1. Introduction
The growth and aging of the world population result in increased occurrence of wounds, including of infected wounds [
1]. Wound infections delay significantly the wound healing process via several mechanisms that may prevent the transition from the wound’s inflammatory phase to the wound’s proliferative phase, leading to wound chronicity [
2]. Successful treatment of many wound infections has become a difficult task with the increasing prevalence of antibiotic resistant microorganisms [
3]. Formation of a microbial biofilm in the wound reduces and may prevent the efficacy of antimicrobial therapy. It has been calculated that one out of 24 surgical interventions will be complicated due to a microbial infection [
4]. Wound infections cause a significant burden to patients and to the health care systems [
5,
6]. Protecting wounds from pathogens is an important clinical practice in the management of wounds, and an important role of wound dressings is to prevent and treat wound infections [
3].
Copper is an essential micronutrient for microorganisms. As copper ions can generate reactive oxygen species (ROS), the microorganisms have different ways to control the intracellular copper concentration, mainly by having copper chaperons and efflux specific copper pumps [
7,
8]. However, when they are exposed to a too high concentration of copper, which varies between microorganisms, they cannot cope with the excess copper and are killed [
9,
10]. The damage to the microorganisms occurs via several non-specific mechanisms, including plasma membrane permeabilization, membrane lipid peroxidation, damage to nucleic acids and inhibition of the biological assembly and activity of intracellular proteins [
11]. The multisite, non-specific copper damaging mechanisms make it very difficult for microorganisms to develop tolerance to copper, and the prevalence of tolerant microorganism to copper is very low [
11,
12].
Antimicrobial wound dressings are increasingly being used in the management of infected wounds and for the protection of wounds from becoming infected [
13]. The development, safety, and biocidal properties of prototype antimicrobial wound dressings containing copper oxide microparticles were previously described [
14]. In the current article, we describe the in vitro biocidal activity of significantly improved wound dressings impregnated with cuprous oxide microparticles that have been cleared for use by several regulatory bodies and started recently to be in use for the management of acute and chronic wounds [
15,
16,
17].
2. Materials and Methods
2.1. Test Dressings
Impregnation of cuprous oxide microparticles in polymeric materials and the production of cuprous oxide-impregnated prototype wound dressings were previously described [
14,
18]. The regulatory cleared cuprous oxide-impregnated dressings (hereafter termed COD) are composed of a highly absorbent layer and one or two external non-binding hydrophilic nonwoven polypropylene layers. All layers are impregnated with cuprous oxide microparticles (
Figure 1). The orange layer, which is placed in contact with the wound bed, allows the passage of the wound exudates into the highly absorbent layer. The wound dressings can absorb ~10 times their own weight. The COD with two external layers are more appropriate for application in wound cavities and deep wounds. COD dressings without an adhesive contour can be cut to the shape and size of the wounds. The COD provided with an adhesive contour are more appropriate for post-operative wounds. The COD were examined by scanning electron microscopy (SEM; Jeol JSM-IT100, Japan) and the presence of copper was confirmed by X-ray photoelectron spectrum analysis (built-in system of JEOL), as can be seen in
Figure 1.
Similar wound dressings (the same construction), but without copper or any other active biocidal component (3M Life Sterile Dressings; Hubei Qianjiang Kingphur Medical Materials Co Ltd., Hubei, China), have been used as negative control dressings. The antimicrobial efficacy of the COD was also compared to the following commercially available antimicrobial wound dressings containing silver: Maxorb extra AG (Medline Industries, Northfield, IL, USA), Puracol Plus (Medline Industries, IL, USA), Optifoam (Medline Industries, IL, USA), Calcium Alginate (McKesson Corporation, Irving, TX, USA), Tegaderm Alginate Ag (3M, Saint Paul, MN, USA), Biatain Alginate Ag (Coloplast, Fredensborg, Denmark), Acticoat Flex 3 (Smith & Nephew, Watford, UK), Mepilex Ag (Mölnlycke Health Care, Gothenburg, Sweden).
