Removal of Pb (II) from Aqueous Solution by a Pectin-Producing Alga, Penium margaritaceum, Immobilized on Filter Paper
Abstract
1. Introduction
2. Materials and Methods
2.1. Algal Strains and Reagents
2.2. Culture Conditions and Exposure of Algal Cells to Pb Solution
2.3. Dry Ashing of Filter Paper and Algal Cell
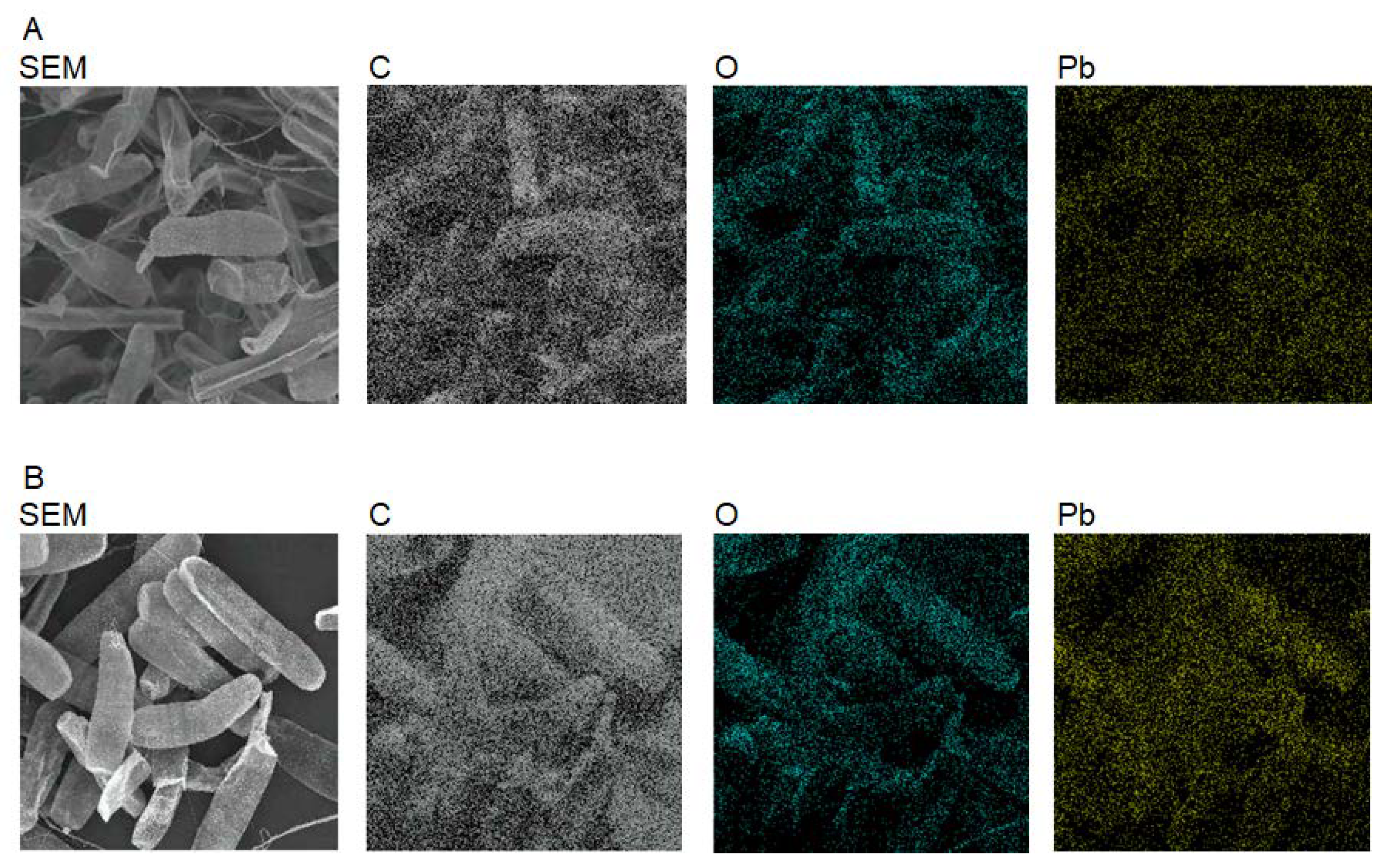
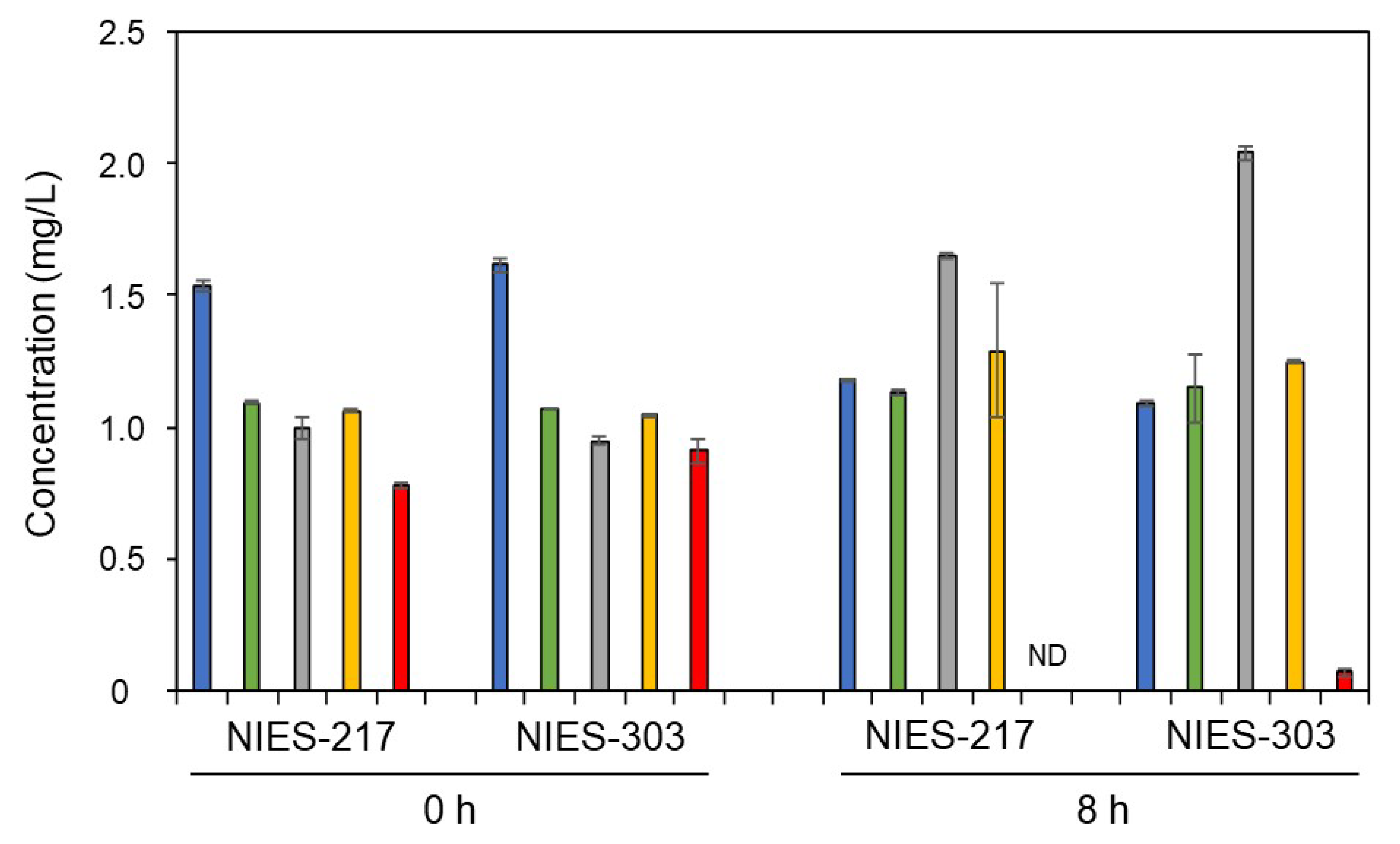
2.4. Elemental Mapping of Algal Cells and Measurements of Pb, Ca, Mg, K, and Na
2.5. Acquisition of Fourier Transform Infrared (FT-IR) Spectra
2.6. Measurements of Pectin Content
3. Results
3.1. Adsorption of Pb on Algal Cells
3.2. Immobilization of Algal Cells on Filter Paper
3.3. Removal and Recovery of Pb by the Immobilized Algal Cells
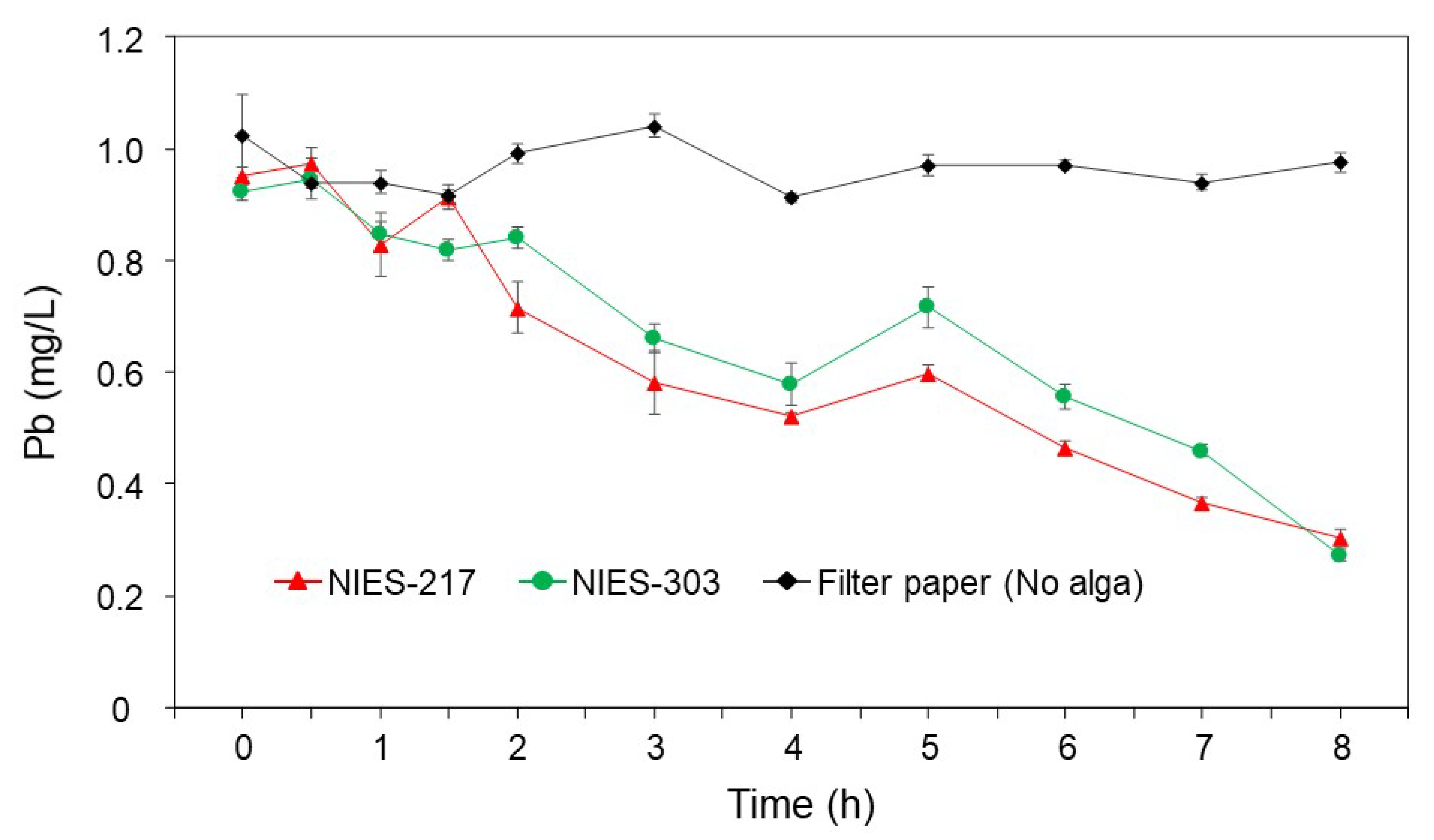
3.4. Time Course and the Specificity of Pb Removal
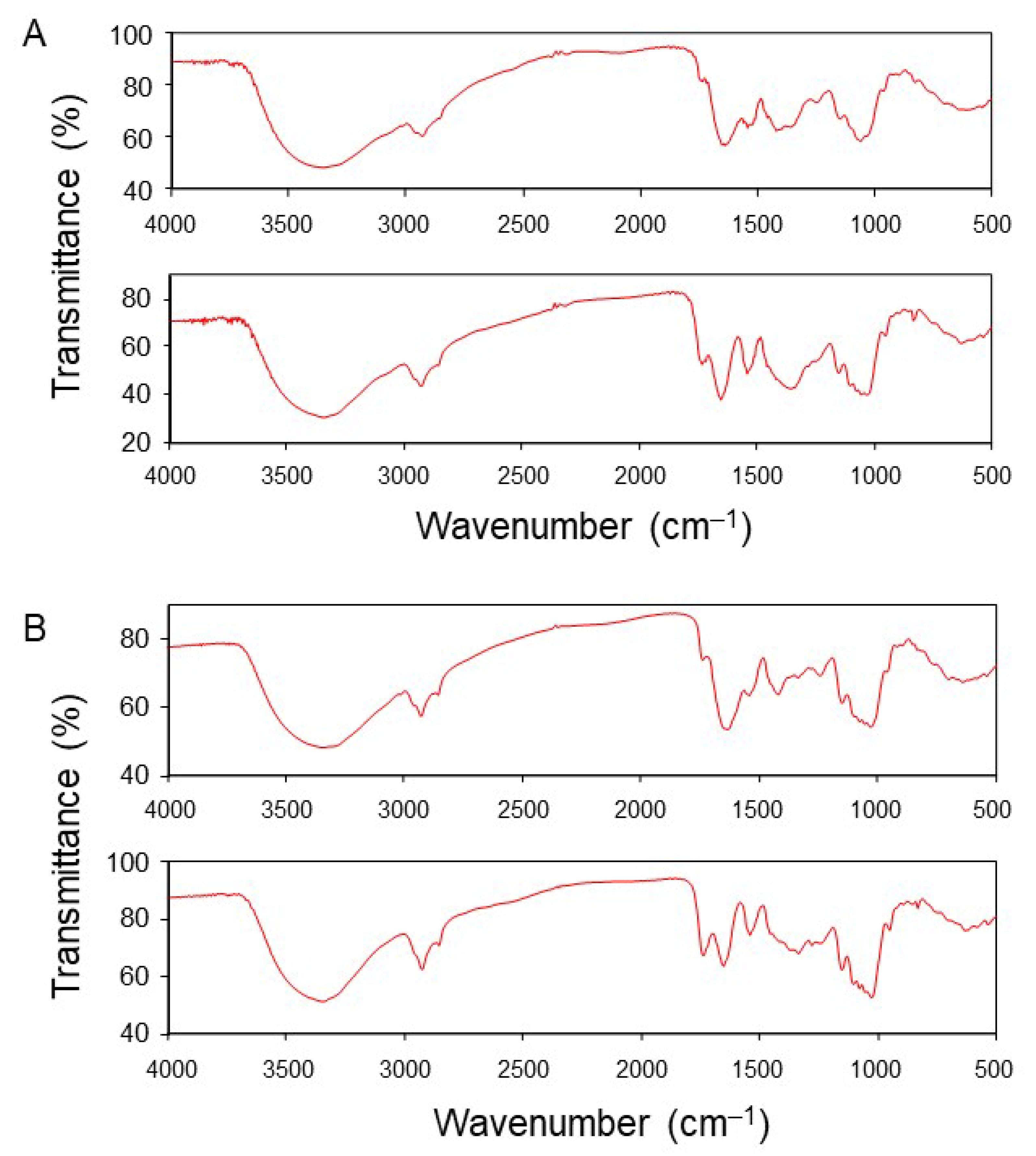
3.5. Detection of a Functional Group Involved in the Adsorption of Pb
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sevak, P.I.; Pushkar, B.K.; Kapadne, P.N. Lead pollution and bacterial bioremediation: A review. Environ. Chem. Lett. 2021, 19, 4463–4488. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Xiong, Z.; Wang, M.; Liu, Q. Environmental pollution effect analysis of lead compounds in China based on life cycle. Int. J. Environ. Res. Public Health 2020, 17, 2184. [Google Scholar] [CrossRef] [PubMed]
- Sikder, M.T.; Kihara, Y.; Yasuda, M.; Mihara, Y.; Tanaka, S.; Odgerel, D.; Mijiddorj, B.; Syawal, S.M.; Hosokawa, T.; Saito, T. River water pollution in developed and developing countries: Judge and assessment of physicochemical characteristics and selected dissolved metal concentration. CLEAN–Soil Air Water 2013, 41, 60–68. [Google Scholar] [CrossRef]
