; Laupland, K.; Misset, B.; Valles, J.; Bruzzi de Carvalho, F.; Paiva, J.A.; Cakar, N.; Ma, X.; Eggimann, P.; et al. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: The EUROBACT International Cohort Study. Intensive Care Med. 2012, 38, 1930–1945. [Google Scholar] [CrossRef] [PubMed]
- Piskin, N.; Aydemir, H.; Oztoprak, N.; Akduman, D.; Comert, F.; Kokturk, F.; Celebi, G. Inadequate treatment of ventilator-associated and hospital-acquired pneumonia: Risk factors and impact on outcomes. BMC Infect. Dis. 2012, 12, 268. [Google Scholar] [CrossRef]
- Chen, H.C.; Lin, W.L.; Lin, C.C.; Hsieh, W.H.; Hsieh, C.H.; Wu, M.H.; Wu, J.Y.; Lee, C.C. Outcome of inadequate empirical antibiotic therapy in emergency department patients with community-onset bloodstream infections. J. Antimicrob. Chemother. 2013, 68, 947–953. [Google Scholar] [CrossRef]
- Cardoso, T.; Ribeiro, O.; Aragao, I.; Costa-Pereira, A.; Sarmento, A. The impact of healthcare-associated infection on mortality: Failure in clinical recognition is related with inadequate antibiotic therapy. PLoS ONE 2013, 8, e58418. [Google Scholar] [CrossRef] [PubMed]
- Retamar, P.; Lopez-Prieto, M.D.; Natera, C.; de Cueto, M.; Nuno, E.; Herrero, M.; Fernandez-Sanchez, F.; Munoz, A.; Tellez, F.; Becerril, B.; et al. Reappraisal of the outcome of healthcare-associated and community-acquired bacteramia: A prospective cohort study. BMC Infect. Dis. 2013, 13, 344. [Google Scholar] [CrossRef]
- Yang, C.J.; Chung, Y.C.; Chen, T.C.; Chang, H.L.; Tsai, Y.M.; Huang, M.S.; Chen, Y.H.; Lu, P.L. The impact of inappropriate antibiotics on bacteremia patients in a community hospital in Taiwan: An emphasis on the impact of referral information for cases from a hospital affiliated nursing home. BMC Infect. Dis. 2013, 13, 500. [Google Scholar] [CrossRef]
- Nygard, S.T.; Langeland, N.; Flaatten, H.K.; Fanebust, R.; Haugen, O.; Skrede, S. Aetiology, antimicrobial therapy and outcome of patients with community acquired severe sepsis: A prospective study in a Norwegian university hospital. BMC Infect. Dis. 2014, 14, 121. [Google Scholar] [CrossRef]
- Esparcia, A.; Artero, A.; Eiros, J.M.; Balaguer, M.; Madrazo, M.; Alberola, J.; Nogueira, J.M. Influence of inadequate antimicrobial therapy on prognosis in elderly patients with severe urinary tract infections. Eur. J. Intern. Med. 2014, 25, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Yokota, P.K.; Marra, A.R.; Martino, M.D.; Victor, E.S.; Durao, M.S.; Edmond, M.B.; dos Santos, O.F. Impact of appropriate antimicrobial therapy for patients with severe sepsis and septic shock—A quality improvement study. PLoS ONE 2014, 9, e104475. [Google Scholar] [CrossRef] [PubMed]
- Membrilla-Fernandez, E.; Sancho-Insenser, J.J.; Girvent-Montllor, M.; Alvarez-Lerma, F.; Sitges-Serra, A.; Secondary Peritonitis Spanish Study, G. Effect of initial empiric antibiotic therapy combined with control of the infection focus on the prognosis of patients with secondary peritonitis. Surg. Infect. (Larchmt) 2014, 15, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Garnacho-Montero, J.; Gutierrez-Pizarraya, A.; Escoresca-Ortega, A.; Fernandez-Delgado, E.; Lopez-Sanchez, J.M. Adequate antibiotic therapy prior to ICU admission in patients with severe sepsis and septic shock reduces hospital mortality. Crit. Care 2015, 19, 302. [Google Scholar] [CrossRef]
- Oshima, T.; Kodama, Y.; Takahashi, W.; Hayashi, Y.; Iwase, S.; Kurita, T.; Saito, D.; Yamaji, Y.; Oda, S. Empiric Antibiotic Therapy for Severe Sepsis and Septic Shock. Surg. Infect. (Larchmt) 2016, 17, 210–216. [Google Scholar] [CrossRef]
