Bioprospecting of a Thermostable L-Methioninase from Alcaligenes aquatilis BJ-1 in Agro-Industrial Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection, Isolation, and Identification of Microbes
2.2. Rapid Screening of Isolates
2.3. Production of L-Methioninase by Submerged Fermentation
2.4. Extraction and Partial Purification of the Enzyme
2.5. Purification of L-Methioninase by Gel Filtration Chromatography and Molecular Weight Determination by SDS-PAGE
2.6. Enzyme Assay
2.7. Confirmatory Test for L-Methioninase Enzyme
2.8. Estimation of Total Protein Content
2.9. Effect of Biotic and Abiotic Factors
2.10. Antioxidant Activity
2.11. Hemolytic Activity of L-Methioninase
3. Results and Discussion
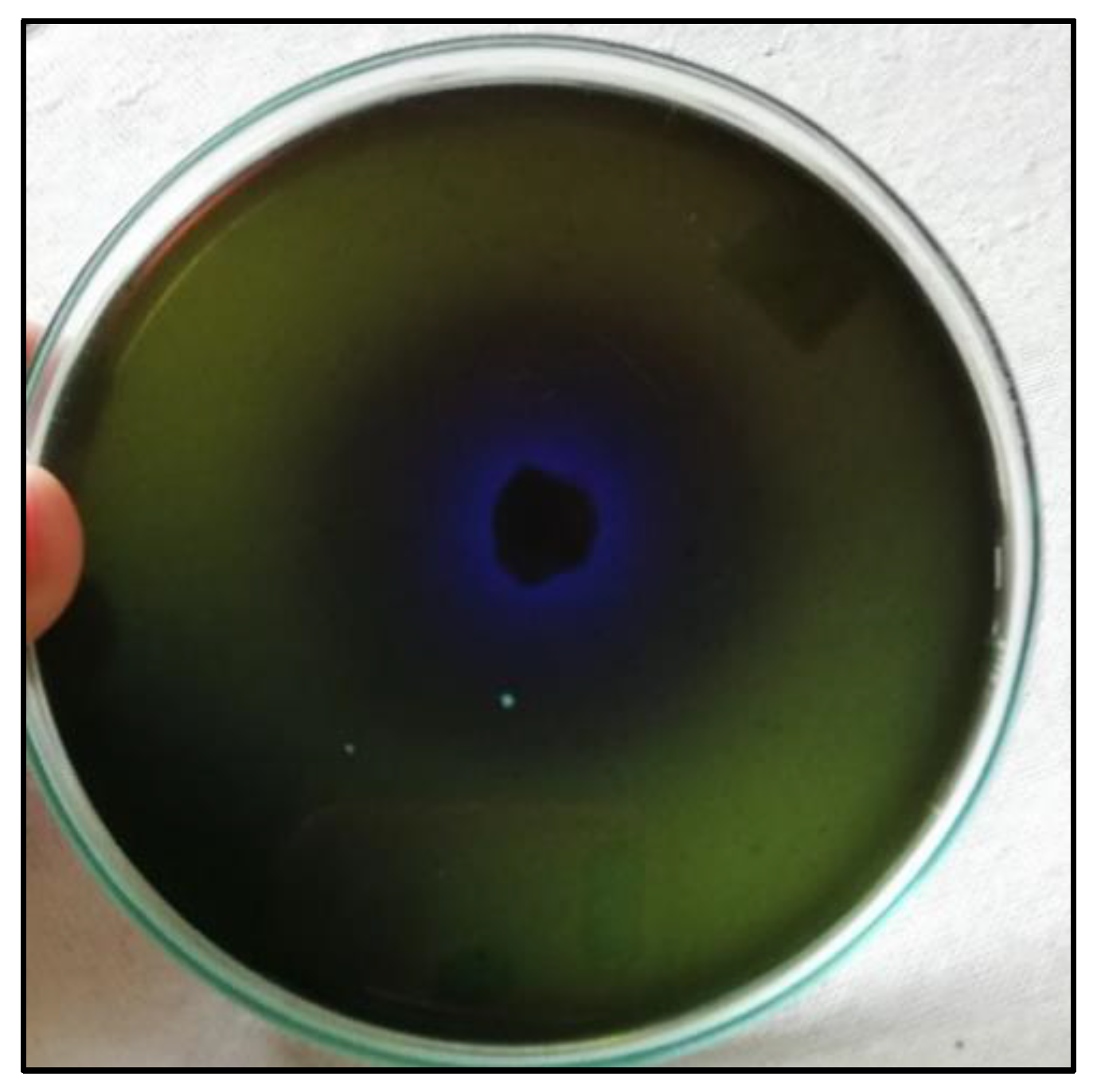
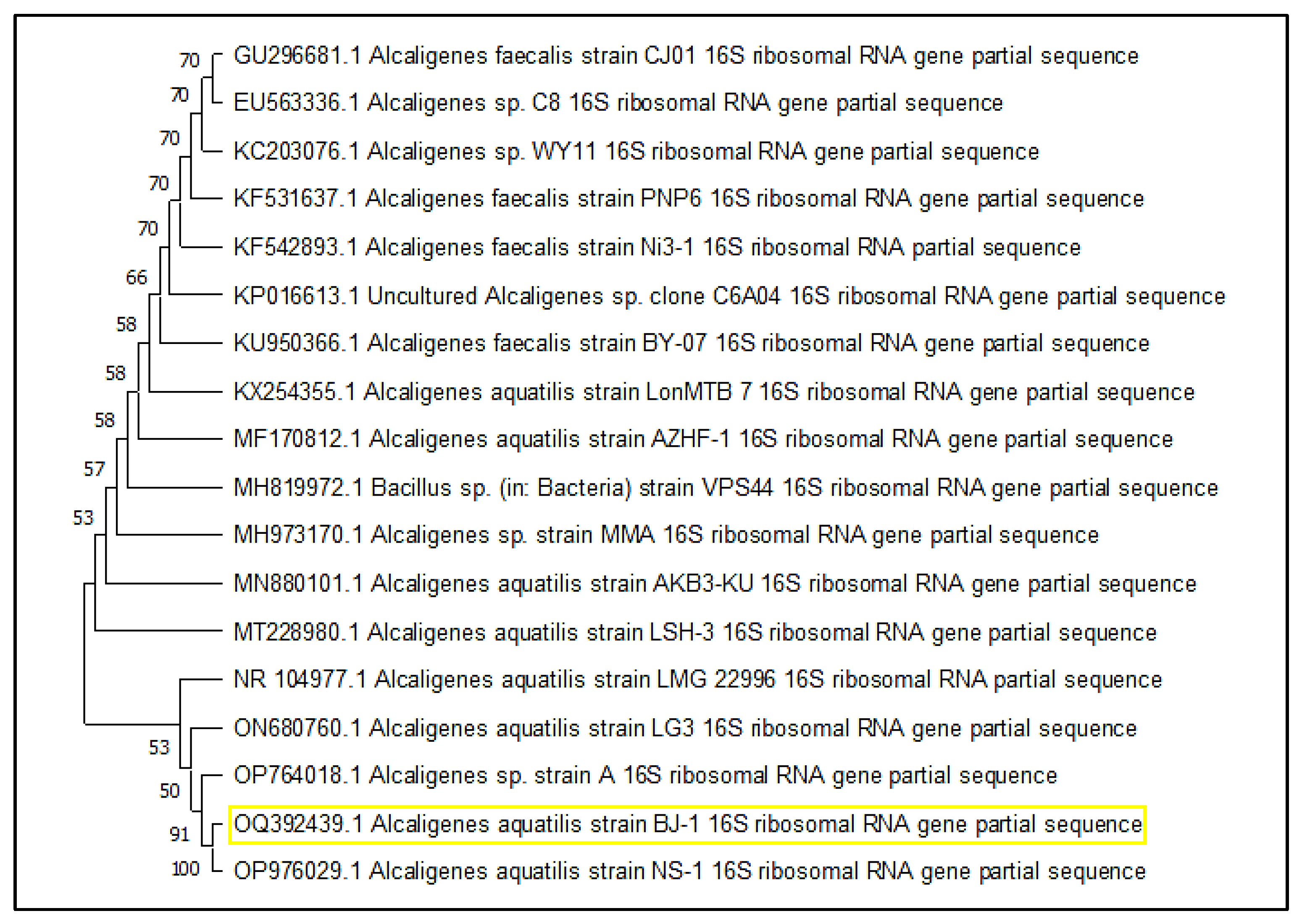
3.1. Isolation, Screening, and Identification of L-Methioninase-Producing Bacteria
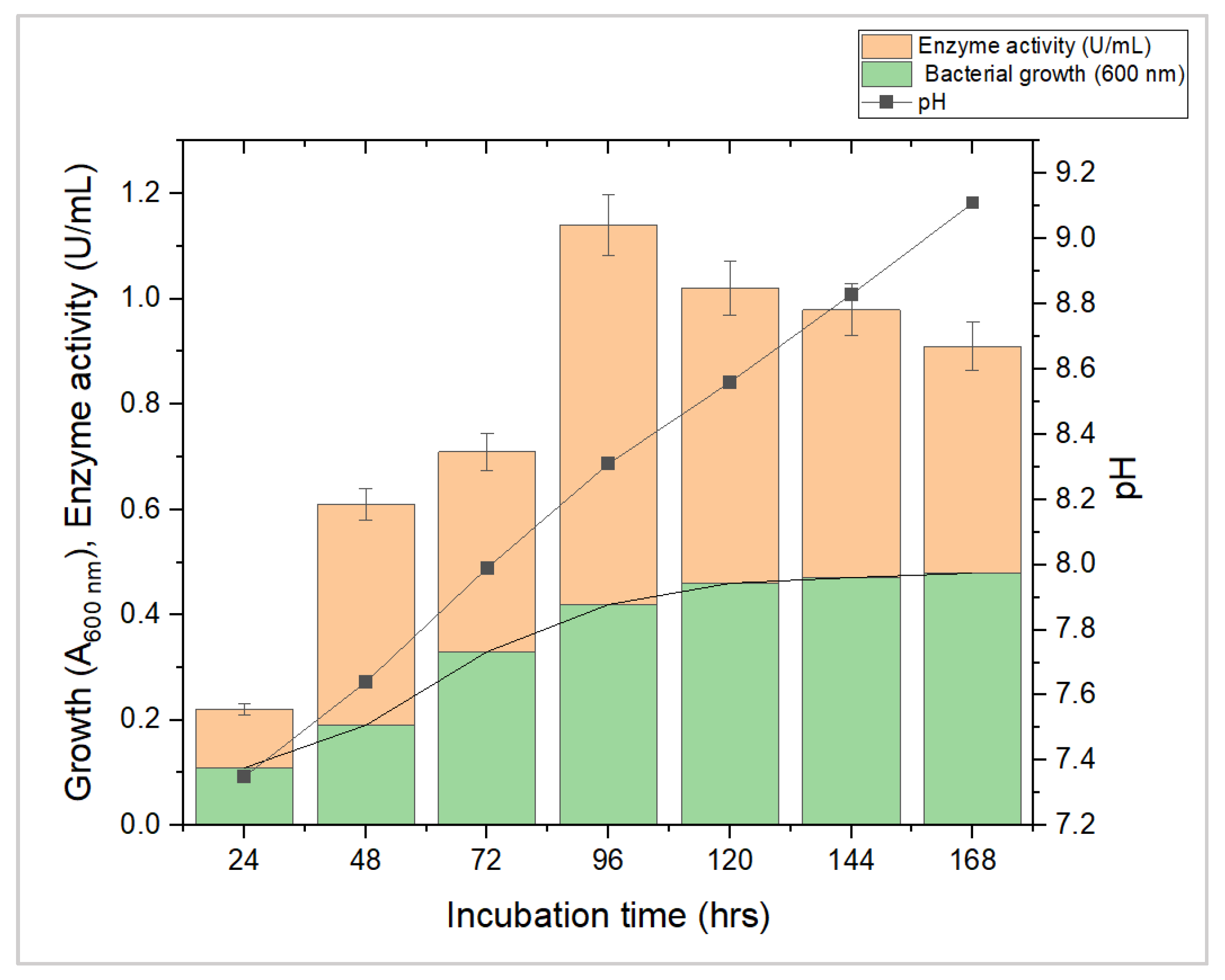
3.2. Production of L-Methioninase by Submerged Fermentation
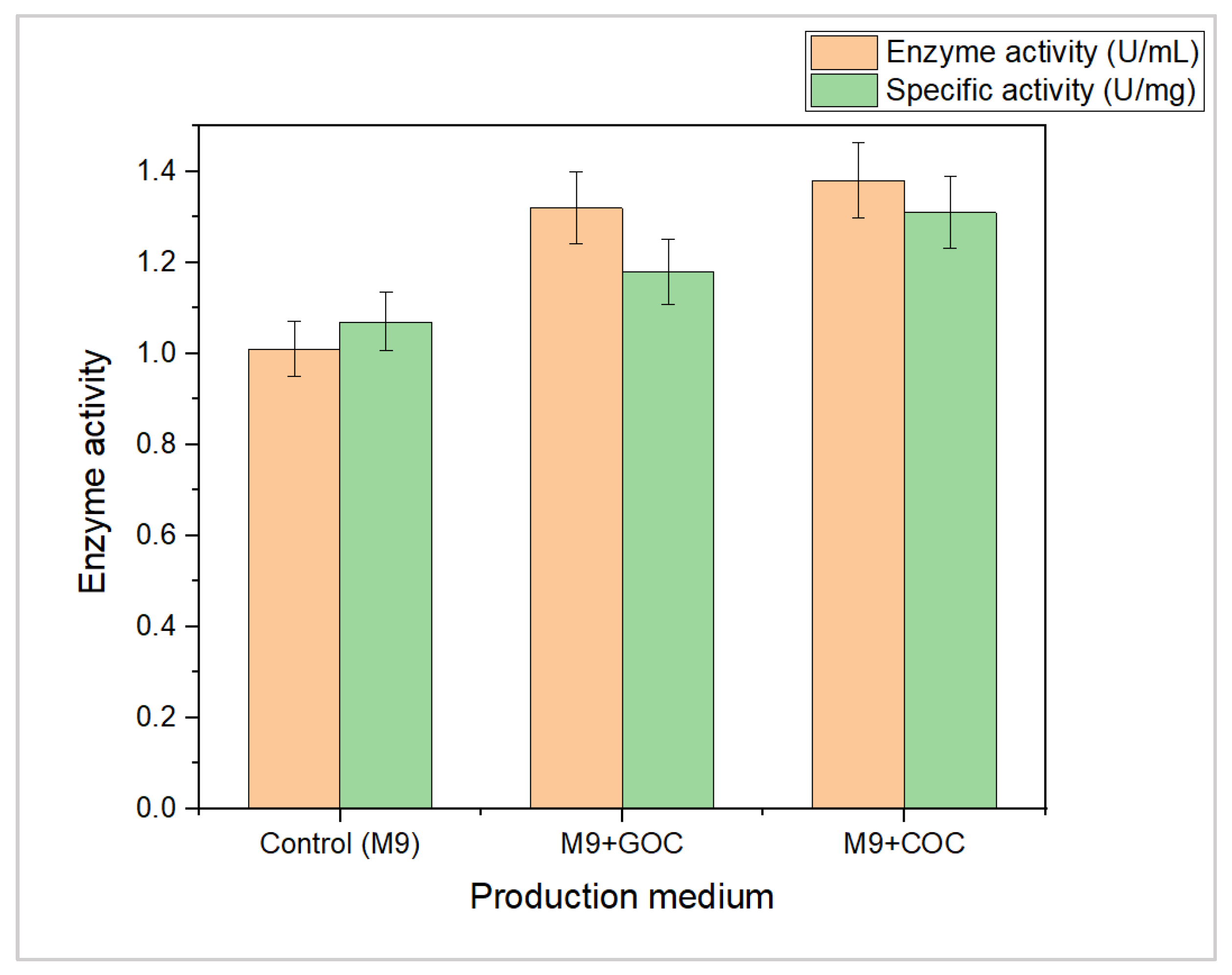
3.3. Production of L-Methioninase by Using Agro-Industrial Waste Material
3.4. Enzyme Extraction and Partial Purification