- Kasimov, N.; Kosheleva, N.; Gunin, P.; Korlyakov, I.; Sorokina, O.; Timofeev, I. State of the environment of urban and mining areas in the Selenga Transboundary River Basin (Mongolia Russia). Environ. Earth Sci. 2016, 75, 1283. [Google Scholar] [CrossRef]
- Rahman, M.A.; Paul, M.; Bhoumik, N.; Hassan, M.; Alam, M.; Aktar, Z. Heavy metal pollution assessment in the groundwater of the Meghna Ghat industrial area, Bangladesh, by using water pollution indices approach. Appl. Water Sci. 2020, 10, 186. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, P.; Yue, X.; Du, X.; Li, W.; Yin, Y.; Yi, C.; Li, Y. Effect of sub-chronic exposure to lead (Pb) and Bacillus subtilis on Carassius auratus gibelio: Bioaccumulation, antioxidant responses and immune responses. Ecotoxicol. Environ. Saf. 2018, 161, 755–762. [Google Scholar] [CrossRef]
- Luo, T.; Shen, M.; Zhou, J.; Wang, X.; Xia, J.; Fu, Z.; Jin, Y. Chronic exposure to low doses of Pb induces hepatotoxicity at the physiological, biochemical, and transcriptomic levels of mice. Environ. Toxicol. 2019, 34, 521–529. [Google Scholar] [CrossRef]
- Satarug, S.; Gobe, G.C.; Vesey, D.A.; Phelps, K.R. Cadmium and lead exposure, nephrotoxicity, and mortality. Toxics 2020, 8, 86. [Google Scholar] [CrossRef]
- Lee, J.-W.; Choi, H.; Hwang, U.-K.; Kang, J.-C.; Kang, Y.J.; Kim, K.I.; Kim, J.-H. Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: A review. Environ. Toxicol. Pharmacol. 2019, 68, 101–108. [Google Scholar] [CrossRef]