2.2. Determination of Log Reduction of Viable Microbial Titers
The antibacterial and antifungal properties of the COD were determined according to the American Association of Textile Chemists and Colorists (AATCC) Test Method 100–1993 [
19]. Unless indicated for specific experiments, we applied the following procedure: Six 3.3 cm × 3.3 cm square swatches from each test and control wound dressing were aseptically cut. Each individual swatch was put in the bottom of a sterile plastic vessel. Two mL of sterile 0.85% saline/0.1% Tween 80 (ST; Sigma Aldrich Israel Ltd., Rehovot, Israel) containing wound exudate surrogate (Biological Industries, Beit-Haemek, Israel) solution was added to each swatch, making sure that all liquid was completely absorbed into the control and test swatch samples. Then, 100 µL of a microbial stock assay solution (a fresh test microorganism transplant grown overnight at 37 ± 2 °C in Tryptic soy broth (TBS; Hy laboratories Ltd., Rehovot, Israel) and adjusted to 2–4 × 10
7 colony forming units (CFU) per mL after being diluted in 5% nutrient broth (NB;Hy laboratories Ltd., Rehovot, Israel) and ST were added to each test swatch sample, making sure that all the liquid was completely absorbed into the control and test samples. Immediately, 100 mL of the neutralizing solution (DeyEngley (D/E) Broth; LAB187, Lab M Limited, Bury, UK) were added to the “Time 0” samples. All other vessels containing the test samples were closed hermetically and put in a 37 °C incubator for 1, 3 (bacteria) or 18 h (
Candida albicans). At the end of the incubation, 100 mL of D/E were added to the samples. Immediately (Time 0), or at the end of the incubation, the D/E neutralized swatches were transferred to sterile Stomager bags (Alex Red Ltd. Mevasseret Zion, Israel). The bags were stomached for 2 min and the liquids were returned to the original vessels. From each vessel, 10 µL, 100 µL, and 1 mL of the above recovered liquids were filtered through 0.45 µm Cellulose Nitrate Filters (Sartorious Stedim Biotech GmbH, Göttingen, Germany) by using a Pall filtration device (Pall Corporation, Port Washington, NY, USA). The filters containing the microorganisms were rinsed twice with 100 ± 5 mL of ST and then the membranes were transferred to Petri dishes containing CHROMagar™ Orientation agar (
http://www.chromagar.com, accessed 10 May 2022) and incubated at 24 °C (
Candida albicans) or 37 °C (bacteria). After 24–48 h of incubation, the CFU were counted. In some experiments, in order to reduce the lower limit of detection from 100 CFU to 10 CFU, 10 mL of each liquid were also filtered. The percent of bacterial or fungal reduction was determined according to the following formula: 100(A − B)/A = %R and the 10-fold reduction was calculated by the following formula: Log A − Log B = Log R to the nearest hundred, where R = 10-fold reduction; A = organism population of the challenge organism; B = the number of test organisms recovered from the inoculated test sample. The test organisms studied are: Methicillin resistant
Staphylococcus aureus (MRSA; ATCC BAA-1708);
Escherichia coli (ATCC 8739);
Klebsiella pneumoniae (ATCC 4352);
Enterobacter aerogenes (ATCC 1304);
Enterococcus faecalis (ATCC 19439);
Pseudomonas aeruginosa (ATCC 15442);
Staphylococcus epidermidis (ATCC 12228) and
Candida albicans (ATCC 10231). All experiments were performed at least twice and the number of replicates per sample per experiment was at least 3. The means ± standard deviations (SD) of all replicates in all experiments are depicted.