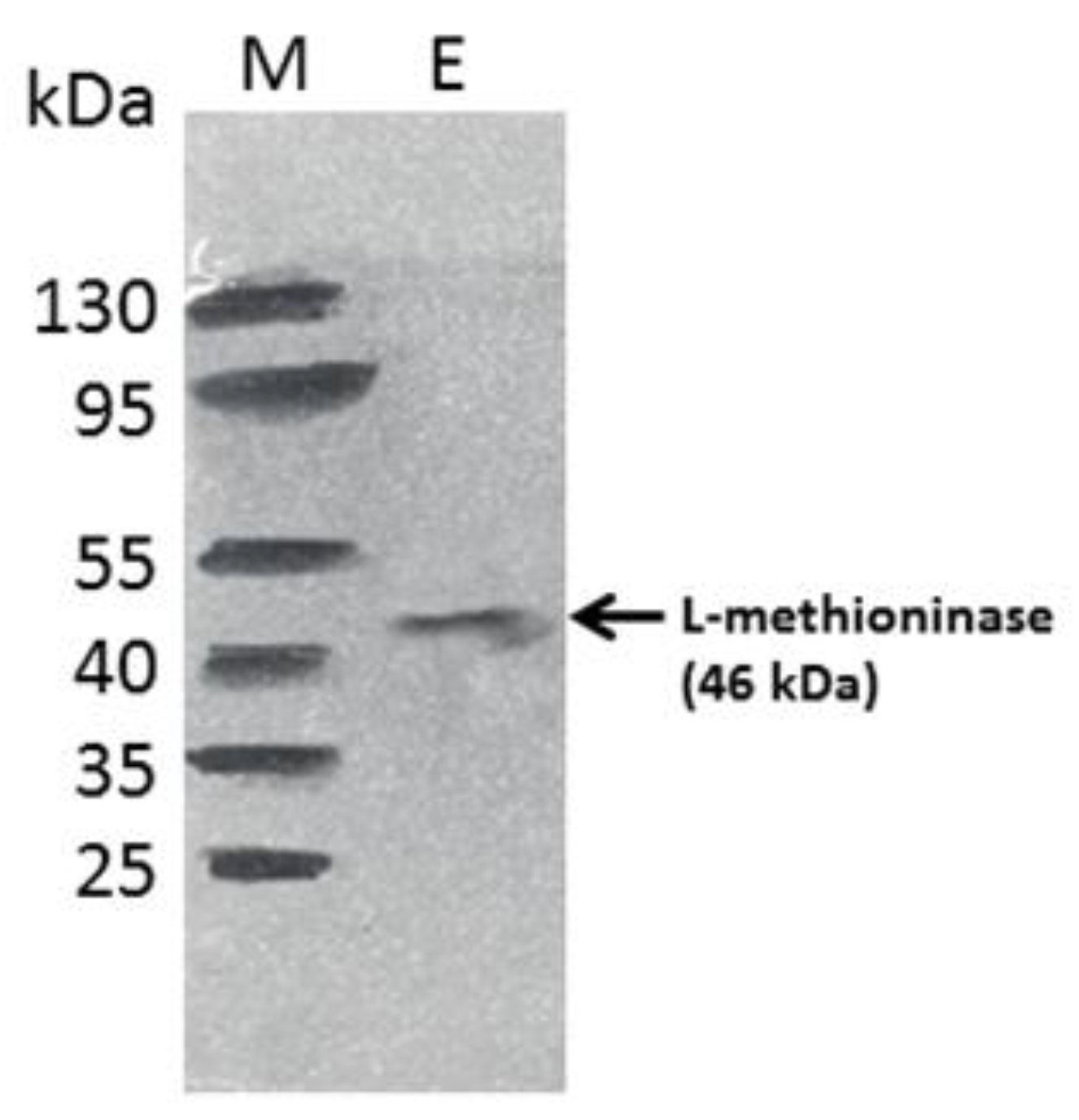
3.5. Purification of L-Methioninase by Gel Filtration Chromatography and Molecular Weight Determination by SDS-PAGE
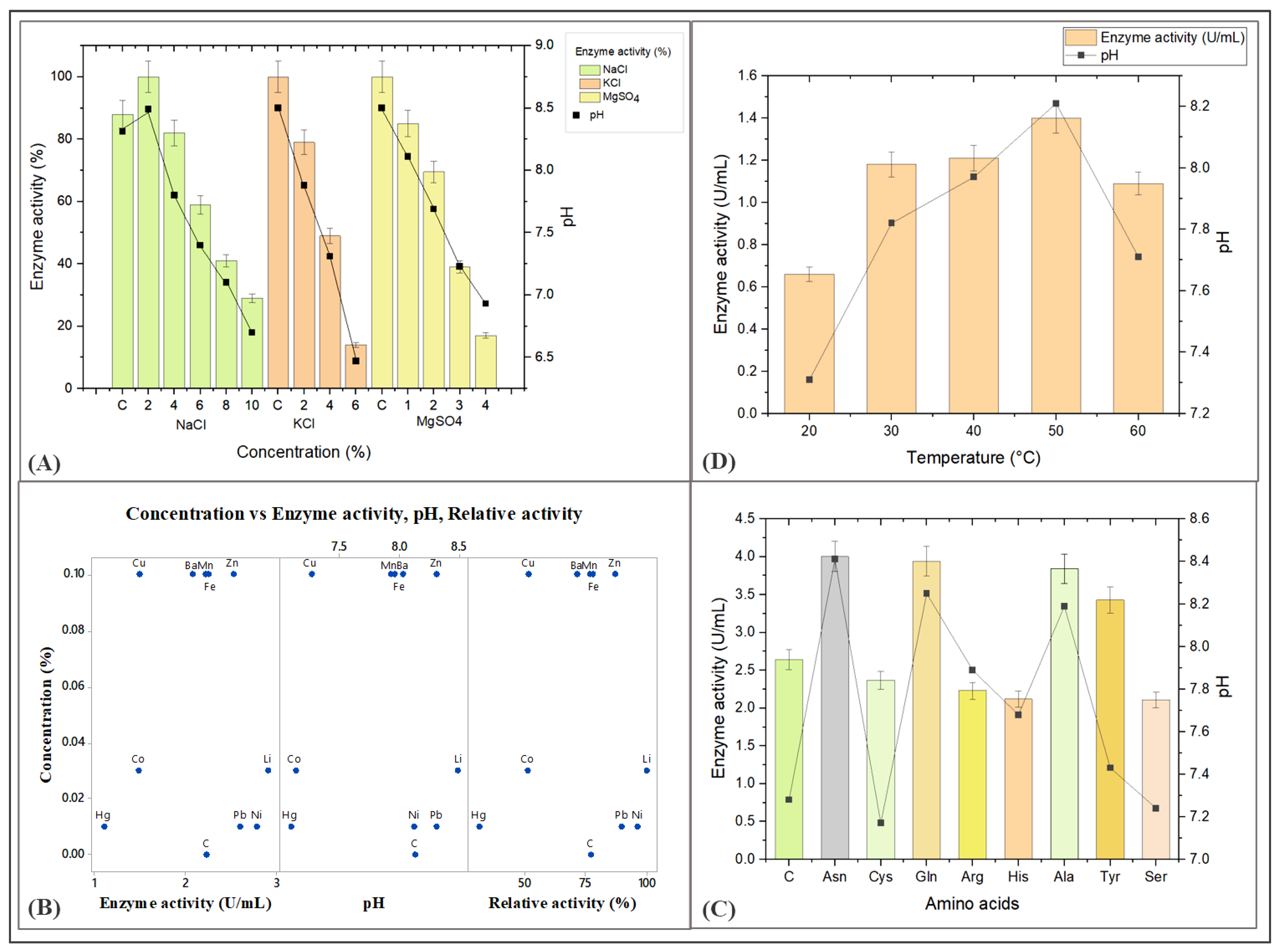
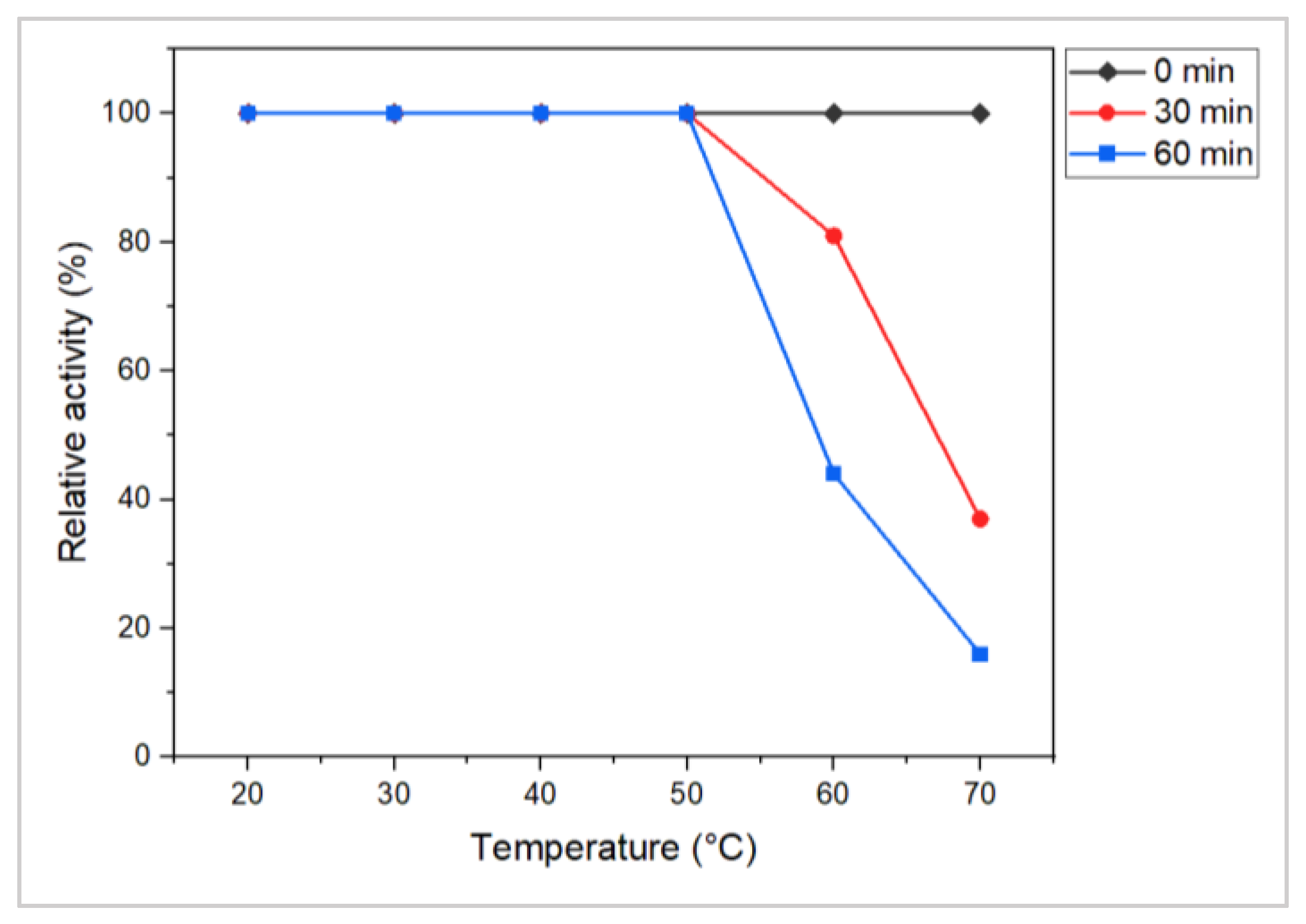
3.6. Effect of Biotic and Abiotic Factors on the Production of L-Methioninase
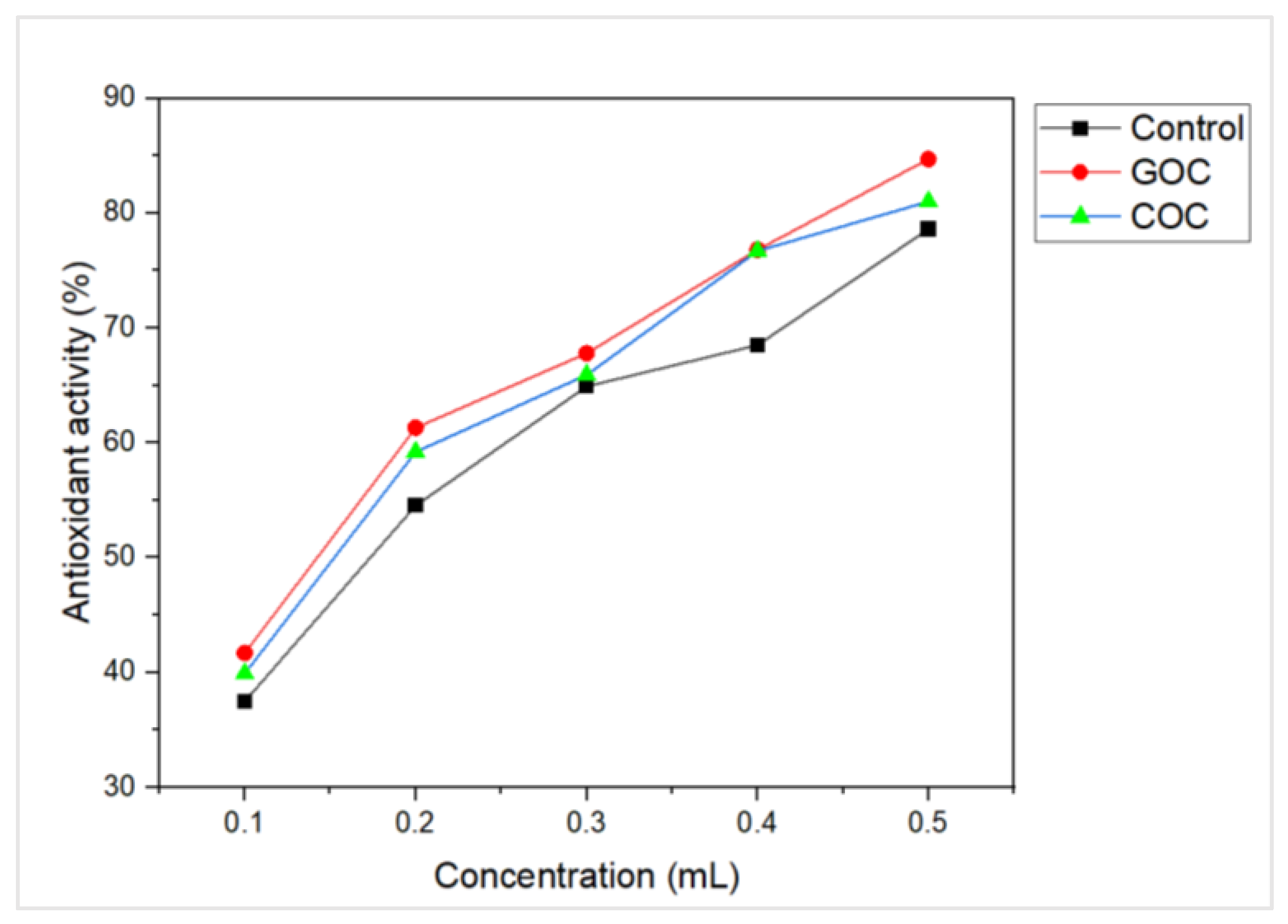
3.7. Antioxidant Activity
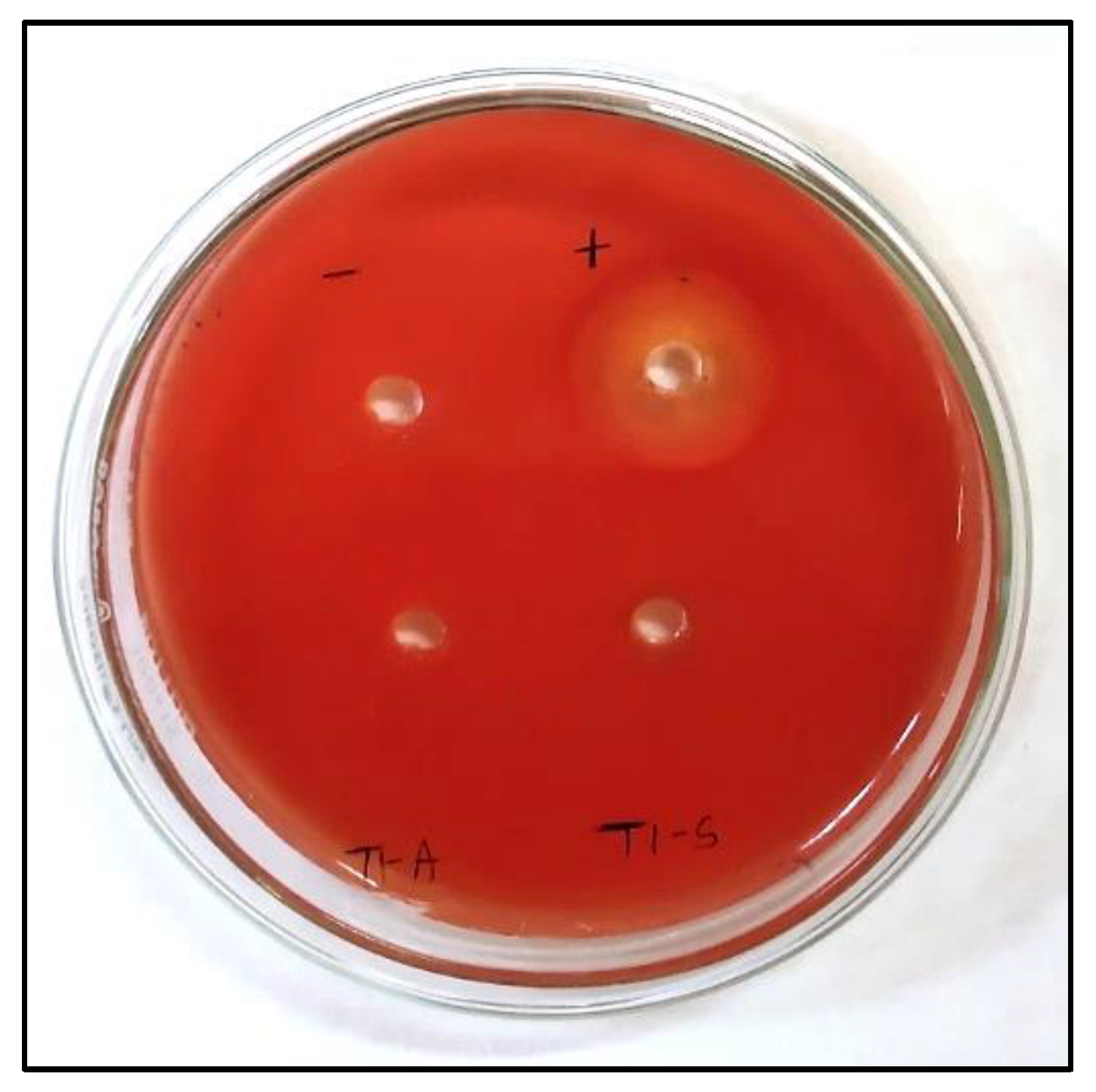
3.8. Hemolytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stratton, M.R.; Campbell, P.J.; Futreal, P.A. The cancer genome. Nature 2009, 458, 719–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittelstein, D.R.; Ye, J.; Schibber, E.F.; Roychoudhury, A.; Martinez, L.T.; Fekrazad, M.H.; Ortiz, M.; Lee, P.P.; Shapiro, M.G.; Gharib, M. Selective ablation of cancer cells with low intensity pulsed ultrasound. Appl. Phys. Lett. 2020, 116, 013701. [Google Scholar] [CrossRef] [Green Version]
- Torchilin, V.P. Enzymes in Medicine: Advantages and Disadvantages. In Immobilized Enzymes in Medicine; Progress in Clinical Biochemistry and Medicine, Vol 11; Springer: Berlin/Heidelberg, Germany, 1991; pp. 3–12. [Google Scholar] [CrossRef]