- Salman, M.S.; Znad, H.; Hasan, M.N.; Hasan, M.M. Optimization of innovative composite sensor for Pb (II) detection and capturing from water samples. Microchem. J. 2021, 160, 105765. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Islam, A.; Rahman, M.M.; Asiri, A.M.; Khaleque, M.A.; Sheikh, M.C. Offering an innovative composited material for effective lead (II) monitoring and removal from polluted water. J. Clean. Prod. 2019, 231, 214–223. [Google Scholar] [CrossRef]
- Awual, M.R. Mesoporous composite material for efficient lead (II) detection and removal from aqueous media. J. Environ. Chem. Eng. 2019, 7, 103124. [Google Scholar] [CrossRef]
- Crist, D.; Crist, R.; Martin, J.; Watson, J. Ion exchange systems in proton—Metal reactions with algal cell walls. FEMS Microbiol. Rev. 1994, 14, 309–313. [Google Scholar] [CrossRef][Green Version]
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef]
- Krzesłowska, M. The cell wall in plant cell response to trace metals: Polysaccharide remodeling and its role in defense strategy. Acta Physiol. Plant. 2011, 33, 35–51. [Google Scholar] [CrossRef]
- Atmodjo, M.A.; Hao, Z.; Mohnen, D. Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol. 2013, 64, 747–779. [Google Scholar] [CrossRef]
- Fürst-Jansen, J.M.; de Vries, S.; de Vries, J. Evo-physio: On stress responses and the earliest land plants. J. Exp. Bot. 2020, 71, 3254–3269. [Google Scholar] [CrossRef]
- Zanchetta, E.; Damergi, E.; Patel, B.; Borgmeyer, T.; Pick, H.; Pulgarin, A.; Ludwig, C. Algal cellulose, production and potential use in plastics: Challenges and opportunities. Algal Res. 2021, 56, 102288. [Google Scholar] [CrossRef]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef]
