Effects of Fructooligosaccharides (FOS) on the Immune Response of the Shrimp Penaeus vannamei and on the Reduction in Vibrio spp. and Pseudomonas spp. in Cultures of Post-Larvae
Abstract
1. Introduction
2. Materials and Methods
2.1. Location
2.2. Experimental Design
2.3. Culture Parameters
2.4. Shrimp Sample Collection and Preservation
2.5. Evaluation of the Quality of Post-Larvae
2.6. Processing of Samples
2.7. Evaluation of Immune Response Parameters
2.7.1. Phenoloxidase-Specific Enzymatic Activity
2.7.2. Superoxide Dismutase-Specific Enzymatic Activity
2.7.3. Peroxidase-Specific Enzymatic Activity
2.7.4. Lysozyme-Specific Enzymatic Activity
2.7.5. Lectin Expression
2.7.6. Bacteriological Analysis
2.8. Statistical Analysis
3. Results and Discussion
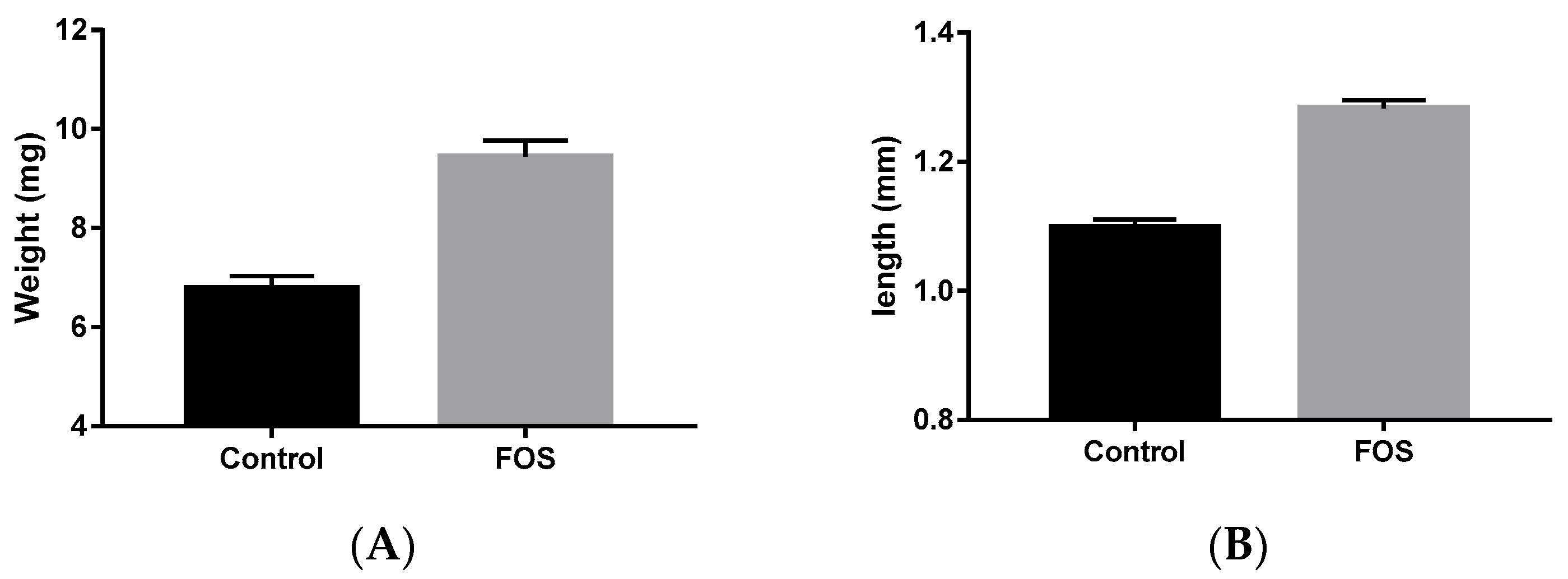
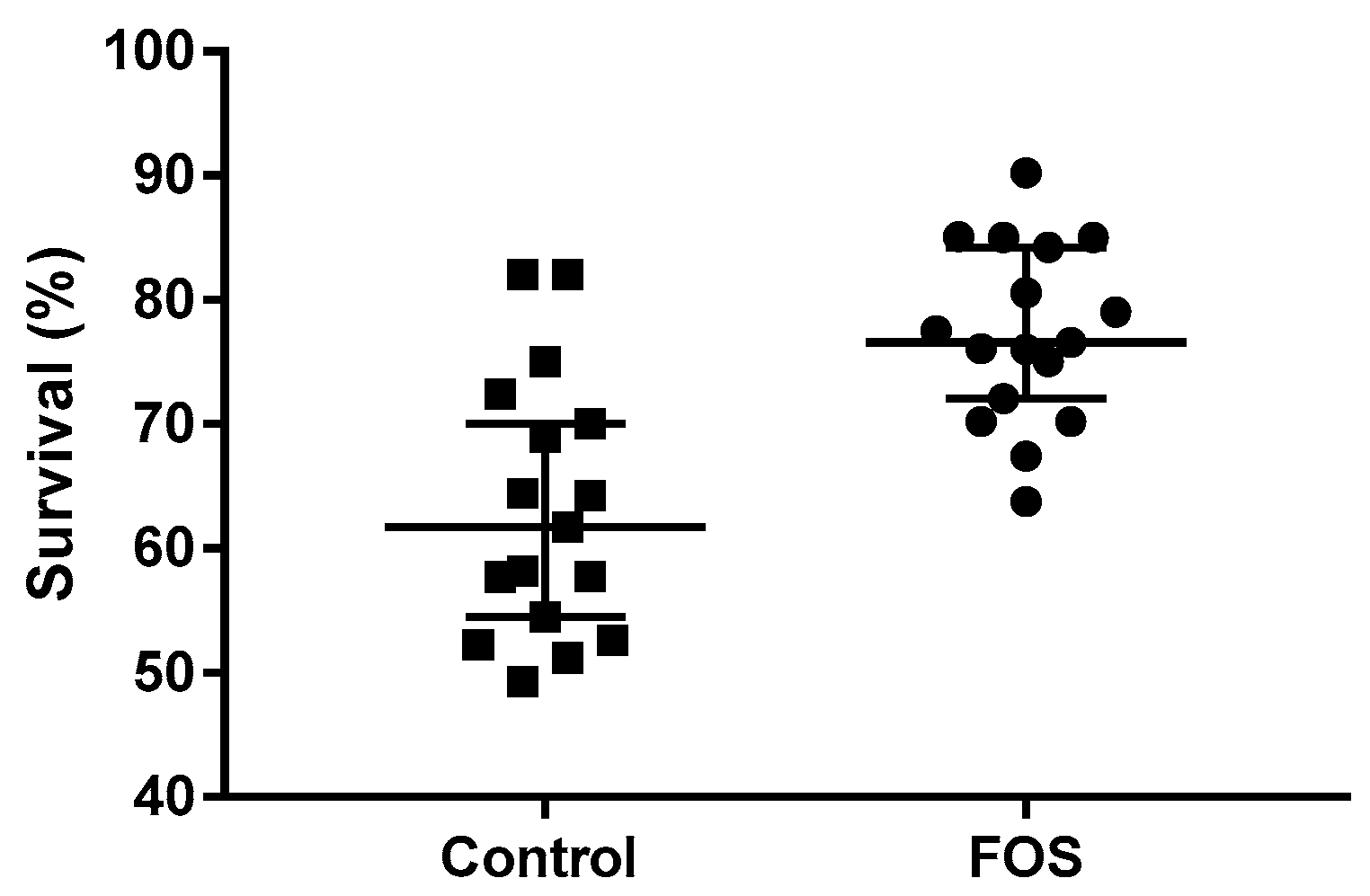
3.1. Length, Weight, and Survival
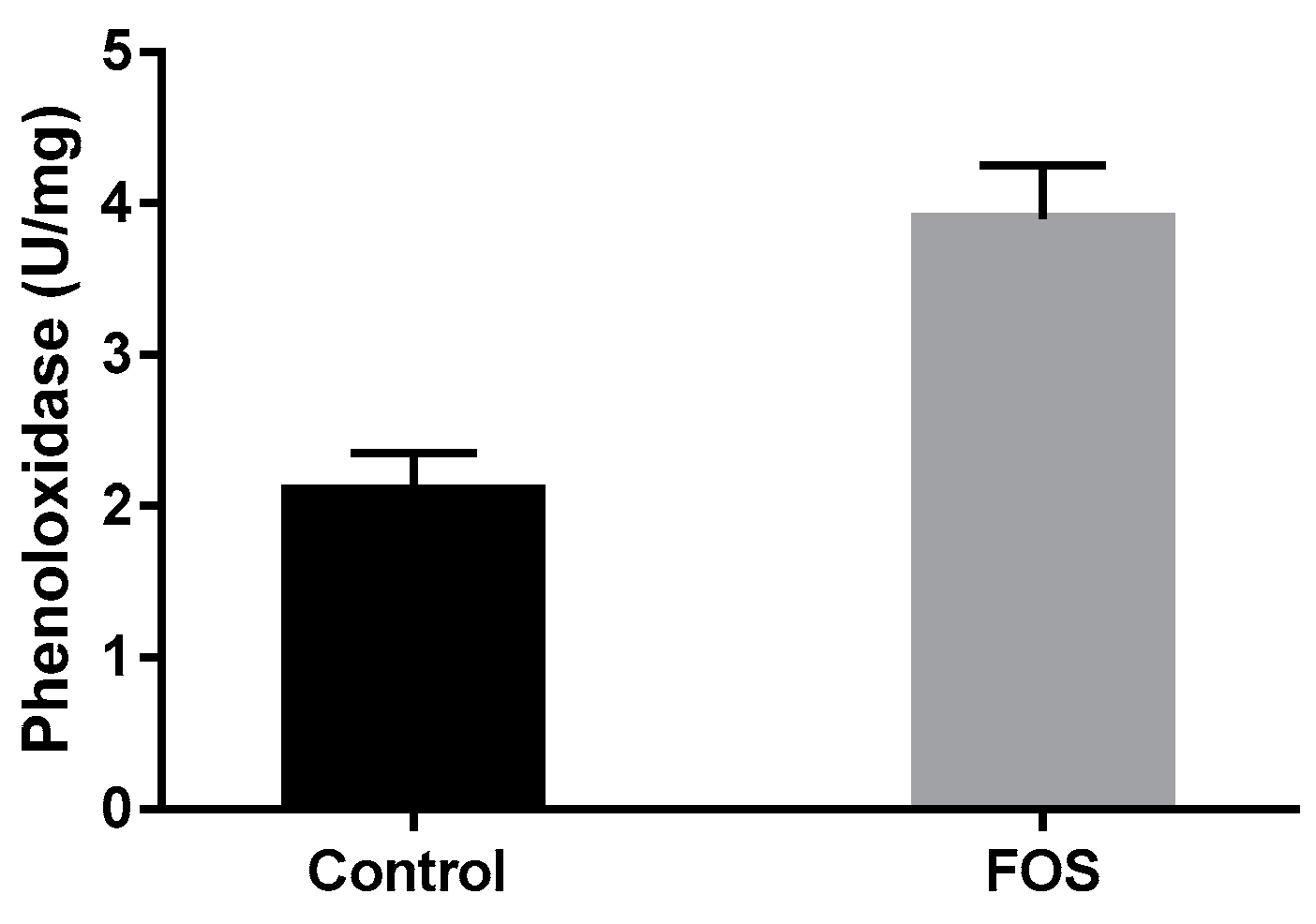
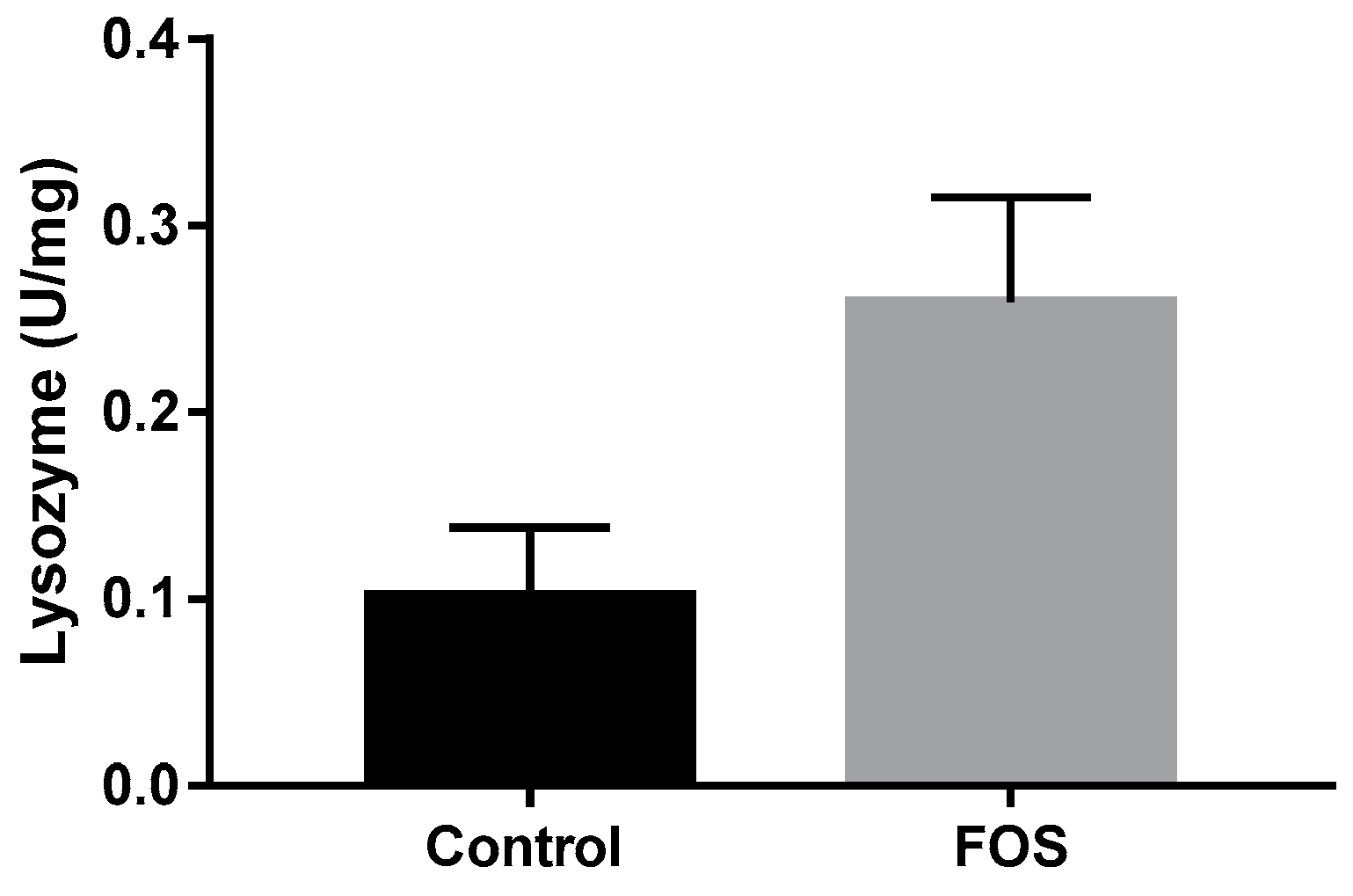
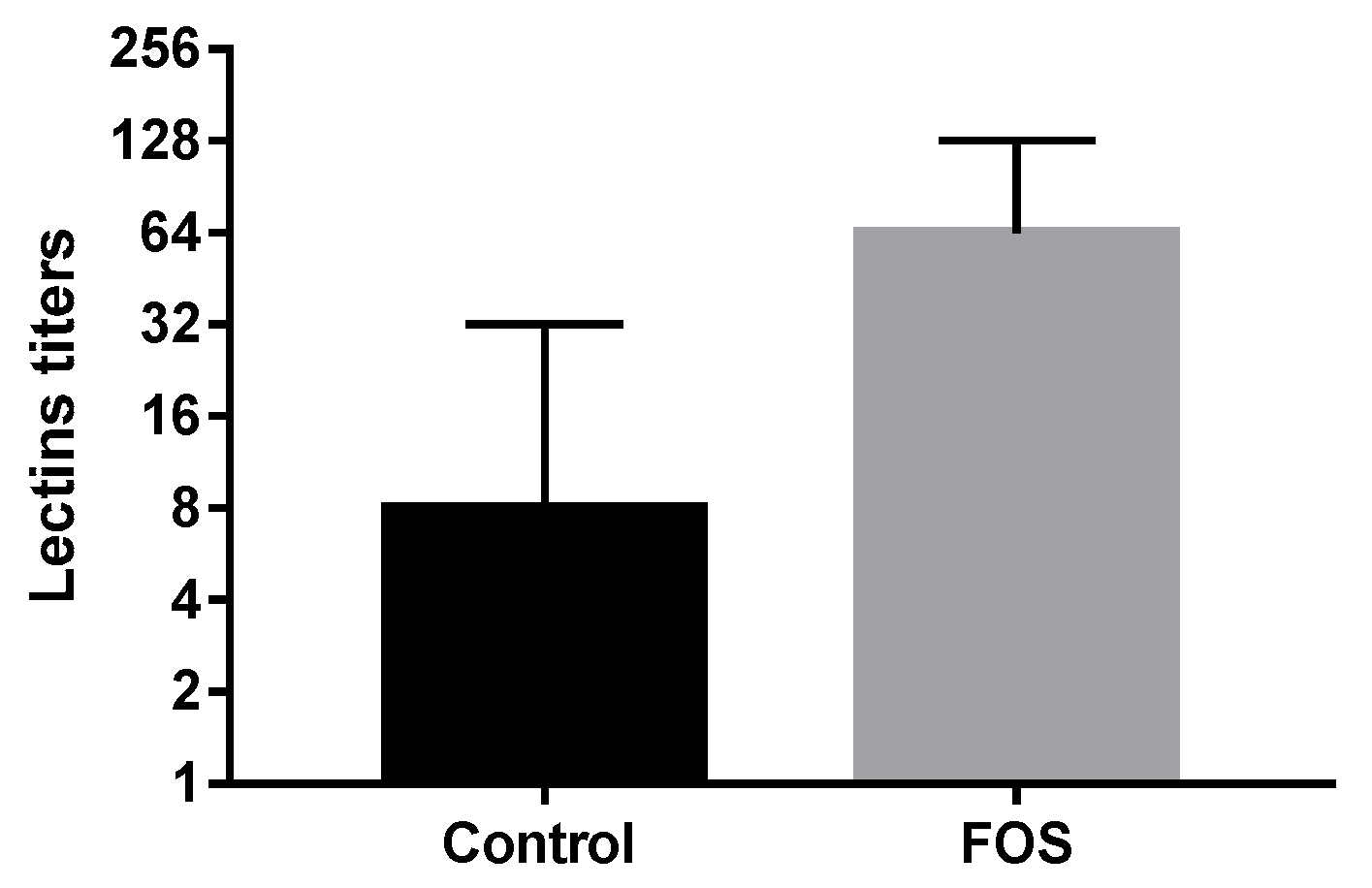
3.2. Innate Immune System
3.3. Protease and Amylase-Specific Enzymatic Activity
3.4. Bacteriological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel-Tawwab, M. Feed Supplementation to Freshwater Fish: Experimental Approaches; LAP Lambert Academic Publishing: Saarbrücken, Germany, 2016. [Google Scholar]
- Aguirre-Guzman, G.; Sanchez-Martinez, J.G.; Campa-Cordova, A.I.; Luna-Gonzalez, A.; Ascencio, F. Penaeid shrimp immune system. Thai J. Vet. Med. 2009, 39, 205–215. [Google Scholar]
- Akhter, N.; Wu, B.; Memon, A.M.; Mohsin, M. Probiotics and prebiotics associated with aquaculture: A review. Fish Shellfish Immunol. 2015, 45, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Anadón, A.; Ares, I.; Martínez-Larrañaga, M.R.; Martínez, M.A. Prebiotics and probiotics in feed and animal health. In Nutraceuticals in Veterinary Medicine; Springer: Berlin/Heidelberg, Germany, 2019; pp. 261–285. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Bi, J.; Ning, M.; Xie, X.; Fan, W.; Huang, Y.; Gu, W.; Wang, W.; Wang, L.; Meng, Q. A typical C-type lectin, perlucin-like protein, is involved in the innate immune defense of whiteleg shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2020, 103, 293–301. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Burge, E.J.; Madigan, D.J.; Burnett, L.E.; Burnett, K.G. Lysozyme gene expression by hemocytes of Pacific white shrimp, Litopenaeus vannamei, after injection with Vibrio. Fish Shellfish Immunol. 2007, 22, 327–339. [Google Scholar] [CrossRef]
