The Morphological and Functional Properties of Lactiplantibacillus plantarum B411 Subjected to Acid, Bile and Heat Multi-Stress Adaptation Process and Subsequent Long-Term Freezing
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
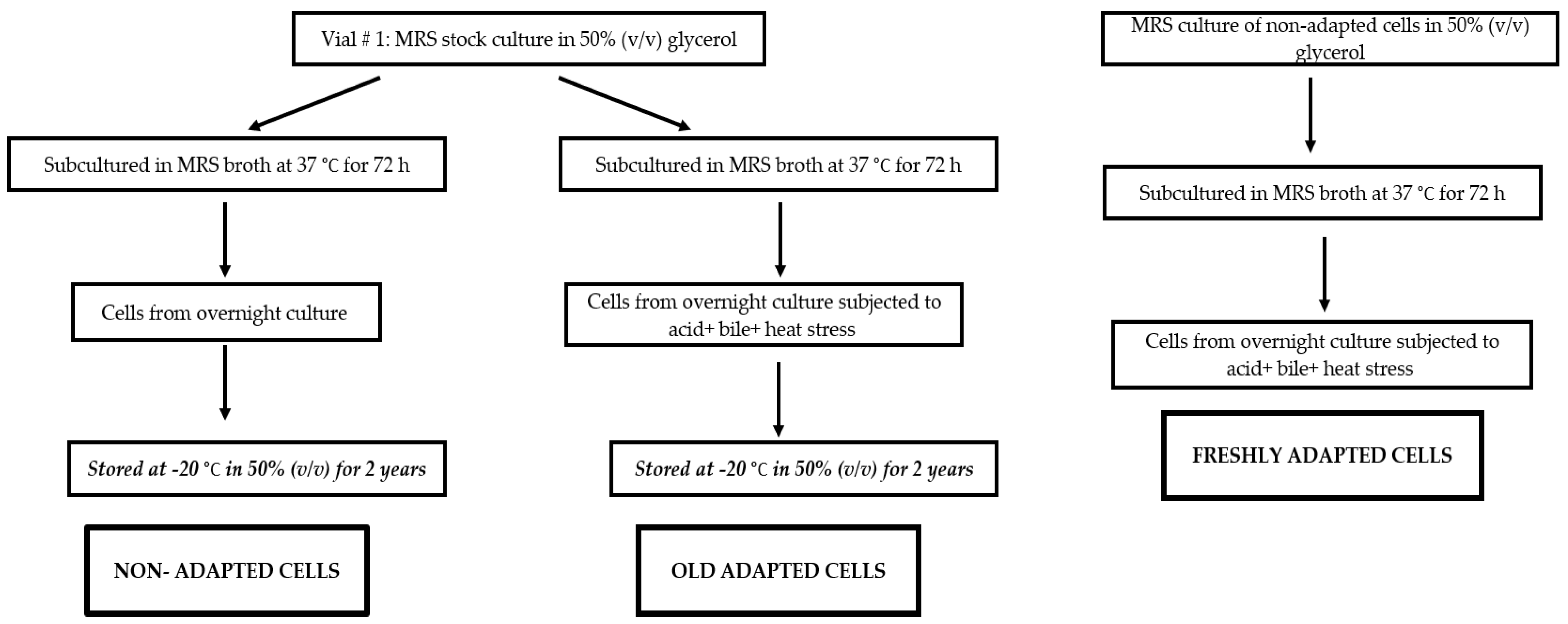
2.2. Acid, Bile and Heat Stress Adaptation of L. plantarum B411
2.3. Tolerance of L. plantarum B411 to Different Stress Factors
2.3.1. Acid Tolerance
2.3.2. Bile Tolerance
2.4. Bile Salt Hydrolase Activity
2.5. Antimicrobial Activity Assay
2.6. Scanning Electron Microscopy (SEM)
2.7. Auto-Aggregation
2.8. Cell Surface Hydrophobicity
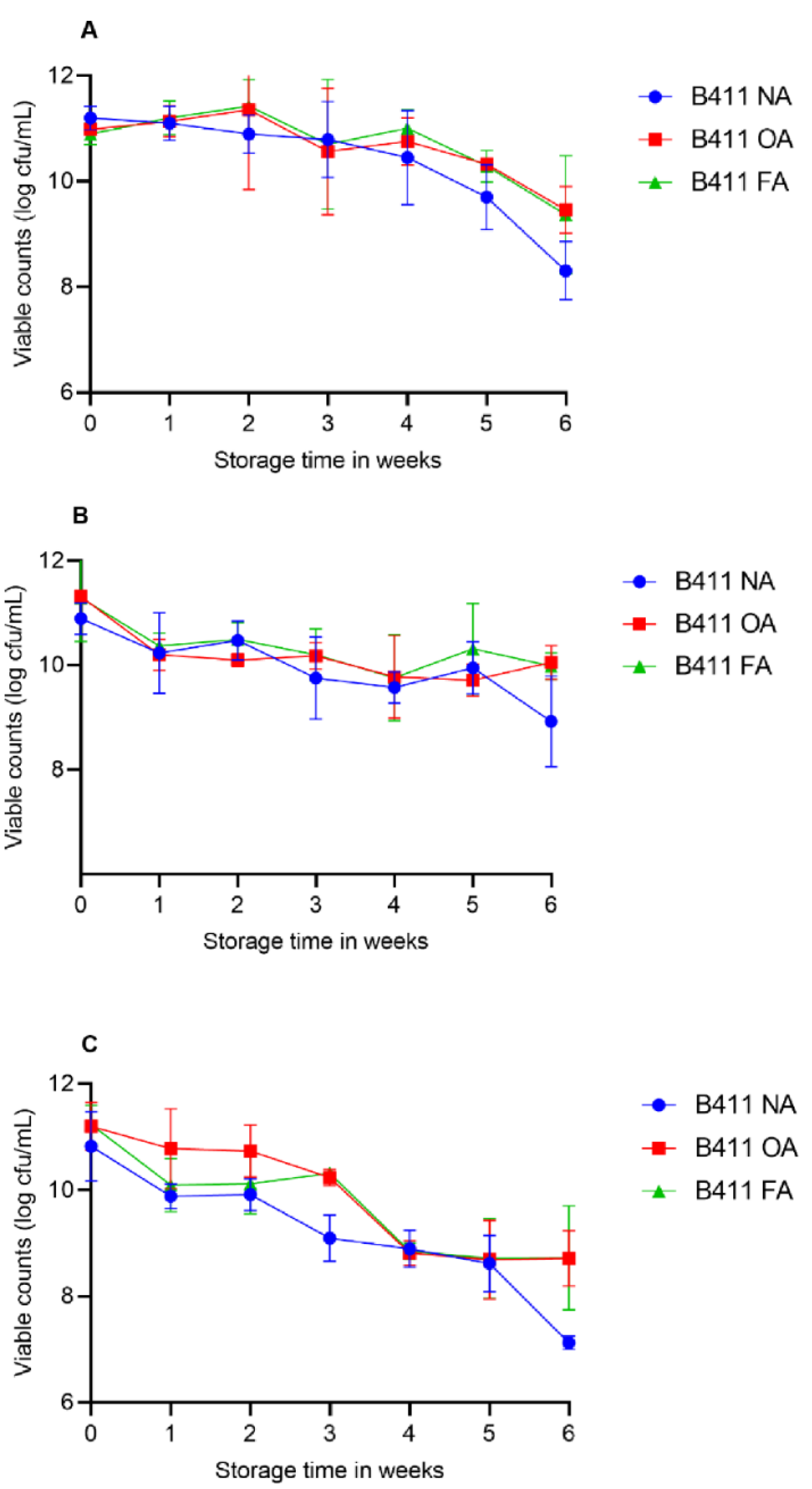
2.9. Survival of L. plantarum B411 in Food during Storage
2.9.1. Preparation of Yoghurt
2.9.2. Selection of Fruit and Vegetable Juice
2.9.3. Preparation of L. plantarum B411
2.9.4. Assessment of L. plantarum B411 Viability in Yoghurt and Juices during Storage
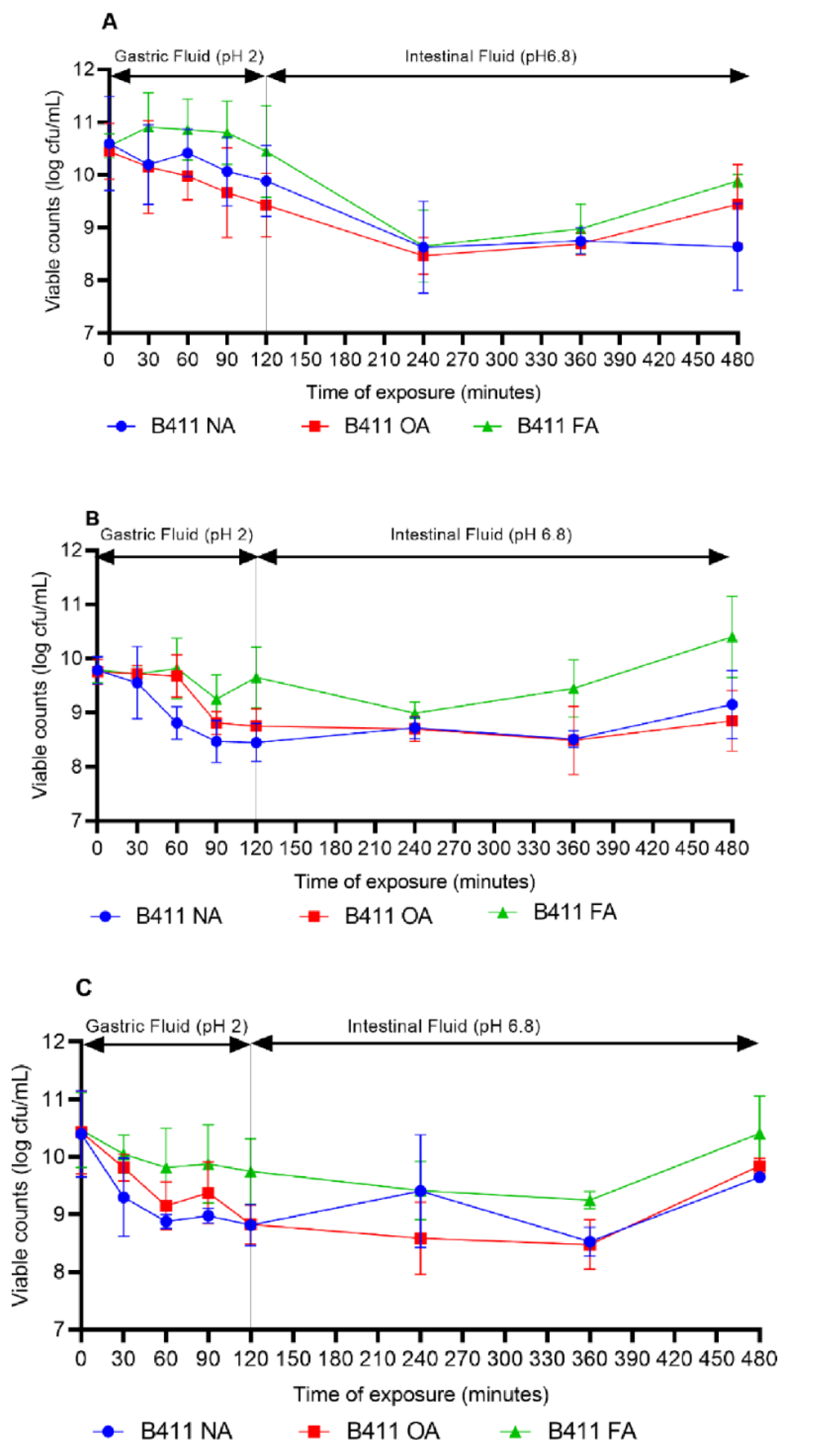
2.10. Effect of Food Matrixes on the Survival of L. plantarum B411 under Simulated GIT Conditions
2.11. Statistical Analysis
3. Results
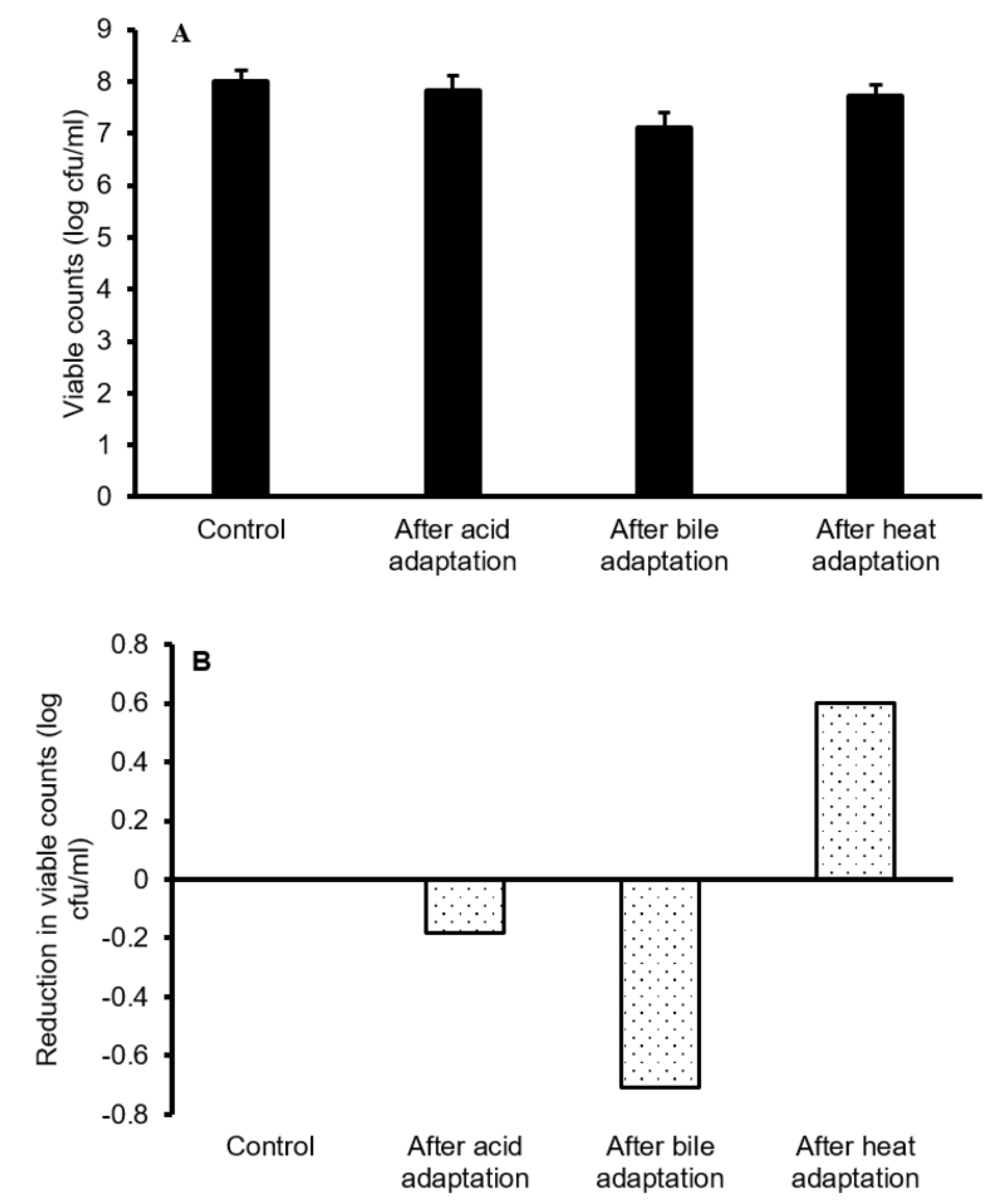
3.1. Adaptation of L. plantarum B411 to Acid, Bile and Heat
3.2. Scanning Electron Microscopy (SEM) of L. plantarum B411 Cells
3.3. Acid and Bile Tolerance by Non-Adapted, Freshly Adapted and Old Adapted L. plantarum B411 Cells
3.4. Bile Salt Hydrolase Activity
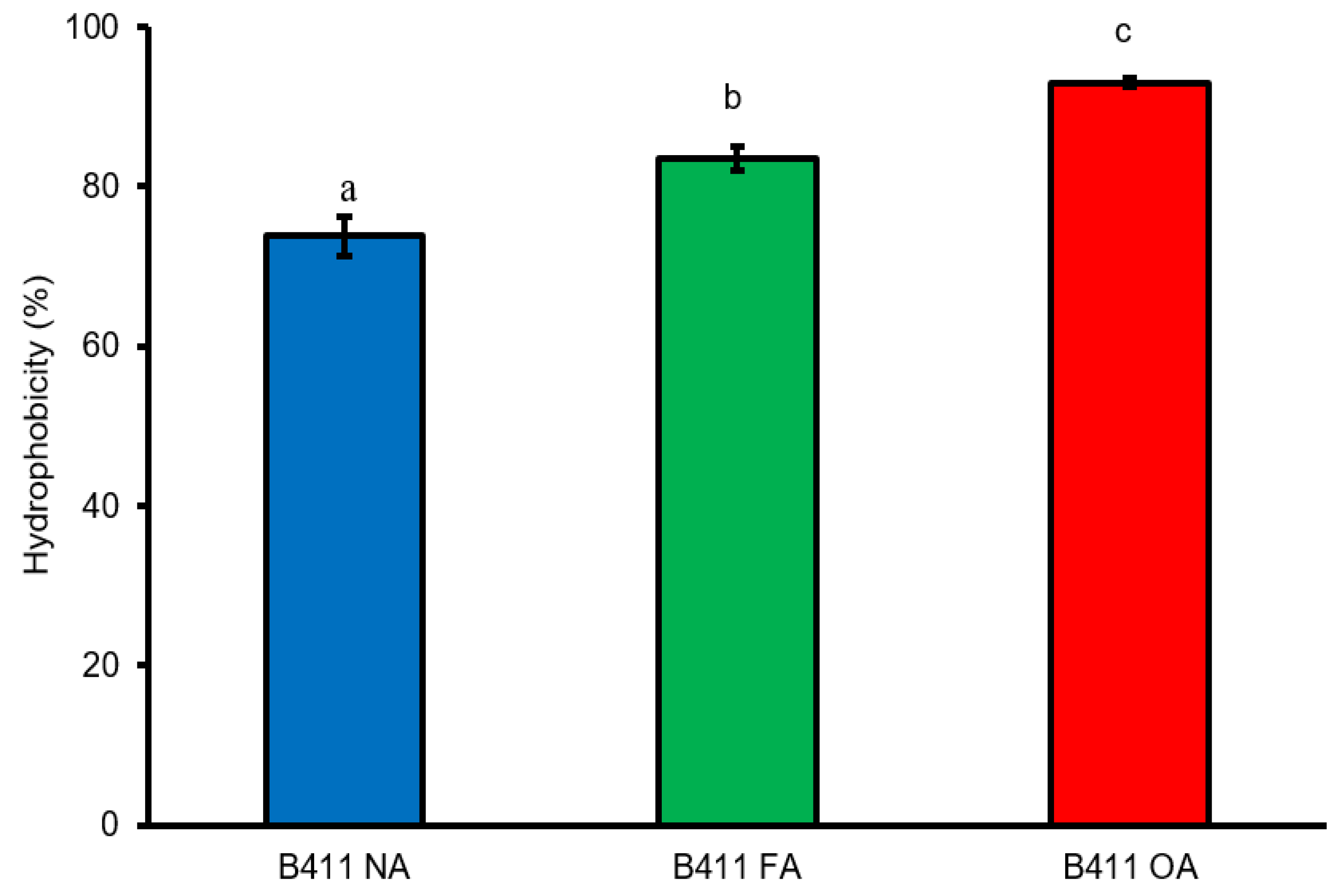
3.5. Auto-Aggregation and Cell Surface Hydrophobicity
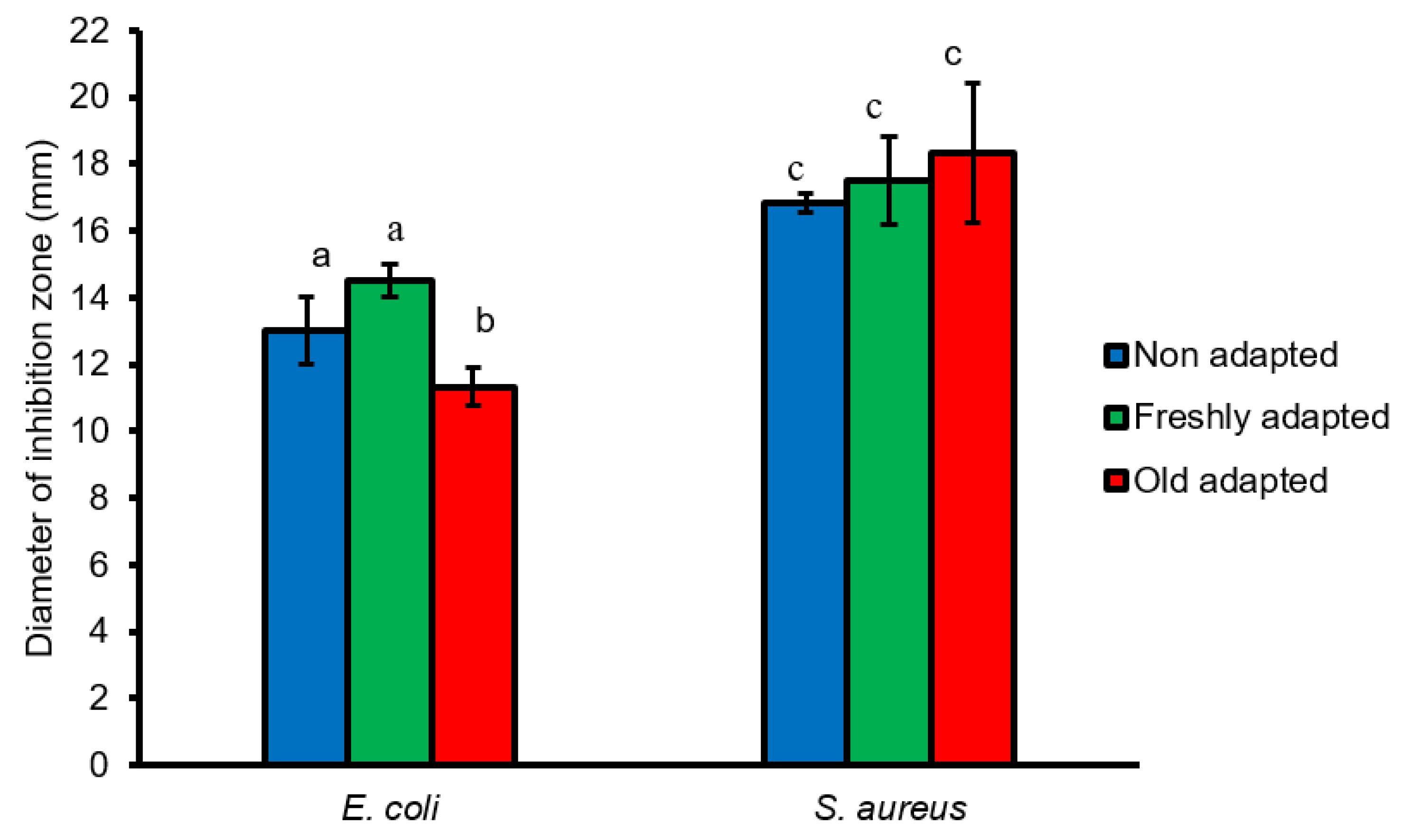
3.6. Antimicrobial Activity Assay of L. plantarum B411 Cells against E. coli ATCC 25922 and S. aureus ATCC 33591
3.7. Survival of L. plantarum B411 in Food Matrixes during Storage and under Simulated GIT Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Xu, C.; Liu, F.; Hou, J.; Shao, H.; Yu, W. Stress adaptation and cross-protection of Lactobacillus plantarum KLDS 1.0628. CyTA-J. Food. 2021, 19, 72–80. [Google Scholar] [CrossRef]