- Ahmad, A.; Bhat, A.; Buang, A. Enhanced biosorption of transition metals by living Chlorella vulgaris immobilized in Ca-alginate beads. Environ. Technol. 2019, 40, 1793–1809. [Google Scholar] [CrossRef]
- Bayramoğlu, G.; Tuzun, I.; Celik, G.; Yilmaz, M.; Arica, M.Y. Biosorption of mercury (II), cadmium (II) and lead (II) ions from aqueous system by microalgae Chlamydomonas reinhardtii immobilized in alginate beads. Int. J. Miner. Process. 2006, 81, 35–43. [Google Scholar] [CrossRef]
- Moreno-Garrido, I.; Campana, O.; Lubián, L.; Blasco, J. Calcium alginate immobilized marine microalgae: Experiments on growth and short-term heavy metal accumulation. Mar. Pollut. Bull. 2005, 51, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Latifi, A.M.; Sadegh Nejad, E.; Babavalian, H. Comparison of extraction different methods of sodium alginate from brown alga Sargassum sp. localized in the Southern of Iran. J. Appl. Biotechnol. Rep. 2015, 2, 251–255. [Google Scholar]
- Papageorgiou, S.; Kouvelos, E.; Katsaros, F. Calcium alginate beads from Laminaria digitata for the removal of Cu+2 and Cd+2 from dilute aqueous metal solutions. Desalination 2008, 224, 293–306. [Google Scholar] [CrossRef]
- Al-Rub, F.A.; El-Naas, M.; Benyahia, F.; Ashour, I. Biosorption of nickel on blank alginate beads, free and immobilized algal cells. Process Biochem. 2004, 39, 1767–1773. [Google Scholar] [CrossRef]