- Savage, R.D.; Fowler, R.A.; Rishu, A.H.; Bagshaw, S.M.; Cook, D.; Dodek, P.; Hall, R.; Kumar, A.; Lamontagne, F.; Lauzier, F.; et al. The Effect of Inadequate Initial Empiric Antimicrobial Treatment on Mortality in Critically Ill Patients with Bloodstream Infections: A Multi-Centre Retrospective Cohort Study. PLoS ONE 2016, 11, e0154944. [Google Scholar] [CrossRef] [PubMed]
- Tagashira, Y.; Sakamoto, N.; Isogai, T.; Hikone, M.; Kosaka, A.; Chino, R.; Higuchi, M.; Uehara, Y.; Honda, H. Impact of inadequate initial antimicrobial therapy on mortality in patients with bacteraemic cholangitis: A retrospective cohort study. Clin. Microbiol. Infect. 2017, 23, 740–747. [Google Scholar] [CrossRef]
- Dokmetas, I.; Hamidi, A.A.; Bulut, M.E.; Cetin, S.; Oncul, A.; Uzun, N. Clinical effect of discordance in empirical treatment of cases with urinary tract infection accompanied by bacteremia. Turk. J. Urol. 2017, 43, 543–548. [Google Scholar] [CrossRef]
- Castano, P.; Plaza, M.; Molina, F.; Hincapie, C.; Maya, W.; Catano, J.; Gonzalez, J.; Leon, A.; Jaimes, F. Antimicrobial agent prescription: A prospective cohort study in patients with sepsis and septic shock. Trop Med. Int Health 2019, 24, 175–184. [Google Scholar] [CrossRef]
- Valles, J.; Fontanals, D.; Oliva, J.C.; Martinez, M.; Navas, A.; Mesquida, J.; Gruartmoner, G.; de Haro, C.; Mestre, J.; Guia, C.; et al. Trends in the incidence and mortality of patients with community-acquired septic shock 2003–2016. J. Crit. Care 2019, 53, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Augustin, P.; Tanaka, S.; Tran-Dinh, A.; Parenti Ribeiro, L.; Arapis, K.; Grall, N.; Al Qarni, A.; Montravers, P. Outcome and Adequacy of Empirical Antibiotherapy in Post-Operative Peritonitis: A Retrospective Study. Surg. Infect. (Larchmt) 2020, 21, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Lambregts, M.M.C.; Wijnakker, R.; Bernards, A.T.; Visser, L.G.; Cessie, S.L.; Boer, M.G.J. Mortality after Delay of Adequate Empiric Antimicrobial Treatment of Bloodstream Infection. J. Clin. Med. 2020, 9, 1378. [Google Scholar] [CrossRef] [PubMed]
- Calo, F.; Retamar, P.; Martinez Perez-Crespo, P.M.; Lanz-Garcia, J.; Sousa, A.; Goikoetxea, J.; Reguera-Iglesias, J.M.; Leon, E.; Arminanzas, C.; Mantecon, M.A.; et al. Catheter-related bloodstream infections: Predictive factors for Gram-negative bacteria aetiology and 30 day mortality in a multicentre prospective cohort. J. Antimicrob. Chemother. 2020, 75, 3056–3061. [Google Scholar] [CrossRef] [PubMed]
- Puzniak, L.; Bauer, K.A.; Yu, K.C.; Moise, P.; Finelli, L.; Ye, G.; De Anda, C.; Vankeepuram, L.; Gupta, V. Effect of Inadequate Empiric Antibacterial Therapy on Hospital Outcomes in SARS-CoV-2-Positive and -Negative US Patients With a Positive Bacterial Culture: A Multicenter Evaluation From March to November 2020. Open Forum Infect. Dis. 2021, 8, ofab232. [Google Scholar] [CrossRef] [PubMed]
- Dietl, B.; Boix-Palop, L.; Gisbert, L.; Mateu, A.; Garreta, G.; Xercavins, M.; Badia, C.; Lopez-Sanchez, M.; Perez, J.; Calbo, E. Risk factors associated with inappropriate empirical antimicrobial treatment in bloodstream infections. A cohort study. Front. Pharm. 2023, 14, 1132530. [Google Scholar] [CrossRef] [PubMed]
- de Miguel-Palacio, M.; Gonzalez-Castillo, A.M.; Membrilla-Fernandez, E.; Pons-Fragero, M.J.; Pelegrina-Manzano, A.; Grande-Posa, L.; Morera-Casaponsa, R.; Sancho-Insenser, J.J. Impact of empiric antibiotic therapy on the clinical outcome of acute calculous cholecystitis. Langenbecks Arch. Surg. 2023, 408, 345. [Google Scholar] [CrossRef] [PubMed]