- Burr, G.; Gatlin, D.M., III; Hume, M. Effects of the prebiotics GroBiotic®-A and inulin on the intestinal microbiota of red drum, Sciaenops ocellatus. J. World Aquac. Soc. 2009, 40, 440–449. [Google Scholar] [CrossRef]
- Bush, S.R.; Belton, B.; Little, D.C.; Islam, M.S. Emerging trends in aquaculture value chain research. Aquaculture 2019, 498, 428–434. [Google Scholar] [CrossRef]
- Campa-Córdova, A.; Hernández-Saavedra, N.; Aguirre-Guzmán, G.; Ascencio, F. Immunomodulatory response of superoxide dismutase in juvenile American white shrimp (Litopenaeus vannamei) exposed to immunostimulants. Cienc. Mar. 2005, 31, 661–669. [Google Scholar] [CrossRef]
- Cerenius, L.; Söderhäll, K. Immune properties of invertebrate phenoloxidases. Dev. Comp. Immunol. 2021, 122, 104098. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Kumar, R.; Ng, T.H.; Wang, H.-C. What vaccination studies tell us about immunological memory within the innate immune system of cultured shrimp and crayfish. Dev. Comp. Immunol. 2018, 80, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Romano, N.; Ebrahimi, M.; Natrah, I. The effects of dietary fructooligosaccharide on growth, intestinal short chain fatty acids level and hepatopancreatic condition of the giant freshwater prawn (Macrobrachium rosenbergii) post-larvae. Aquaculture 2017, 100, 95–101. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Chen, J.-C.; Tseng, K.-C.; Lin, Y.-C.; Huang, C.-L. Activation of immunity, immune response, antioxidant ability, and resistance against Vibrio alginolyticus in white shrimp Litopenaeus vannamei decrease under long-term culture at low pH. Fish Shellfish Immunol. 2015, 46, 192–199. [Google Scholar] [CrossRef]
- De-La-Re-Vega, E.; García-Galaz, A.; Díaz-Cinco, M.E.; Sotelo-Mundo, R.R. White shrimp (Litopenaeus vannamei) recombinant lysozyme has antibacterial activity against Gram negative bacteria: Vibrio alginolyticus, Vibrio parahemolyticus and Vibrio cholerae. Fish Shellfish Immunol. 2006, 20, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Denis, M.; Thayappan, K.; Ramasamy, S.; Munusamy, A. Lectin in innate immunity of Crustacea. Austin Biol 2016, 1, 1001. [Google Scholar]