- Hameed, M.A. Continuous removal and recovery of lead by alginate beads, free and alginate-immobilized Chlorella vulgaris. Afr. J. Biotechnol. 2006, 5, 1819–1823. [Google Scholar]
- Nichols, H.W. Growth media-freshwater. In Handbook of Phycological Methods: Culture Methods and Growth Measurements, 1st ed.; Stein, J.R., Ed.; Cambridge University Press: New York, NY, USA, 1973; Volume 1, pp. 39–78. [Google Scholar]
- Sato, M.d.F.; Rigoni, D.C.; Canteri, M.H.G.; Petkowicz, C.L.d.O.; Nogueira, A.; Wosiacki, G. Chemical and instrumental characterization of pectin from dried pomace of eleven apple cultivars. Acta Scientiarum. Agron. 2011, 33, 383–389. [Google Scholar]
- Lasheen, M.R.; Ammar, N.S.; Ibrahim, H.S. Adsorption/desorption of Cd (II), Cu (II) and Pb (II) using chemically modified orange peel: Equilibrium and kinetic studies. Solid State Sci. 2012, 14, 202–210. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Hubicki, Z.; Pasieczna-Patkowska, S. FT-IR/PAS Studies of Cu (II)-EDTA Complexes Sorption οn the Chelating Ion Exchangers. Acta Phys. Pol. A 2009, 116, 340–343. [Google Scholar] [CrossRef]
- Abe, K.; Matsumura, I.; Imamaki, A.; Hirano, M. Removal of inorganic nitrogen sources from water by the algal biofilm of the aerial microalga Trentepohlia aurea. World J. Microbiol. Biotechnol. 2003, 19, 325–328. [Google Scholar] [CrossRef]
- Tüzün, I.; Bayramoğlu, G.; Yalçın, E.; Başaran, G.; Celik, G.; Arıca, M.Y. Equilibrium and kinetic studies on biosorption of Hg (II), Cd (II) and Pb (II) ions onto microalgae Chlamydomonas reinhardtii. J. Environ. Manag. 