- Champagne, C.P.; Ross, R.P.; Saarela, M.; Hansen, K.F.; Charalampopoulos, D. Recommendations for the viability assessment of probiotics as concentrated cultures and in food matrices. Int. J. Food Microbiol. 2011, 149, 185–193. [Google Scholar] [CrossRef]
- Chen, M.-J.; Tang, H.-Y.; Chiang, M.-L. Effects of heat, cold, acod and bile salt adaptations on the stress tolerance and protein expression of kefir-isolated probiotic Lactobacillus kefiranofaciens M1. Food Microbiol. 2017, 66, 20–27. [Google Scholar] [CrossRef]
- Gaucher, F.; Bonnasie, S.; Rabah, H.; Marchand, P.; Blanc, P.; Jeantet, R.; Jan, G. Review: Adaptation of beneficial Propionibacteria, lactobacilli, and bifidobacteria improves tolerance toward technological and digestive stresses. Front. Microbiol. 2019, 10, 841. [Google Scholar] [CrossRef]
- Liu, S.; Ma, Y.; Zheng, Y.; Zhao, W.; Luo, T.; Zhang, J.; Yang, Z. Cold-stress response of probiotic Lactobacillus plantarum K25 by iTRAQ proteomic analysis. J. Microbiol. Biotechnol. 2020, 30, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Mbye, M.; Baig, M.F.; AbuQamar, S.F.; El-Tarabily, K.A.; Obaid, R.S.; Osaili, T.M.; Al-Nabulsi, A.A.; Turner, M.S.; Shah, N.P.; Ayyash, M.M. Updates on understanding of probiotic lactic acid bacteria responses to environmental stresses and highlights on proteomic analyses. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1110–1124. [Google Scholar] [CrossRef]
- Min, M.; Bunt, C.R.; Mason, S.L.; Hussain, M.A. Non-dairy probiotic food products: An emerging group of functional foods. Crit. Rev. Food Sci. Nutr. 2018, 59, 2626–2641. [Google Scholar] [CrossRef]
- Yang, H.; He, M.; Wu, C. Cross protection of lactic acid bacteria during environmetal stresses: Stress responses and underlying mechanisms. LWT-Food Sci. Technol. 2021, 144, 111203. [Google Scholar] [CrossRef]
- Ma, J.; Wang, W.; Sun, C.; Gu, L.; Liu, Z.; Yu, W.; Chen, L.; Jing, Z.; Hou, J. Effects of environmental stresses on the physiological characteristics, adhesion ability and pathogen adhesion inhibition of Lactobacillus plantarum KLDS 1.0328. Process Biochem. 2020, 92, 426–436. [Google Scholar] [CrossRef]