- Dimitroglou, A.; Merrifield, D.L.; Spring, P.; Sweetman, J.; Moate, R.; Davies, S.J. Effects of mannan oligosaccharide (MOS) supplementation on growth performance, feed utilisation, intestinal histology and gut microbiota of gilthead sea bream (Sparus aurata). Aquaculture 2010, 300, 182–188. [Google Scholar] [CrossRef]
- Divya, M.; Vaseeharan, B.; Anjugam, M.; Iswarya, A.; Karthikeyan, S.; Velusamy, P.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; et al. Phenoloxidase activation, antimicrobial, and antibiofilm properties of β-glucan binding protein from Scylla serrata crab hemolymph. Int. J. Biol. Macromol. 2018, 114, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wang, J. Immunostimulatory effects of dietary fructooligosaccharides on red swamp crayfish, Procambarus clarkii (Girard). Aquac. Res. 2013, 44, 1416–1424. [Google Scholar] [CrossRef]
- Elshopakey, G.E.; Risha, E.F.; Abdalla, O.A.; Okamura, Y.; Hanh, V.D.; Ibuki, M.; Sudhakaran, R.; Itami, T. Enhancement of immune response and resistance against Vibrio parahaemolyticus in kuruma shrimp (Marsupenaeus japonicus) by dietary supplementation of β-1, 4-mannobiose. Fish Shellfish Immunol. 2018, 74, 26–34. [Google Scholar] [CrossRef]
- Fan, J.; Li, B.; Hong, Q.; Yan, Z.; Yang, X.; Lu, K.; Chen, G.; Wang, L.; Chen, Y. A glutathione peroxidase gene from Litopenaeus vannamei is involved in oxidative stress responses and pathogen infection resistance. Int. J. Mol. Sci. 2022, 23, 567. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Franco, R.; Martín, L.; Arenal, A.; Santiesteban, D.; Sotolongo, J.; Cabrera, H.; Mejías, J.; Rodríguez, G.; Moreno, A.G.; Pimentel, E.; et al. Evaluation of two probiotics used during farm production of white shrimp Litopenaeus vannamei (Crustacea: Decapoda). Aquac. Res. 2017, 48, 1936–1950. [Google Scholar] [CrossRef]
- Fuandila, N.N.; Widanarni, W.; Yuhana, M. Growth performance and immune response of prebiotic honey fed pacific white shrimp Litopenaeus vannamei to Vibrio parahaemolyticus infection. J. Appl. Aquac. 2020, 32, 221–235. [Google Scholar] [CrossRef]