2005, 77, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ciempiel, W.; Czemierska, M.; Szymańska-Chargot, M.; Zdunek, A.; Wiącek, D.; Jarosz-Wilkołazka, A.; Krzemińska, I. Soluble Extracellular Polymeric Substances Produced by Parachlorella kessleri and Chlorella vulgaris: Biochemical Characterization and Assessment of Their Cadmium and Lead Sorption Abilities. Molecules 2022, 27, 7153. [Google Scholar] [CrossRef]
- Shanab, S.; Essa, A.; Shalaby, E. Bioremoval capacity of three heavy metals by some microalgae species (Egyptian Isolates). Plant Signal. Behav. 2012, 7, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.-L.; Dao, T.-S.; Bui, H.N.; Pham, T.K.N.; Ngo, T.T.H.; Bui, H.M. Lipid production combined with removal and bioaccumulation of Pb by Scenedesmus sp. Green Alga. Pol. J. Environ. Stud. 2020, 29, 1785–1791. [Google Scholar] [CrossRef]
- Sumranwanich, T.; Boonthaworn, K.; Singh, A. The roles of plant cell wall as the first-line protection against lead (Pb) toxicity. Appl. Sci. Eng. Prog. 2018, 11, 239–245. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Xie, X.; Wu, Y.; Liang, F.; Tang, M. Arbuscular mycorrhizal fungi promote lead immobilization by increasing the polysaccharide content within pectin and inducing cell wall peroxidase activity. Chemosphere 2021, 267, 128924. [Google Scholar] [CrossRef]
- Chudzik, B.; Szczuka, E.; Leszczuk, A.; Strubińska, J. Modification of pectin distribution in sunflower (Helianthus annuus L.) roots in response to lead exposure. Environ. Exp. Bot. 2018, 155, 251–259. [Google Scholar] [CrossRef]
- Domozych, D.; Kort, S.; Benton, S.; Yu, T. The extracellular polymeric substance of the green alga Penium margaritaceum and its role in biofilm formation. Biofilms 2005, 2, 129–144. [Google Scholar] [CrossRef]