- Fernandes, H.S.; Silva Teixeira, C.S.; Fernandes, P.A.; Ramos, M.J.; Cerqueira, N.M.F.S.A. Amino acid deprivation using enzymes as a targeted therapy for cancer and viral infections. Expert Opin. Ther. Pat. 2017, 27, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Kiriyama, Y.; Kubota, M.; Takimoto, T.; Kitoh, T.; Tanizawa, A.; Akiyama, Y.; Mikawa, H. Biochemical characterization of U937 cells resistant to L-asparaginase: The role of asparagine synthetase. Leukemia 1989, 3, 294–297. [Google Scholar]
- Prajapati, B.; Supriya, N.R. Review on anticancer enzymes and their targeted amino acids. World J. Pharm. Res. 2017, 6, 268–284. [Google Scholar]
- El-Asmar, F.A.; Greenberg, D.M.; Amand, G.S. Studies on the mechanism of inhibition of tumor growth by the enzyme glutaminase. Cancer Res. 1966, 26, 116–122. [Google Scholar]
- Ghosh, S.; Murthy, S.; Govindasamy, S.; Chandrasekaran, M. Optimization of L-asparaginase production by Serratia marcescens (NCIM 2919) under solid state fermentation using coconut oil cake. Sustain. Chem. Process. 