- Corcoran, B.; Stanton, C.; Fitzgerald, G.; Ross, R. Life under stress: The probiotic stress response and how it may be manipulated. Curr. Pharm. Des. 2008, 14, 1382–1399. [Google Scholar] [CrossRef] [PubMed]
- Sanz, Y. Ecological and functional implications of the acid-adaptation ability of Bifidobacterium: A way of selecting improved probiotic strains. Int. Dairy J. 2007, 17, 1284–1289. [Google Scholar] [CrossRef]
- Champagne, C.P.; Gardner, N. Effect of storage in a fruit drink on subsequent survival of probiotic lactobacilli to gastro-intestinal stresses. Food Res. Int. 2008, 41, 539–543. [Google Scholar] [CrossRef]
- Kulkarni, S.; Haq, S.F.; Samant, S.; Sukumaran, S. Adaptation of Lactobacillus acidophilus to thermal stress yields a thermotolerant variant which also exhibits improved survival at pH 2. Probiotics Antimicro. Prot. 2018, 10, 717–727. [Google Scholar] [CrossRef]
- Sánchez, B.; Ruiz, L.; Gueimonde, M.; Ruas-Madiedo, P.; Margolles, A. Toward improving technological and functional properties of probiotics in foods. Trends Food Sci. Technol. 2012, 26, 56–63. [Google Scholar] [CrossRef]
- Sánchez, B.; Champomier-Vergès, M.C.; Collado, M.; Anglade, P.; Baraige, F.; Sanz, Y.; de los Reyes-Gavilán, C.G.; Margolles, A.; Zagorec, M. Low-pH adaptation and the acid tolerance response of Bifidobacterium longum biotype longum. Appl. Environ. Microbiol. 2007, 73, 6450–6459. [Google Scholar] [CrossRef]
- Mathipa, M.G.; Thantsha, M.S. Cocktails of probiotics pre-adapted to multiple stress factors are more robust under simulated gastrointestinal conditions than their parental counterparts and exhibit enhanced antagonistic capabilities against Escherichia coli and Staphylococcus aureus. Gut Pathog. 2015, 7, 5. [Google Scholar] [CrossRef]
- Ruiz, L.; Margolles, A.; Sánchez, B. Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front. Microbiol. 2013, 4, 396. [Google Scholar] [CrossRef]
- Haddaji, N.; Mahdhi, A.B.; Ismaiil, M.B.; Bakhrouf, A. Effect of environmental stress on cell surface and membrane fatty acids of Lactobacillus plantarum. Arch. Microbiol. 2017, 199, 1243–1250. [Google Scholar] [CrossRef]