- Domozych, D.; Serfis, A.; Kiemle, S.; Gretz, M. The structure and biochemistry of charophycean cell walls: I. Pectins of Penium margaritaceum. Protoplasma 2007, 230, 99–115. [Google Scholar] [CrossRef]
- Domozych, D.S.; Sørensen, I.; Popper, Z.A.; Ochs, J.; Andreas, A.; Fangel, J.U.; Pielach, A.; Sacks, C.; Brechka, H.; Ruisi-Besares, P. Pectin metabolism and assembly in the cell wall of the charophyte green alga Penium margaritaceum. Plant Physiol. 2014, 165, 105–118. [Google Scholar] [CrossRef]
- Mohammed, C.; Mahabir, S.; Mohammed, K.; John, N.; Lee, K.-Y.; Ward, K. Calcium alginate thin films derived from Sargassum natans for the selective adsorption of Cd2+, Cu2+, and Pb2+ ions. Ind. Eng. Chem. Res. 2018, 58, 1417–1425. [Google Scholar] [CrossRef]
- Mousa, N.E.; Simonescu, C.M.; Pătescu, R.-E.; Onose, C.; Tardei, C.; Culiţă, D.C.; Oprea, O.; Patroi, D.; Lavric, V. Pb2+ removal from aqueous synthetic solutions by calcium alginate and chitosan coated calcium alginate. React. Funct. Polym. 2016, 109, 137–150. [Google Scholar] [CrossRef]
- Hasan, M.N.; Salman, M.S.; Islam, A.; Znad, H.; Hasan, M.M. Sustainable composite sensor material for optical cadmium (II) monitoring and capturing from wastewater. Microchem. J. 2021, 161, 105800. [Google Scholar] [CrossRef]

| Immobilization | Filter Paper (mg) * | Flask Bottom (mg) * | OD600 at 0 h | OD600 at 8 h |
|---|---|---|---|---|
| NIES-217 | 9.5 ± 1.1 | 1.6 ± 0.7 | 0.018 ± 0.002 | 0.016 ± 0.001 |
| NIES-303 | 17.5 ± 3.0 | 0.4 ± 0.4 | 0.016 ± 0.001 | 0.016 ± 0.002 |