- García-Carreño, F.L.; Cota, K.; Navarrete Del Toro, M.A. Phenoloxidase activity of hemocyanin in whiteleg shrimp Penaeus vannamei: Conversion, characterization of catalytic properties, and role in postmortem melanosis. J. Agric. Food Chem. 2008, 56, 6454–6459. [Google Scholar] [CrossRef] [PubMed]
- Genc, M.; Aktas, M.; Genc, E.; Yilmaz, E. Effects of dietary mannan oligosaccharide on growth, body composition and hepatopancreas histology of Penaeus semisulcatus (de Haan 1844). Aquac. Nutr. 2007, 13, 156–161. [Google Scholar] [CrossRef]
- Hasyimi, W.; Widanarni, W.; Yuhana, M. Growth Performance and Intestinal Microbiota Diversity in Pacific White Shrimp Litopenaeus vannamei Fed with a Probiotic Bacterium, Honey Prebiotic, and Synbiotic. Curr. Microbiol. 2020, 77, 2982–2990. [Google Scholar] [CrossRef]
- Hernández-López, J.; Gollas-Galván, T.; Vargas-Albores, F. Activation of the prophenoloxidase system of the brown shrimp Penaeus californiensis Holmes. Comp. Biochem. Physiol. Part C Pharmacol. 1996 Toxicol. Endocrinol. 1996, 113, 61–66. [Google Scholar] [CrossRef]
- Hu, F.; Wang, Y.; Hu, J.; Bao, Z.; Wang, M. A novel c-type lysozyme from Litopenaeus vannamei exhibits potent antimicrobial activity. Fish Shellfish Immunol. 2022, 131, 729–735. [Google Scholar] [CrossRef]
- Hu, X.; Yang, H.L.; Yan, Y.Y.; Zhang, C.X.; Ye, J.D.; Lu, K.L.; Hu, L.H.; Zhang, J.J.; Ruan, L.; Sun, Y.Z. Effects of fructooligosaccharide on growth, immunity and intestinal microbiota of shrimp (Litopenaeus vannamei) fed diets with fish meal partially replaced by soybean meal. Aquac. Nutr. 2019, 25, 194–204. [Google Scholar] [CrossRef]
- Li, H.; Parisi, M.-G.; Toubiana, M.; Cammarata, M.; Roch, P. Lysozyme gene expression and hemocyte behaviour in the Mediterranean mussel, Mytilus galloprovincialis, after injection of various bacteria or temperature stresses. Fish Shellfish Immunol. 2008, 25, 143–152. [Google Scholar] [CrossRef]
- Li, P.; Burr, G.S.; Gatlin, D.M., III; Hume, M.E.; Patnaik, S.; Castille, F.L.; Lawrence, A.L. Dietary supplementation of short-chain fructooligosaccharides influences gastrointestinal microbiota composition and immunity characteristics of Pacific white shrimp, Litopenaeus vannamei, cultured in a recirculating system. J. Nutr. 2007, 137, 2763–2768. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lee, F.F.; Wu, C.-L.; Chen, J.-C. Molecular cloning and characterization of a cytosolic manganese superoxide dismutase (cytMnSOD) and mitochondrial manganese superoxide dismutase (mtMnSOD) from the kuruma shrimp Marsupenaeus japonicus. Fish Shellfish Immunol. 2010, 28, 143–150. [Google Scholar] [CrossRef]