2013, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- Bopaiah, B.B.K.; Kumar, D.A.N.; Balan, K.; Dehingia, L.; Reddy, M.K.R.V.; Suresh, A.B.; Nadumane, V.K. Purification, characterization, and antiproliferative activity of L-methioninase from a new isolate of Bacillus haynesii JUB2. J. Appl. Pharm. Sci. 2020, 10, 054–061. [Google Scholar] [CrossRef]
- Sharma, B.; Singh, S.; Kanwar, S.S. L-methioninase: A therapeutic enzyme to treat malignancies. BioMed Res. Int. 2014, 2014, 506287. [Google Scholar] [CrossRef]
- Anderson, M.E. Glutathione: An overview of biosynthesis and modulation. Chem. Biol. Interact. 1998, 111, 1–14. [Google Scholar] [CrossRef]
- Kahraman, H.; Aytan, E.; Kurt, A.G. Production of methionine γ-lyase in recombinant Citrobacter freundii bearing the hemoglobin gene. BMB Rep. 2011, 44, 590–594. [Google Scholar] [CrossRef] [Green Version]
- Cellarier, E.; Durando, X.; Vasson, M.P.; Farges, M.C.; Demiden, A.; Maurizis, J.C.; Madelmont, J.C.; Chollet, P. Methionine dependency and cancer treatment. Cancer Treat. Rev. 2003, 29, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.M. Altered methionine metabolism, DNA methylation and oncogene expression in carcinogenesis: A review and synthesis. Biochim. Biophys. Acta 1984, 738, 49–87. [Google Scholar] [CrossRef] [PubMed]