- da Cruz, A.G.; Faria, D.A.F.; Van Dender, A.G.F. Packaging system and probiotic dairy foods. Food Res. Int. 2007, 40, 951–956. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Nazzaro, F.; Cruz, A.G.; Faria, J.A.F. Functional foods and nondairy probiotic food development: Trends, concepts, and products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Homayoni, R.A.; Vaghef, M.E.; Alipoor, B.; Vaghef, M.L. The comparison of food and supplement as probiotic delivery vehicles. Crit. Rev. Food Sci. Nutr. 2016, 56, 896–909. [Google Scholar] [CrossRef] [PubMed]
- Shori, A.B. Influence of food matrix on the viability of probiotic bacteria: A review based on dairy and non-dairy beverages. Food Biosci. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Kumar, V.B.; Vijayendra, S.V.N.; Reddy, O.V.S. Trends in dairy and non-dairy probiotic products—A review. J. Food Sci. Technol. 2015, 52, 6112–6124. [Google Scholar] [CrossRef] [PubMed]
- Sedláčková, P.; Horáčková, S.; Shi, T.; Kosová, M.; Plocková, M. Two different methods for screening of bile salt hydrolase activity in Lactobacillus Strains. Czech. J. Food Sci. 2015, 33, 13–18. [Google Scholar] [CrossRef]
- Mohankumar, A.; Murugalatha, N. Characterization and antibacterial activity of bacteriocin producing Lactobacillus isolated from raw cattle milk sample. Int. J. Biol. 2011, 3, 128. [Google Scholar] [CrossRef]
- Booyens, J.; Labuschagne, M.C.; Thantsha, M.S. In vitro antibacterial mechanism of action of crude garlic (Allium sativum) clove extract on selected probiotic Bifidobacterium species as revealed by SEM, TEM, and SDS-PAGE analysis. Probiotics Antimicrob. Proteins 2014, 6, 82–87. [Google Scholar] [CrossRef]
- Li, Q.; Liu, X.; Dong, M.; Zhou, J.; Wang, Y. Aggregation and adhesion abilities of 18 Lactic acid bacteria strains isolated from traditional fermented food. Int. J. Agric. Policy Res. 2015, 3, 84–92. [Google Scholar]
