Baicalin Attenuated PANX-1/P2X7 Axis, P2Y6, and NLRP3/Caspase-1 Signaling Pathways in Peritonitis Induced by Glaesserella parasuis
Abstract
:1. Introduction
2. Meterials and Methods
2.1. Bacterial Strains
2.2. Drugs
2.3. Experiment Design
2.4. Western Blot
2.5. Statistical Analysis
3. Results
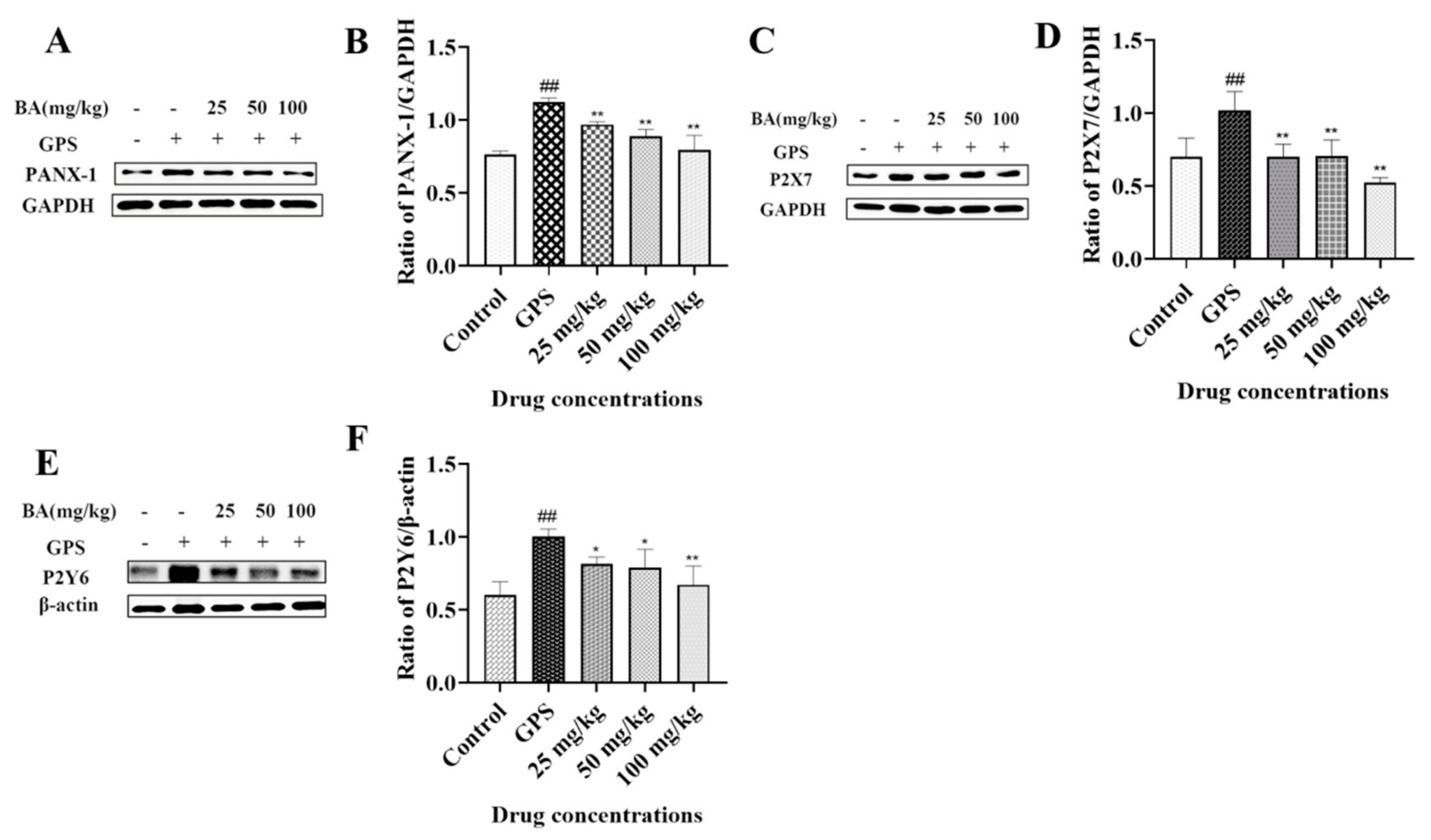
3.1. The Effects of Baicalin on PANX-1, P2X7, and P2Y6 Expression in Peritoneum Induced by G. parasuis