- Liu, C.-H.; Tseng, M.-C.; Cheng, W. Identification and cloning of the antioxidant enzyme, glutathione peroxidase, of white shrimp, Litopenaeus vannamei, and its expression following Vibrio alginolyticus infection. Fish Shellfish Immunol. 2007, 23, 34–45. [Google Scholar] [CrossRef]
- Lu, X.; Wang, C.; Liu, B. The role of Cu/Zn-SOD and Mn-SOD in the immune response to oxidative stress and pathogen challenge in the clam Meretrix meretrix. Fish Shellfish Immunol. 2015, 42, 58–65. [Google Scholar] [CrossRef]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: A review of the top 15 major producers. Rev. Aquac. 2020, 12, 640–663. [Google Scholar] [CrossRef]
- Mahious, A.S.; Gatesoupe, F.J.; Hervi, M.; Metailler, R.; Ollevier, F. Effect of dietary inulin and oligosaccharides as prebiotics for weaning turbot, Psetta maxima (Linnaeus, C. 1758). Aquac. Int. 2006, 14, 219–229. [Google Scholar] [CrossRef]
- Martín, L.; Castillo, N.M.; Arenal, A.; Rodríguez, G.; Franco, R.; Santiesteban, D.; Sotolongo, J.; Forrellat, A.; Espinosa, G.; Carrillo, O.; et al. Ontogenetic changes of innate immune parameters from eggs to early postlarvae of white shrimp Litopenaeus vannamei (Crustacea: Decapoda). Aquaculture 2012, 358, 234–239. [Google Scholar] [CrossRef]
- Martínez, R.; Carpio, Y.; Arenal, A.; Lugo, J.M.; Morales, R.; Martín, L.; Rodríguez, R.F.; Acosta, J.; Morales, A.; Duconge, J.; et al. Significant improvement of shrimp growth performance by growth hormone-releasing peptide-6 immersion treatments. Aquac. Res. 2017, 48, 4632–4645. [Google Scholar] [CrossRef]
- Martinez, R.; Carpio, Y.; Morales, A.; Lugo, J.M.; Herrera, F.; Zaldívar, C.; Carrillo, O.; Arenal, A.; Pimentel, E.; Estrada, M.P. Oral administration of the growth hormone secretagogue-6 (GHRP-6) enhances growth and non-specific immune responses in tilapia (Oreochromis sp.). Aquaculture 2016, 452, 304–310. [Google Scholar] [CrossRef]
- Mastan, S. Use of immunostimulants in aquaculture disease management. Int. J. Fish. Aquat. Stud. 2015, 2, 277–280. [Google Scholar]