- Amakiri, A.C.; Thantsha, M.S. Survival of Bifidobacterium longum LMG 13197 microencapsulated in vegetal or vegetal-inulin matrix in simulated gastrointestinal fluids and yoghurt. Springerplus 2016, 5, 1343. [Google Scholar] [CrossRef]
- Ding, W.K.; Shah, N.P. Survival of free and microencapsulated probiotic bacteria in orange and apple juices. Int. Food Res. J. 2008, 15, 219–232. [Google Scholar] [CrossRef]
- Young, K.D. Bacterial morphology: Why have different shapes? Curr. Opin. Microbiol. 2007, 10, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; McMullen, L.; Jeon, B. Impact of oxidative stress defense on bacterial survival and morphological change in Campylobacter jejuni under aerobic conditions. Front. Microbiol. 2015, 6, 295. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, J.; Pan, D.; Wu, Z.; Guo, Y.; Zeng, X.; Lian, L. Metabolomics analysis of Lactobacillus plantarum ATCC 14917 adhesion activity under initial acid and alkali stress. PLoS ONE 2018, 13, e0196231. [Google Scholar] [CrossRef] [PubMed]
- Chaiyanan, S.; Chaiyanan, S.; Grim, C.; Maugel, T.; Huq, A.; Colwell, R.R. Ultrastructure of coccoid viable but non-culturable Vibrio cholerae. Environ. Microbiol. 2007, 9, 393–402. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Q.; Liu, H.; Kong, B.; Chen, Q. In vitro growth performance, antioxidant activity and cell surface physiological characteristics of Pediococcus pentosaceus R1 and Lactobacillus fermentum R6 stressed at different NaCl concentration. Food Funct. 2020, 11, 6376–6386. [Google Scholar] [CrossRef]
- Mills, S.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Enhancing the stress responses of probiotics for a lifestyle from gut to product and back again. Microb. Cell Fact. 2011, 10, S19. [Google Scholar] [CrossRef]
- Urdaneta, V.; Casadesús, J. Interactions between bacteria and bile salts in the gastrointestinal and hepatobiliary tracts. Front. Med. 2017, 4, 163. [Google Scholar] [CrossRef]
- Sahadeva, R.P.K.; Leong, S.F.; Chua, K.H.; Tan, C.H.; Chan, H.Y.; Tong, E.V.; Wong, S.Y.; Chan, H.K. Survival of commercial probiotic strains to pH and bile. Int. Food Res. J. 2011, 18, 1515–1522. [Google Scholar]