- Bateman, R.M.; Sharpe, M.D.; Jagger, J.E.; Ellis, C.G.; Sole-Violan, J.; Lopez-Rodriguez, M.; Herrera-Ramos, E.; Ruiz-Hernandez, J.; Borderias, L.; Horcajada, J.; et al. 36th International Symposium on Intensive Care and Emergency Medicine: Brussels, Belgium. 15–18 March 2016. Crit. Care 2016, 20, 94. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Tuon, F.F.; Rymsza, A.M.; Penteado-Filho, S.R.; Pilonetto, M.; Arend, L.N.; Levin, A.S. Should polymyxin be used empirically to treat infections in patients under high risk for carbapenem-resistant Acinetobacter? J. Infect. 2011, 62, 246–249. [Google Scholar] [CrossRef]
- Orsatti, V.N.; Ribeiro, V.S.T.; de Oliveira Montenegro, C.; Costa, C.J.; Raboni, E.A.; Sampaio, E.R.; Michielin, F.; Gasparetto, J.; Telles, J.P.; Tuon, F.F. Sepsis death risk factor score based on systemic inflammatory response syndrome, quick sequential organ failure assessment, and comorbidities. Med. Intensiva. 2024, 48, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Tuon, F.F.; Kruger, M.; Terreri, M.; Penteado-Filho, S.R.; Gortz, L. Klebsiella ESBL bacteremia-mortality and risk factors. Braz. J. Infect. Dis. 2011, 15, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Tuon, F.F.; Bianchet, L.C.; Penteado-Filho, S.R. Epidemiology of extended spectrum beta-lactamase producing Enterobacter bacteremia in a brazilian hospital. Rev. Soc. Bras. Med. Trop. 2010, 43, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Telles, J.P.; Leme, R.C.P.; Campos, M.L.; Ito, C.; Bail, L.; Nogueira, K.D.S.; Tuon, F.F. Ceftriaxone and methicillin-susceptible staphylococcus aureus: A perspective from pharmacokinetics/pharmacodynamics studies. Expert. Opin. Drug Metab. Toxicol. 2021, 17, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Reiber, C.; Bodendoerfer, E.; Brugger, S.D.; Eberhard, N.; Hitz, E.; Hofmaenner, D.A.; Herren, S.; Kolesnik-Goldmann, N.; Manicini, S.; Zbinden, R.; et al. Rapid antimicrobial susceptibility testing in patients with bacteraemia due to Enterobacterales: An implementation study. Swiss Med. Wkly. 2023, 153, 40066. [Google Scholar] [CrossRef]
- Cartuliares, M.B.; Rosenvinge, F.S.; Mogensen, C.B.; Skovsted, T.A.; Andersen, S.L.; Østergaard, C.; Pedersen, A.K.; Skjøt-Arkil, H. Evaluation of point-of-care multiplex polymerase chain reaction in guiding antibiotic treatment of patients acutely admitted with suspected community-acquired pneumonia in Denmark: A multicentre randomised controlled trial. PLoS Med. 2023, 20, e1004314. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuon, F.F.; Zequinao, T.; da Silva, M.S.; Silva, K.O. eHealth and mHealth in Antimicrobial Stewardship to Reduce Mortality in Empirical Antimicrobial Therapy and a Systematic Review with a Meta-Analysis of Adequate Therapy. Infect. Dis. Rep. 2024, 16, 707-723. https://doi.org/10.3390/idr16040054
Tuon FF, Zequinao T, da Silva MS, Silva KO. eHealth and mHealth in Antimicrobial Stewardship to Reduce Mortality in Empirical Antimicrobial Therapy and a Systematic Review with a Meta-Analysis of Adequate Therapy. Infectious Disease Reports. 2024; 16(4):707-723. https://doi.org/10.3390/idr16040054
Chicago/Turabian StyleTuon, Felipe Francisco, Tiago Zequinao, Marcelo Silva da Silva, and Kleber Oliveira Silva. 2024. "eHealth and mHealth in Antimicrobial Stewardship to Reduce Mortality in Empirical Antimicrobial Therapy and a Systematic Review with a Meta-Analysis of Adequate Therapy" Infectious Disease Reports 16, no. 4: 707-723. https://doi.org/10.3390/idr16040054