- Mustafa, A.; Buentello, A.; Gatlin, D.; Lightner, D.; Hume, M.; Lawrence, A. Dietary supplementation of galactooligosaccharides (GOS) in Pacific white shrimp, Litopenaeus vannamei, cultured in a recirculating system and its effects on gut microflora, growth, stress, and immune response. J. Immunoass. Immunochem. 2019, 40, 662–675. [Google Scholar] [CrossRef]
- Mustafa, A.; Buentello, A.; Gatlin, D., III; Lightner, D.; Hume, M.; Lawrence, A. Effects of fructooligosaccharides (FOS) on growth, survival, gut microflora, stress, and immune response in Pacific white shrimp, Litopenaeus vannamei, cultured in a recirculating system. J. Immunoass. Immunochem. 2020, 41, 45–59. [Google Scholar] [CrossRef]
- Reznichenko, A.; Reznichenko, L.; Dorozhkin, V.; Noskov, S.; Vodianitskaia, S. Prospects of the use of prebiotics in broiler poultry farming as an alternative to antibiotics. In Proceedings of the International Scientific-Practical Conference “Agriculture and Food Security: Technology, Innovation, Markets, Human Resources” (FIES 2021), Kazan, Russia, 26–28 May 2021. [Google Scholar]
- Ringø, E.; Olsen, R.; Gifstad, T.; Dalmo, R.; Amlund, H.; Hemre, G.-I.; Bakke, A. Prebiotics in aquaculture: A review. Aquac. Nutr. 2010, 16, 117–136. [Google Scholar] [CrossRef]
- Ringø, E.; Song, S. Application of dietary supplements (synbiotics and probiotics in combination with plant products and β-glucans) in aquaculture. Aquac. Nutr. 2016, 22, 4–24. [Google Scholar] [CrossRef]
- Ríos, L.D.M.; Barrios, Y.C.; Salotén, G.; Olimpia, M.C.F.; Alarcón, H.C.; Arenal, A. Principales factores que modifican el sistema inmune en camarones peneidos estrategias para un cultivo sostenible. Rev. Prod. Anim. 2022, 34, 103–126. [Google Scholar]
- Safari, O.; Shahsavani, D.; Paolucci, M.; Atash, M.M.S. Single or combined effects of fructo-and mannan oligosaccharide supplements on the growth performance, nutrient digestibility, immune responses and stress resistance of juvenile narrow clawed crayfish, Astacus leptodactylus leptodactylus Eschscholtz, 1823. Aquaculture 2014, 432, 192–203. [Google Scholar] [CrossRef]
- Salze, G.; McLean, E.; Schwarz, M.; Craig, S. Dietary mannan oligosaccharide enhances salinity tolerance and gut development of larval cobia. Aquaculture 2008, 274, 148–152. [Google Scholar] [CrossRef]