- Bi, J.; Liu, S.; Du, G.; Chen, J. Bile tolerance of Lactococcus lactis is enhanced by expresssion of bile salt hydrolase threby producing less bile acid in the cells. Biotechnol. Lett. 2016, 38, 659–665. [Google Scholar] [CrossRef]
- Kusada, H.; Morinaga, K.; Tamaki, H. Identification of bile salt hydrolase and bile salt resistance in a probiotic bacterium Lactobacillus gasseri JCM1131T. Microorganisms 2021, 9, 1011. [Google Scholar] [CrossRef]
- Moser, S.A.; Savage, D.C. Bile salt hydrolase activity and resistance to toxicity of conjugated bile salts are unrelated properties in Lactobacilli. Appl. Environ. Microbiol. 2001, 67, 3476–3480. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.-X.; Yan, R.; Shi, H.-Y.; Shi, D.; Fang, D.-Q.; Jiang, H.-Y.; Wu, W.-R.; Guo, F.-F.; Jiang, X.-W.; Gu, S.-L.; et al. Integrated transcriptomic and proteomic analysis of the bile stress response in probiotic Lactobacillus salivarius LI01. J. Proteomics 2017, 150, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Sridevi, N.; Vishwe, P.; Prabhune, A. Hypocholesteremic effect of bile salt hydrolase from Lactobacillus buchneri ATCC 4005. Food Res. Int. 2009, 42, 516–520. [Google Scholar] [CrossRef]
- Gong, X.; Yu, H.; Chen, J.; Han, B. Cell surface properties of Lactobacillus salivarius under osmotic stress. Eur. Food Res. Technol. 2012, 234, 671–678. [Google Scholar] [CrossRef]
- Haddaji, N.; Boubaker, K.; Lagha, R.; Khouadja, S.; Bakhrouf, A. Effect of high temperature on viability of Lactobacillus casei and analysis of secreted and GroEL proteins profiles. African J. Bacteriol. Res. 2015, 7, 29–34. [Google Scholar] [CrossRef]
- Shakirova, L.; Grube, M.; Gavare, M.; Auzina, L.; Zikmanis, P. Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 cell surface hydrophobicity and survival of the cells under adverse environmental conditions. J. Ind. Microbiol. Biotechnol. 2013, 40, 85–93. [Google Scholar] [CrossRef]