- Carr, K.A.; Arduino, M.J. Isolation and characterization of bacterial L-Methioninase as an effective anti-tumor agent. Bios 1981, 52, 13–22. Available online: http://www.jstor.org/stable/4607648 (accessed on 20 June 2023).
- Moinard, C.; Cynober, L.; de Bandt, J.P. Polyamines: Metabolism and implications in human diseases. Clin. Nutr. 2005, 24, 184–197. [Google Scholar] [CrossRef]
- Gilmour, S.K. Polyamines and nonmelanoma skin cancer. Toxicol. Appl. Pharmacol. 2007, 224, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breillout, F.; Antoine, E.; Poupon, M.F. Methionine dependency of malignant tumors: A possible approach for therapy. J. Natl. Cancer Inst. 1990, 82, 1628–1632. [Google Scholar] [CrossRef]
- Yvon, M.; Thirouin, S.; Rijnen, L.; Fromentier, D.; Gripon, J.C. An aminotransferase from Lactococcus lactis initiates conversion of amino acids to cheese flavor compounds. Appl. Environ. Microbiol. 1997, 63, 414–419. [Google Scholar] [CrossRef]
- Amarita, F.; Yvon, M.; Nardi, M.; Chambellon, E.; Delettre, J.; Bonnarme, P. Identification and functional analysis of the gene encoding methionine-γ-lyase in Brevibacterium linens. Appl. Environ. Microbiol. 2004, 70, 7348–7354. [Google Scholar] [CrossRef] [Green Version]
- Dias, B.; Weimer, B. Purification and Characterization of l-Methionine γ-Lyase from Brevibacterium linens BL2. Appl. Environ. Microbiol. 1998, 64, 3327–3331. [Google Scholar] [CrossRef] [Green Version]
- Nakayma, T.; Esaki, N.; Lee, W.J.; Tanaka, I.; Tanaka, H.; Soda, K. Purification and properties of l-methionine γ-lyase from Aeromonas sp. Agric. Biol. Chem. 1894, 48, 2367–2369. [Google Scholar] [CrossRef]
- Khan, M.A.; Lopez-Munoz, M.M.; Kaspar, C.W.; Hung, K.F. Activities of methionine-γ-lyase in the acidophilic archaeon “Ferroplasma acidarmanus” strain fer1. Res. Rep. Biol. 2013, 4, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Sundar, W.A.; Nellaiah, H. A rapid method for screening of methioninase producing Serratia marcescens species from soil. Int. J. Pharm. Pharm. Sci. 2013, 5, 426–427. Available online: https://innovareacademics.in/journal/ijpps/Vol5Issue2/6615.pdf (accessed on 20 June 2023).