- Song, S.K.; Beck, B.R.; Kim, D.; Park, J.; Kim, J.; Kim, H.D.; Ringø, E. Prebiotics as immunostimulants in aquaculture: A review. Fish Shellfish Immunol. 2014, 40, 40–48. [Google Scholar] [CrossRef]
- Sritunyalucksana, K.; Söderhäll, K. The proPO and clotting system in crustaceans. Aquaculture 2000, 191, 53–70. [Google Scholar] [CrossRef]
- Varela-Granados, Y.; Frías-Gómez, S.A.; Hernández-Hernández, L.H.; Powell, M.S.; Vega-Villasante, F. Effects of mannan oligosaccharides and fructooligosaccharides on the growth and nonspecific immune responses of juvenile freshwater prawn Macrobrachium acanthurus. Lat. Am. J. Aquat. Res. 2021, 49, 299–306. [Google Scholar] [CrossRef]
- Vaseeharan, B.; Thaya, R. Medicinal plant derivatives as immunostimulants: An alternative to chemotherapeutics and antibiotics in aquaculture. Aquac. Int. 2014, 22, 1079–1091. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.-N.; Liu, Y.; Cai, D.-X.; Li, J.-Z.; Wang, A.-L. Two types of ATPases from the Pacific white shrimp, Litopenaeus vannamei in response to environmental stress. Mol. Biol. Rep. 2012, 39, 6427–6438. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-N.; Zhou, J.; Wang, P.; Tian, T.-T.; Zheng, Y.; Liu, Y.; Mai, W.-J.; Wang, A.-L. Oxidative stress, DNA damage and antioxidant enzyme gene expression in the Pacific white shrimp, Litopenaeus vannamei when exposed to acute pH stress. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, J.; Liu, C.; Xue, Z. Application of immunostimulants in aquaculture: Current knowledge and future perspectives. Aquac. Res. 2017, 48, 1–23. [Google Scholar] [CrossRef]
- Wee, W.; Hamid, N.K.A.; Mat, K.; Khalif, R.I.A.R.; Rusli, N.D.; Rahman, M.M.; Kabir, M.A.; Wei, L.S. The effects of mixed prebiotics in aquaculture: A review. Aquac. Fish. 2022. [Google Scholar] [CrossRef]
- Włodarczyk, A.; Wilczek, G.; Wilczek, P.; Student, S.; Ostróżka, A.; Tarnawska, M.; Rost-Roszkowska, M. Relationship between ROS production, MnSOD activation and periods of fasting and re-feeding in freshwater shrimp Neocaridina davidi (Crustacea, Malacostraca). PeerJ 2019, 7, e7399. [Google Scholar] [CrossRef]