- Tareb, R.; Bernardeau, M.; Gueguen, M.; Vernoux, J.-P. In vitro characterization of aggregation and adhesion properties of viable and heat-killed forms of two probiotic Lactobacillus strains and interaction with foodborne zoonotic bacteria, especially Campylobacter jejuni. J. Med. Microbiol. 2013, 62, 637–649. [Google Scholar] [CrossRef]
- Gueimonde, M.; Sánchez, B. Enhancing probiotic stability in industrial processes. Microb. Ecol. Health Dis. 2012, 23, 18562. [Google Scholar] [CrossRef][Green Version]
- Amund, O.D. Exploring the relationship between exposure to technological and gastrointestinal stress and probiotic functional properties of lactobacilli and bifidobacteria. Can. J. Microbiol. 2016, 62, 715–725. [Google Scholar] [CrossRef]
- Vizoso Pinto, M.G.; Holzapfel, W.H.; Franz, C.; Schillinger, U. Lactobacillus spp. with in vitro probiotic properties from human faeces and traditional fermented products. Int. J. Food Microbiol. 2006, 109, 205–214. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Prasanna, P.H.B.; Vidanarachchi, J.K. Fruit juices as probiotic carriers. In Fruit Juices: Types, Nutritional Composition and Health Benefits; Nova Science: New York, NY, USA, 2014; pp. 253–267. [Google Scholar]
- Patel, A.R. Probiotic fruit and vegetable juices—Recent advances and future perspective. Int. Food Res. J. 2017, 24, 1850–1857. [Google Scholar]

| L. plantarum Cells | pH 2 | pH 2.5 | pH 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Time (min) | |||||||||
| 60 | 120 | 180 | 60 | 120 | 180 | 60 | 120 | 180 | |
| Viable Counts Log (cfu/mL) | |||||||||
| Non-adapted | 7.88 ± 1.11 | 7.71 ± 1.25 | 8.11 ± 1.23 | 8.21 ± 0.78 | 7.77 ± 1.78 | 8.07 ± 1.69 | 8.16 ± 1.15 | 8.28 ± 1.11 | 7.94 ± 1.98 |
| Freshly adapted | 8.22 ± 1.54 | 8.26 ± 1.71 | 7.90 ± 1.89 | 7.60 ± 1.23 | 7.90 ± 1.45 | 6.99 ± 1.78 | 8.22 ± 2.42 | 8.18 ± 1.23 | 8.02 ± 1.87 |
| Old adapted | 7.89 ± 0.99 | 6.60 ± 1.32 | 7.86 ± 1.20 | 8.01 ± 1.47 | 7.86 ± 1.24 | 7.28 ± 2.36 | 8.29 ± 1.89 | 8.25 ± 1.74 | 7.83 ± 1.28 |
| Bile concentrations (%) | |||||||||
| 0.3 | 0.5 | 2 | |||||||
| Time (min) | |||||||||
| 60 | 120 | 180 | 60 | 120 | 180 | 60 | 120 | 180 | |