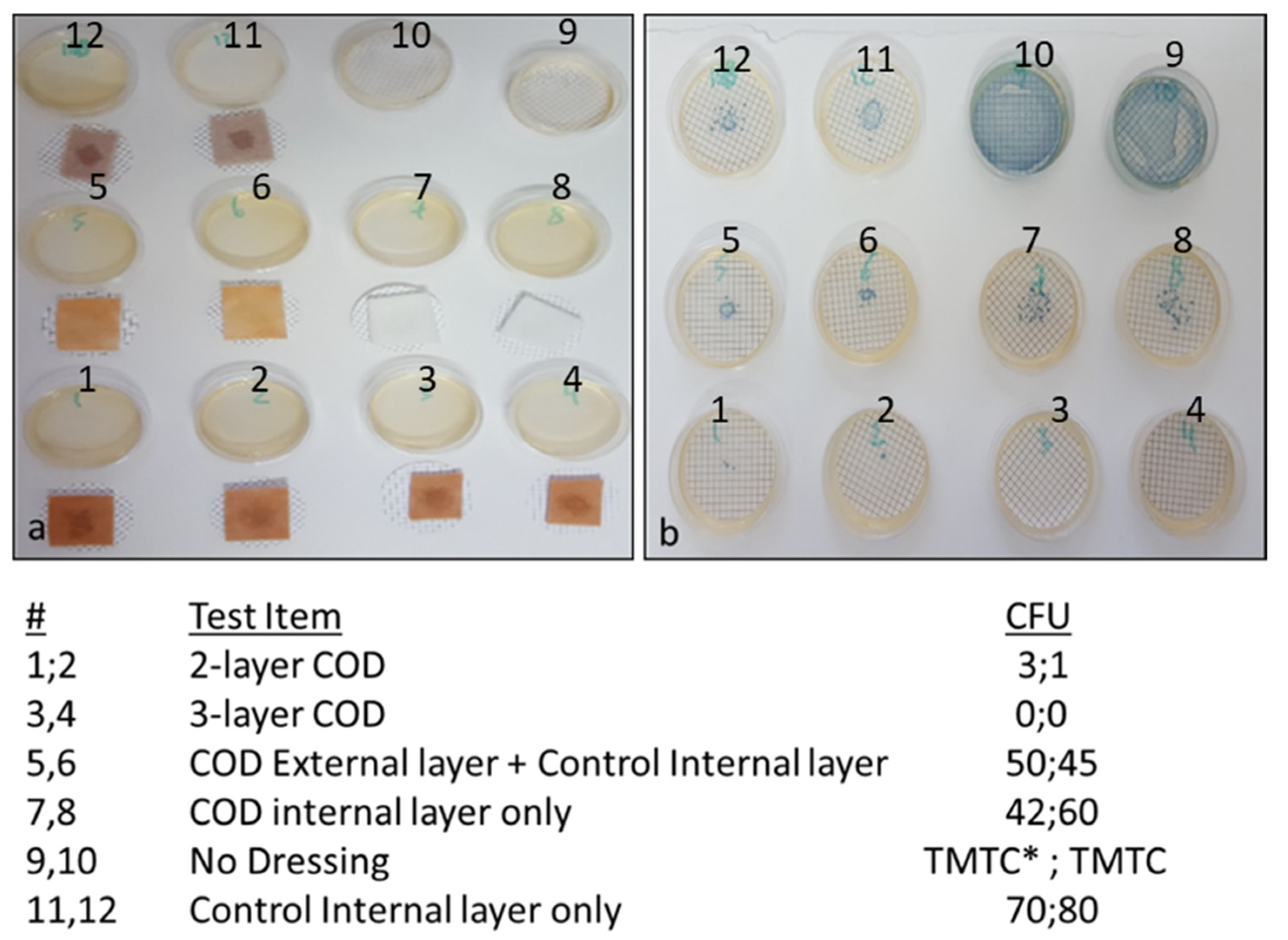
2.3. Determination of COD Microbial Barrier Properties
In order to determine the dressing construction that is capable of reducing significantly the capacity of viable bacteria to pass from the exterior surface to the interior surface of the dressings, several different COD constructions and controls were tested in the study. The test samples included the 2-and 3-layer COD; a dressing composed of the exterior cuprous oxide-impregnated layer and an absorbent internal control layer (without copper); only the internal layer containing cuprous oxide microparticles; and the internal absorbent layer but without copper. Klebsiella pneumoniae, grown overnight with TSB at 37 °C, and diluted in saline to a final concentration of ~800,000 CFU/mL, served as the bacterial inoculum. Duplicate swatches (1.5 cm × 1.5 cm) of each test item were placed on top of sterile cellulose nitrate filters (pore size 0.45 µm, Sartorious Stedim Biotech GmbH, Germany). One mL of the bacterial inoculum was then spiked in the middle area on top of each test item. In parallel, the number of CFU in 1 mL was determined by making serial dilutions and culturing the appropriate dilutions overnight at 37 °C. Ten minutes after the test items were inoculated with the bacteria, the filters were removed and placed on top of agar plates containing CHROMagar™ Orientation agar. The plates were incubated overnight at 37 °C and the number of CFU was then calculated. As an additional control, 1 mL aliquots from the stock bacterial solution were spiked directly on 2 agar plates.