- Revtovich, S.; Anufrieva, N.; Morozova, E.; Kulikova, V.; Nikulin, A.; Demidkina, T. Structure of methionine γ-lyase from Clostridium sporogenes. Acta Crystallogr. F Struc. Biol. Commun. 2016, 72, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Cuesta, M.C.; Peláez, C.; Eagles, J.; Gasson, M.J.; Requena, T.; Hanniffy, S.B. YtjE from Lactococcus lactis IL1403 is a C-S lyase with α, γ-elimination activity toward methionine. Appl. Environ. Microbiol. 2016, 72, 4878–4884. [Google Scholar] [CrossRef] [Green Version]
- Fukumoto, M.; Kudou, D.; Murano, S.; Shiba, T.; Sato, D.; Tamura, T.; Harada, S.; Inagaki, K. The role of amino acid residues in the active site of L-methionine γ-lyase from Pseudomonas putida. Biosci. Biotechnol. Biochem. 2012, 76, 1275–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selim, M.H.; Elshikh, H.H.; Saad, M.M.; Mostafa, E.E.; Mahmoud, M.A. Purification and characterization of a novel thermo stable L-methioninase from Streptomyces sp. DMMMH4 and its evaluation for anticancer activity. J. Appl. Pharm. Sci. 2016, 6, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Xu, R.; Guo, Z. Identification and characterization of a methionine γ-lyase in the calicheamicin biosynthetic cluster of Micromonospora echinospora. Chembiochem 2015, 16, 100–109. [Google Scholar] [CrossRef]
- Prihanto, A.A.; Nursyam, H.; Yufidasari, H.S.; Lutfiana, L. Mangrove ecosystem as a source of L-methioninase-producing bacteria. Malays. Appl. Biol. 2018, 47, 141–145. Available online: https://www.mabjournal.com/images/47_3_June_2018/47_03_18.pdf (accessed on 20 June 2023).
- Nikulin, A.; Revtovich, S.; Morozova, E.; Nevskaya, N.; Nikonov, S.; Garber, M.; Demidkina, T. High-resolution structure of methionine γ-lyase from Citrobacter freundii. Acta Crystallogr. D Biol. Crystallogr. 2008, 64, 211–218. [Google Scholar] [CrossRef]
- Kavya, D.; Nadumane, V.K. Identification of highest L-Methioninase enzyme producers among soil microbial isolates, with potential antioxidant and anticancer properties. J. Appl. Biol. Biotechnol. 2020, 8, 21–27. [Google Scholar] [CrossRef]
- Huang, K.Y.; Hu, H.Y.; Tang, Y.L.; Xia, F.G.; Luo, X.Q.; Liu, J.Z. High-level expression, purification and large-scale production of L-methionine γ-lyase from Idiomarina as a novel anti-leukemic drug. Mar. Drugs 2015, 13, 5492–5507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukamachi, H.; Nakano, Y.; Okano, S.; Shibata, Y.; Abiko, Y.; Yamashita, Y. High production of methyl mercaptan by L-methionine-α-deamino-γ-mercaptomethane lyase from Treponema denticola. Biochem. Biophys. Res. Commun. 2005, 331, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, W.A. Bacterium Hafnia alvei secretes L-methioninase enzyme: Optimization of the enzyme secretion conditions. Saudi J. Biol. Sci. 2020, 27, 1222–1227. [Google Scholar] [CrossRef]
- Abu-Tahon, M.A.; Isaac, G.S. Purification and characterization of new alkaline L-methioninase from Aspergillus ustus AUMC 1051 grown under solid-state fermentation conditions. Egypt. J. Bot. 2016, 56, 785–798. [Google Scholar] [CrossRef]
- El-Sayed, A.S. Purification and characterization of a new L-methioninase from solid cultures of Aspergillus flavipes. J. Microbiol. 2011, 49, 130–140. [Google Scholar] [CrossRef]
- Hendy, M.H.; Hashem, A.H.; Sulieman, W.B.; Sultan, M.H.; Abdelraof, M. Purification, Characterization and anticancer activity of L-methionine γ-lyase from thermo-tolerant Aspergillus fumigatus. Microb. Cell Fact. 2023, 22, 1–11. [Google Scholar] [CrossRef]
- Salim, N.; Santhiagu, A.; Joji, K. Purification, characterization and anticancer evaluation of l-methioninase from Trichoderma harzianum. 3 Biotech 2020, 10, 501. [Google Scholar] [CrossRef]
- Khalaf, S.A.; El-Sayed, A.S. L-Methioninase production by filamentous Saccharomyces: I-screening and optimization under submerged conditions. Curr. Microbiol. 2009, 58, 219–226. [Google Scholar] [CrossRef]
- Bonnarme, P.; Arfi, K.; Dury, C.; Helinck, S.; Yvon, M.; Spinnler, H.E. Sulfur compound production by Geotrichum candidum from L-methionine: Importance of the transamination step. FEMS Microbiol. Lett. 2001, 205, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Selim, M.H.; Karm Eldin, E.Z.; Saad, M.M.; Mostafa, E.S.E.; Shetia, Y.H.; Anise, A.A.H. Purification, characterization of L-methioninase from Candida tropicalis, and its application as an anticancer. Biotechnol. Res. Int. 2015, 2015, 173140. [Google Scholar] [CrossRef] [Green Version]
- Rajauria, G.; Jaiswal, A.K.; Abu-Gannam, N.; Gupta, S. Antimicrobial, antioxidant and free radical-scavenging capacity of brown seaweed Himanthalia elongata from western coast of Ireland. J. Food Biochem. 2013, 37, 322–335. [Google Scholar] [CrossRef]
- Mohkam, M.; Taleban, Y.; Golkar, N.; Berenjian, A.; Dehshahri, A.; Mobasher, M.A.; Ghasemi, Y. Isolation and identification of novel L-methioninase producing bacteria and optimization of its production by experimental design method. Biocatal. Agric. Biotechnol. 2020, 26, 101566. [Google Scholar] [CrossRef]
- Nejadi, N.; Masti, S.M.; Tavirani, M.R.; Golmohammadi, T. Comparison of three routine protein precipitation methods: Acetone, TCA/acetone wash and TCA/acetone. Arch. Adv. Biosci. 2014, 5. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Spinnler, H.E.; Berger, C.; Lapadatescu, C.; Bonnarme, P. Production of sulfur compounds by several yeasts of technological interest for cheese ripening. Int. Dairy J. 2001, 11, 245–252. [Google Scholar] [CrossRef]
- Arfi, K.; Tâche, R.; Spinnler, H.E.; Bonnarme, P. Dual influence of the carbon source and L-methionine on the synthesis of sulphur compounds in the cheese-ripening yeast Geotrichum candidum. Appl. Microbiol. Biotechnol. 2003, 61, 359–365. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. Available online: https://www.jbc.org/article/S0021-9258(19)52451-6/pdf (accessed on 20 June 2023). [CrossRef]