| Immobilization | Time | Solution (µg) * | Filter Paper (µg) * | Flask Bottom (µg) * | Recovery (µg) * | Recovery % |
|---|---|---|---|---|---|---|
| NIES-217 | 0 h | 90.9 ± 1.4 | – | – | – | – |
| 8 h | 41.0 ± 11.6 | 32.7 ± 1.8 | 22.7 ± 6.4 | 96.3 ± 16.0 | 96.3 | |
| NIES-303 | 0 h | 92.0 ± 2.1 | – | – | – | – |
| 8 h | 46.7 ± 10.0 | 31.8 ± 6.4 | 20.4 ± 15.6 | 98.9 ± 6.1 | 98.9 | |
| No alga | 0 h | 96.9 ± 5.8 | – | – | – | – |
| 8 h | 96.2 ± 1.2 | 0.2 ± 0.4 | ND | 96.4 ± 2.7 | 96.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byamba, T.; Hasegawa, K.; Maeda, I. Removal of Pb (II) from Aqueous Solution by a Pectin-Producing Alga, Penium margaritaceum, Immobilized on Filter Paper. Microbiol. Res. 2022, 13, 1007-1017. https://doi.org/10.3390/microbiolres13040073
Byamba T, Hasegawa K, Maeda I. Removal of Pb (II) from Aqueous Solution by a Pectin-Producing Alga, Penium margaritaceum, Immobilized on Filter Paper. Microbiology Research. 2022; 13(4):1007-1017. https://doi.org/10.3390/microbiolres13040073
Chicago/Turabian StyleByamba, Tsogjargal, Kazutoshi Hasegawa, and Isamu Maeda. 2022. "Removal of Pb (II) from Aqueous Solution by a Pectin-Producing Alga, Penium margaritaceum, Immobilized on Filter Paper" Microbiology Research 13, no. 4: 1007-1017. https://doi.org/10.3390/microbiolres13040073
APA StyleByamba, T., Hasegawa, K., & Maeda, I. (2022). Removal of Pb (II) from Aqueous Solution by a Pectin-Producing Alga, Penium margaritaceum, Immobilized on Filter Paper. Microbiology Research, 13(4), 1007-1017. https://doi.org/10.3390/microbiolres13040073