In a similar experiment, triplicate swatches of COD with an adhesive contour and control dressings without copper (3M Life Sterile Dressings) were placed on top of Petri dishes containing CHROMagar™ Orientation agar. The dressings were then spiked with 0.5 mL of 5% NB in saline containing ~2 × 106 microorganisms/mL of the tested microorganism. The inoculation was repeated every 24 h 7 consecutive times. One hour after the 7th inoculation, the wound dressings were removed from the plates and the plates were incubated for 18 h at 37 °C, and the microorganism growth was examined.
3. Results
As can be seen in the graphs presented in
Figure 2, the exposure of all the microorganisms to the COD resulted in a dramatic reduction in their viable titer, reaching >10,000-fold reduction (4-logs) at 3 h of incubation as compared to the initial titer, with the exception of
Candida albicans that reached the 10,000-fold reduction at 18 h of incubation. In contrast, the titers of all microorganisms exposed to the negative control dressings at 37 °C were either not affected or even increased (negative log reduction) over time.
No difference in the biocidal efficacy was found between dressings naturally aged for 7 months, 15 months or 3 years, as shown in a representative experiment in
Figure 3.
The capacity of the COD to achieve a >10,000-fold reduction in bacterial titers when daily exposed to a high titer of bacteria for 7 consecutive days was examined by inoculating 4 replicate COD swatches daily with ~10
6 CFU of
Klebsiella pneumoniae. Then, three hours after the 7th bacterial inoculation, the bacteria were retrieved from the COD and control dressing swatches and the CFU were determined after 24 h of incubation at 37 °C. While in the control samples the CFU was ~10
6, no CFU were recovered from all 4 COD swatches, demonstrating an above 10,000-fold reduction in the viable bacterial titer (
Figure 4).
We also compared the antimicrobial efficacy of the COD against several antimicrobial commercially available wound dressings containing silver by spiking the dressings with high microorganism titers. After 1 h of incubation at 37 °C, the microorganisms were retrieved, and their viability was determined as described in the
Section 2. While the COD as well as Mepilex Ag and Acticoat Ag demonstrated good biocidal efficacy against the tested microorganisms, as no viable microorganisms were recovered after 1 h of incubation, the other tested dressings showed significantly lower biocidal efficacy (
Table 1).
In order to determine the capacity of the various COD constructions without an adhesive contour to reduce the passage of bacteria from the exterior environment to the wound bed, the swatches of various test items, as described in the
Section 2 and as shown in
Figure 5, were spiked with 1 mL containing ~8 × 10
5 CFU of
Klebsiella pneumoniae. As can be seen in
Figure 5, a similar number of bacteria passed through the COD internal layer only, the COD external layer only and control internal layer alone, and the control internal layer alone without cuprous oxide microparticles. However, through the 2-layer COD only 1 and 3 viable bacteria, and from the 3-layer COD no viable bacteria, passed from one side of the fabric to the other side.
Figure 6 depicts the capacity of the COD with an adhesive contour to block the passage of bacteria from the exterior environment to the wound bed even after 7 daily consecutive inoculations of different representative gram-negative and gram-positive bacteria on the outer surface of the dressings. After the 7th and last bacterial inoculation, and after wound dressings were removed from the agar, no growth was observed on the agar plates under the removed tested COD. In contrast, bacterial growth was observed on the agar under all the control wound dressings (
Figure 6).
4. Discussion
Wound dressings impregnated with cuprous oxide microparticles have been cleared by the USA FDA (510(k) K180643), EU and other regulatory bodies worldwide to be used in the management of acute and chronic wounds, after their biocompatibility, safety, and efficacy were demonstrated. These wound dressings have already been in clinical use for more than 2 years in several geographies, enhancing wound healing of both infected and non-infected wounds, including chronic wounds [
15,
16,
17].
The data obtained in our study clearly indicate that COD possess a strong biocidal activity against a broad spectrum of bacteria on the dressings and confer protection to the wounds from external microbial contamination.