- El-Sayed, A.S. L-methioninase production by Aspergillus flavipes under solid-state fermentation. J. Basic Microbiol. 2009, 49, 331–341. [Google Scholar] [CrossRef]
- Apostolou, A.; Stagos, D.; Galitsiou, E.; Spyrou, A.; Haroutounian, S.; Portesis, N.; Trizoglou, I.; Hayes, A.W.; Tsatsakis, A.M.; Kouretas, D. Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced D.N.A. damage and anticancer activity of Vitis vinifera stem extracts. Food Chem. Toxicol. 2013, 61, 60–68. [Google Scholar] [CrossRef]
- Huang, S.; Pan, S.; Chen, G.; Huang, S.; Zhang, Z.; Li, Y.; Liang, Z. Biochemical characteristics of a fibrinolytic enzyme purified from a marine bacterium, Bacillus subtilis HQS-3. Int. J. Biol. Macromol. 2013, 62, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Bulmus, V.; Woodward, M.; Lin, L.; Murthy, N.; Stayton, P.; Hoffman, A. A new pH-responsive and glutathione-reactive, endosomal membrane-disruptive polymeric carrier for intracellular delivery of biomolecular drugs. J. Control. Release 2003, 93, 105–120. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Sridhar, K.; Inbaraj, B.S.; Singh, R.; Kamma, S.; Tripathi, M.; Sharma, M. Transformation of agro-waste into value-added bioproducts and bioactive compounds: Micro/nano formulations and application in the agri-food-pharma sector. Bioengineering 2023, 10, 152. [Google Scholar] [CrossRef]
- Ramachandran, S.; Patel, A.K.; Nampoothiri, K.M.; Francis, F.; Nagy, V.; Szakacs, G.; Pandey, A. Coconut oil cake—A potential raw material for the production of α-amylase. Bioresour. Technol. 2004, 93, 169–174. [Google Scholar] [CrossRef]
- Rekha, K.S.S.; Lakshmi, M.V.C.; Devi, V.S.; Siddartha Kumar, M. Production and optimization of lipase from Candida rugosa using groundnut oilcake under solid state fermentation. Int. J. Res. Eng. Technol. 2012, 1, 571–577. [Google Scholar] [CrossRef]
- Abdelraof, M.; Selim, M.H.; Elsoud, M.M.A.; Ali, M.M. Statistically optimized production of extracellular l-methionine γ-lyase by Streptomyces Sp. DMMMH60 and evaluation of purified enzyme in sub-culturing cell lines. Biocatal. Agric. Biotechnol. 2019, 18, 101074. [Google Scholar] [CrossRef]
- Joseph, B.; Upadhyaya, S.; Ramteke, P. Production of cold-active bacterial lipases through semisolid state fermentation using oil cakes. Enzym. Res. 2011, 2011, 796407. [Google Scholar] [CrossRef] [Green Version]
- Manukhov, I.V.; Mamaeva, D.V.; Rastorguev, S.M.; Faleev, N.G.; Morozova, E.A.; Demidkina, T.V.; Zavilgelsky, G.B. A gene encoding l-methionine γ-lyase is present in Enterobacteriaceae family genomes: Identification and characterization of Citrobacter freundii l-methionine γ-lyase. J. Bacteriol. 2005, 187, 3889–3893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, M.S.; Kanwal, H.K. Influence of carbon, nitrogen sources, inducers, and substrates on lignocellulolytic enzyme activities of Morchella spongiola. J. Agric. Food Res. 2022, 7, 100271. [Google Scholar] [CrossRef]
- Yadav, N.; Sarkar, S. Production of L-asparaginase by Fusarium oxysporum using submerged fermentation. Int. J. Pharm. Sci. Invent. 2014, 3, 32–40. Available online: http://www.ijpsi.org/Papers/Vol3(6)/G0361032040.pdf (accessed on 20 June 2023).
- Fatima, N.; Khan, M.M.; Khan, I.A. L-asparaginase produced from soil isolates of Pseudomonas aeruginosa shows potent anticancer activity on HeLa cells. Saudi J. Biol. Sci. 2019, 26, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, M.M.A.A.; Awad, M.F.; El-Shenawy, F.S.; El-Bondkly, A.M.A. Production, purification, characterization, antioxidant and antiproliferative activities of extracellular L-asparaginase produced by Fusarium equiseti AHMF4. Saudi J. Biol. Sci. 2021, 28, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Dange, V.; Peshwe, S. Purification and biochemical characterization of L-asparaginase from Aspergillus niger and evaluation of its antineoplastic activity. Int. J. Sci. Res. 2015, 4, 564–569. Available online: https://www.ijsr.net/getabstract.php?paperid=OCT141573 (accessed on 20 June 2023).
- Dias, F.F.G.; Santos Aguilar, J.G.D.; Sato, H.H. L-Asparaginase from Aspergillus spp.: Production based on kinetics, thermal stability and biochemical characterization. 3 Biotech 2019, 9, 289. [Google Scholar] [CrossRef]
- Lincoln, L.; Niyonzima, F.N.; More, S.S. Purification and properties of a fungal L-asparaginase from Trichoderma viride pers: SF GREY. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 310–316. [Google Scholar]
- Tan, Y.; Xu, M.; Tan, X.; Tan, X.; Wang, X.; Saikawa, Y.; Nagahama, T.; Sun, X.; Lenz, M.; Hoffman, R.M. Overexpression and large-scale production of recombinant L-methionine-α-deamino-γ-mercaptomethane-lyase for novel anticancer therapy. Protein Expr. Purif. 1997, 9, 233–245. [Google Scholar] [CrossRef]
| Enzyme Treatments | Enzyme Activity (U/mL) | Total Protein (mg/mL) | Specific Activity (U/mg) | Purification Fold |
|---|---|---|---|---|
| Crude extract | 1.01 | 0.95 | 1.07 | - |
| Acetone purified | 0.6 | 0.13 | 4.61 | 4.30 |
| Sephadex G-100 | 0.23 | 0.03 | 7.66 | 7.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javia, B.; Gadhvi, M.; Vyas, S.; Dudhagara, P.; Shyu, D.J.H.; Chen, Y.-Y.; Dudhagara, D. Bioprospecting of a Thermostable L-Methioninase from Alcaligenes aquatilis BJ-1 in Agro-Industrial Waste. Microbiol. Res. 2023, 14, 959-976. https://doi.org/10.3390/microbiolres14030066
Javia B, Gadhvi M, Vyas S, Dudhagara P, Shyu DJH, Chen Y-Y, Dudhagara D. Bioprospecting of a Thermostable L-Methioninase from Alcaligenes aquatilis BJ-1 in Agro-Industrial Waste. Microbiology Research. 2023; 14(3):959-976. https://doi.org/10.3390/microbiolres14030066
Chicago/Turabian StyleJavia, Bhumi, Megha Gadhvi, Suhas Vyas, Pravin Dudhagara, Douglas J. H. Shyu, Yih-Yuan Chen, and Dushyant Dudhagara. 2023. "Bioprospecting of a Thermostable L-Methioninase from Alcaligenes aquatilis BJ-1 in Agro-Industrial Waste" Microbiology Research 14, no. 3: 959-976. https://doi.org/10.3390/microbiolres14030066