| Viable counts log (cfu/mL) | |||||||||
| Non-adapted | 7.85 ± 2.0 | 7.33 ± 1.11 | 7.31 ± 1.69 | 6.51 ± 0.89 | 5.87 ± 1.78 | 5.55 ± 1.47 | - | - | - |
| Freshly adapted | 6.81 ± 1.1 | 6.53 ± 1.50 | 6.51 ± 1.10 | 5.91 ± 1.25 | 5.98 ± 1.96 | 4.55 ± 1.91 | - | - | - |
| Old adapted | 7.10 ± 1.96 | 6.90 ± 2.36 | 6.81 ± 2.79 | 5.77 ± 1.68 | 5.20 ± 2.14 | 4.69 ± 1.23 | - | - | - |
| L. plantarum B411 Cells | Auto-Aggregation (%) | ||
|---|---|---|---|
| 0 h | 3 h | 6 h | |
| Non-adapted | 0.00 a | 15.80 a ± 1.06 | 10.33 a ± 2.88 |
| Freshly adapted | 0.00 a | 8.73 b ± 4.03 | 9.04 a ± 1.41 |
| Old adapted | 0.00 a | 9.33 b ± 3.09 | 7.06 b ± 1.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dlangalala, T.N.; Mathipa-Mdakane, M.G.; Thantsha, M.S. The Morphological and Functional Properties of Lactiplantibacillus plantarum B411 Subjected to Acid, Bile and Heat Multi-Stress Adaptation Process and Subsequent Long-Term Freezing. Microbiol. Res. 2022, 13, 909-927. https://doi.org/10.3390/microbiolres13040064
Dlangalala TN, Mathipa-Mdakane MG, Thantsha MS. The Morphological and Functional Properties of Lactiplantibacillus plantarum B411 Subjected to Acid, Bile and Heat Multi-Stress Adaptation Process and Subsequent Long-Term Freezing. Microbiology Research. 2022; 13(4):909-927. https://doi.org/10.3390/microbiolres13040064
Chicago/Turabian StyleDlangalala, Thobeka N., Moloko G. Mathipa-Mdakane, and Mapitsi S. Thantsha. 2022. "The Morphological and Functional Properties of Lactiplantibacillus plantarum B411 Subjected to Acid, Bile and Heat Multi-Stress Adaptation Process and Subsequent Long-Term Freezing" Microbiology Research 13, no. 4: 909-927. https://doi.org/10.3390/microbiolres13040064
APA StyleDlangalala, T. N., Mathipa-Mdakane, M. G., & Thantsha, M. S. (2022). The Morphological and Functional Properties of Lactiplantibacillus plantarum B411 Subjected to Acid, Bile and Heat Multi-Stress Adaptation Process and Subsequent Long-Term Freezing. Microbiology Research, 13(4), 909-927. https://doi.org/10.3390/microbiolres13040064