Cuprous oxide microparticles were chosen as the active biocidal ingredient to be used in the COD dressings for two main reasons: they have very powerful wide spectrum biocide properties (e.g., [
20]), and they are non-soluble copper particles. The cuprous oxide microparticles serve as a reservoir of copper ions that are slowly and constantly released [
16,
21], endowing the wound dressing with prolonged and stable biocidal properties for at least 7 consecutive days that protect the dressings from bio-contamination and reduction of passage of the viable microorganism through them from the exterior environment into the wound bed.
The potent biocidal efficacy of the dressings was proved against both gram-positive and gram-negative bacteria, including against MRSA, an antibiotic-resistant bacterium. The bacteria that we choose to use in our antimicrobial tests are known wound pathogens [
22,
23]. In some of the experiments we chose to test the antimicrobial efficacy of the COD against
Enterobacter aerogenes and
Klebsiella pneumoniae as representative microorganisms (
Figure 3,
Figure 4 and
Figure 5), because these bacteria produce blue colonies when using CHROMagar™ Orientation agar that are easy to count for determining the CFU. In order to be able to demonstrate at least 10,000-fold reductions, we inoculated the dressings with an initial inoculum titer of ~10
6 CFU, and the lower limit of detection was 10
2 CFU, allowing us to detect up to 10,000-fold microbial titer reductions. Indeed, with all bacteria tested, ≥10,000-fold titer reductions were achieved within 3 h of the bacterial exposure to the dressings. Even after 7 consecutive daily spikings of the COD to high bacterial titers, a 10,000-fold reduction was achieved after the 7th bacterial inoculation, indicating that the amount of cuprous oxide microparticles in the dressings is sufficient to endow the dressings with prolonged potent biocidal efficacy. The reason we chose to inoculate the dressings for 7 consecutive days was because each individual COD is cleared to be used on a wound for up to 7 days by the regulatory bodies. A longer exposure time was required to achieve 10,000-fold reduction against
Candida albicans. Yeast and fungi seem to be less sensitive to copper exposure than bacteria [
24]. It should be mentioned that the lower limit of detection of most assays was 100 CFU (2-logs). Therefore, when no CFU were found, the number of bacteria recovered was considered to be ≤100 CFU. Since the initial inoculum was ~10
6 CFU, the maximum fold reduction detected in these cases was ≥10,000. The exact 10-fold reduction may have been significantly higher as was indeed the case in several experiments (data not shown).
Today most antimicrobial dressings in clinical use contain silver, as silver also has antimicrobial properties. However, there are increasing reports of potential wound-healing inhibition and toxicity by silver dressings [
25,
26,
27,
28,
29]. In our hands, the COD showed significantly better antimicrobial efficacy than most commercially available antimicrobial wound dressings containing silver that we tested (
Table 1), indicating that the potential of the COD to confer antimicrobial protection in clinical use is not less and most probably greater than most currently clinically used silver dressings.
The COD have two basic layers. A highly absorbent layer capable of absorbing ~10 times its own weight (data not shown). This allows the absorption of the wound exudates, which is mainly secreted from infected wounds. As wound exudate management is important for allowing wound healing processes to occur, the capacity to absorb and retain wound exudates is an important wound-dressing attribute [
30,
31]. The presence of the cuprous oxide microparticles in this layer prevents the proliferation of the absorbed bacteria secreted from the wound, suggesting reducing the risk of cross-contamination and foul odor. The second layer is a thin, non-stick, non-woven fabric that allows the passage of the wound’s exudate to the absorbent layer, while also allowing easier removal from the wound bed of the dressing after its use. This layer, which also contains cuprous oxide microparticles, additionally enhances the capacity of the dressings to prevent the passage of viable bacteria from the exterior to the wound bed (
Figure 5). In one of the COD constructions, used mainly to manage wound cavities and wound tunnels, this thin nonwoven layer is present on both sides of the absorbent fabric, in order to prevent the wound dressings from adhering to the surrounding wound bed. The COD with an adhesive contour also confer a mechanical barrier for at least 7 days from microbial contamination (
Figure 6). The described COD have significantly better properties than the prototypes previously described [
14]— they have significantly better biocidal properties and better exudate management properties, and the COD with an adhesive contour also serves as a mechanical barrier to microbial contamination.
Taken together, the data obtained in the study, demonstrate the high broad spectrum and prolonged biocidal and wound microbial protection efficacy of wound dressings impregnated with cuprous oxide microparticles as well as the potential to manage infected wounds with COD.