- Xiong, J.; Dai, W.; Zhu, J.; Liu, K.; Dong, C.; Qiu, Q. The underlying ecological processes of gut microbiota among cohabitating retarded, overgrown and normal shrimp. Microb. Ecol. 2017, 73, 988–999. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ding, Z.; Huiyuan, L. Effects of dietary short-chain fructooligosaccharides on intestinal microflora, survival, and growth performance of juvenile white shrimp, Litopenaeus vannamei. J. World Aquac. Soc. 2007, 38, 296–301. [Google Scholar] [CrossRef]
- Zhu, F.; Zhang, X. Protection of shrimp against white spot syndrome virus (WSSV) with β-1, 3-D-glucan-encapsulated vp28-siRNA particles. Mar. Biotechnol. 2012, 14, 63–68. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrales Barrios, Y.; Roncarati, A.; Martín Ríos, L.D.; Rodríguez González, M.; González Salotén, M.; López Zaldívar, Y.; Arenal, A. Effects of Fructooligosaccharides (FOS) on the Immune Response of the Shrimp Penaeus vannamei and on the Reduction in Vibrio spp. and Pseudomonas spp. in Cultures of Post-Larvae. Microbiol. Res. 2023, 14, 870-882. https://doi.org/10.3390/microbiolres14030060
Corrales Barrios Y, Roncarati A, Martín Ríos LD, Rodríguez González M, González Salotén M, López Zaldívar Y, Arenal A. Effects of Fructooligosaccharides (FOS) on the Immune Response of the Shrimp Penaeus vannamei and on the Reduction in Vibrio spp. and Pseudomonas spp. in Cultures of Post-Larvae. Microbiology Research. 2023; 14(3):870-882. https://doi.org/10.3390/microbiolres14030060
Chicago/Turabian StyleCorrales Barrios, Yulaine, Alessandra Roncarati, Leonardo Davier Martín Ríos, Maikelis Rodríguez González, Marbelys González Salotén, Yeidel López Zaldívar, and Amilcar Arenal. 2023. "Effects of Fructooligosaccharides (FOS) on the Immune Response of the Shrimp Penaeus vannamei and on the Reduction in Vibrio spp. and Pseudomonas spp. in Cultures of Post-Larvae" Microbiology Research 14, no. 3: 870-882. https://doi.org/10.3390/microbiolres14030060
APA StyleCorrales Barrios, Y., Roncarati, A., Martín Ríos, L. D., Rodríguez González, M., González Salotén, M., López Zaldívar, Y., & Arenal, A. (2023). Effects of Fructooligosaccharides (FOS) on the Immune Response of the Shrimp Penaeus vannamei and on the Reduction in Vibrio spp. and Pseudomonas spp. in Cultures of Post-Larvae. Microbiology Research, 14(3), 870-882. https://doi.org/10.3390/microbiolres14030060