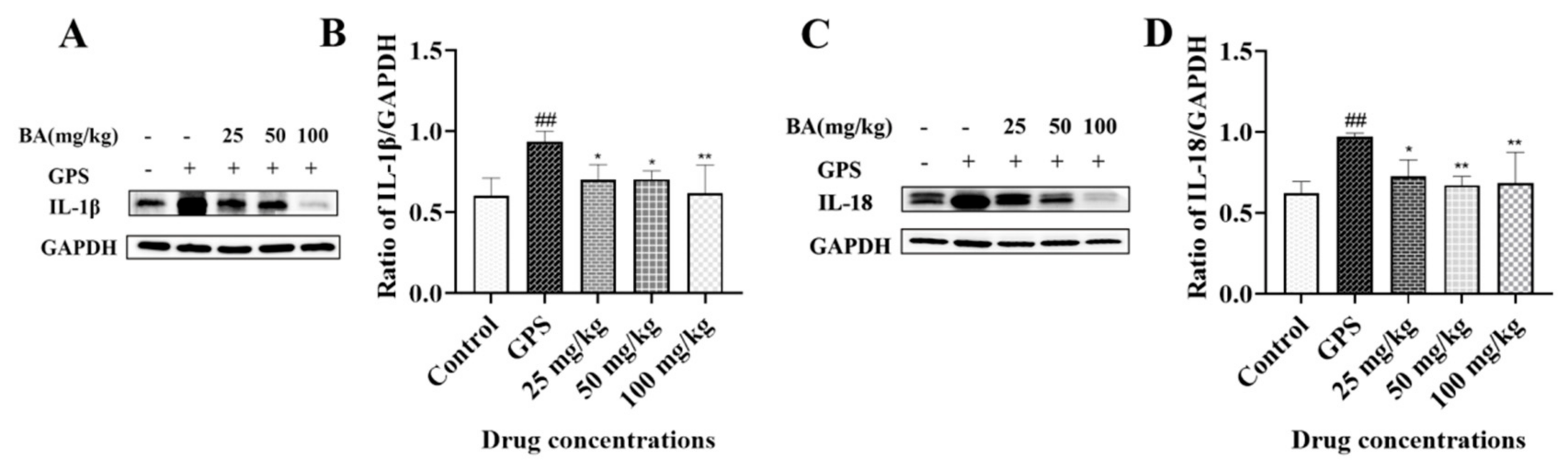
3.2. The Effects of Baicalin on NOD-like Receptor Thermal Protein Domain-Associated Protein3 (NLRP3) Inflammasome Activation Triggered by G. parasuis in Peritoneum
3.3. The Effects of Baicalin on IL-1β and IL-18 Release in Peritoneum Elicited by G. parasuis
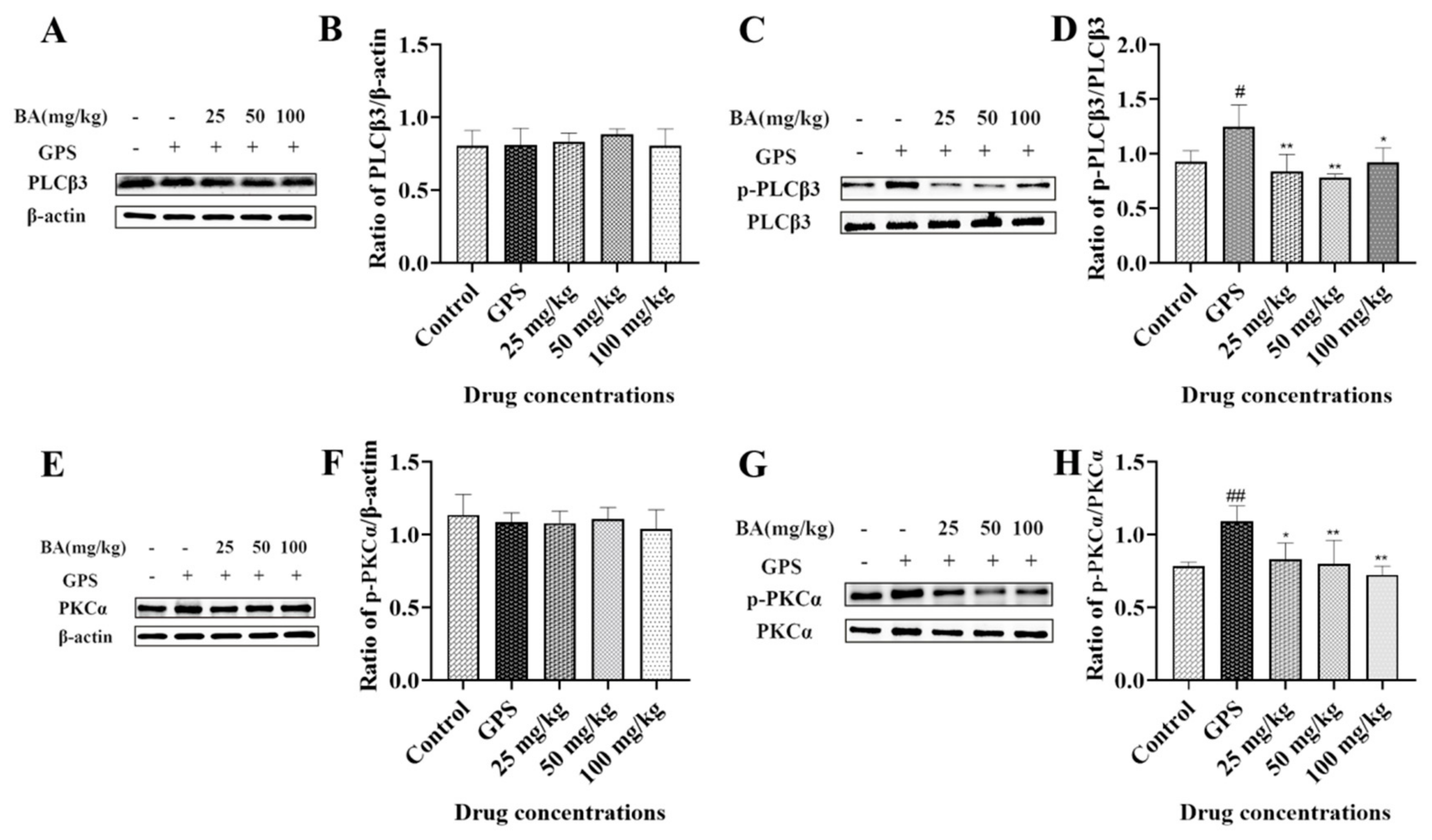
3.4. The Effects of Baicalin on Phospholipase C (PLC) and PKC Activation in Peritoneum Induced by G. parasuis
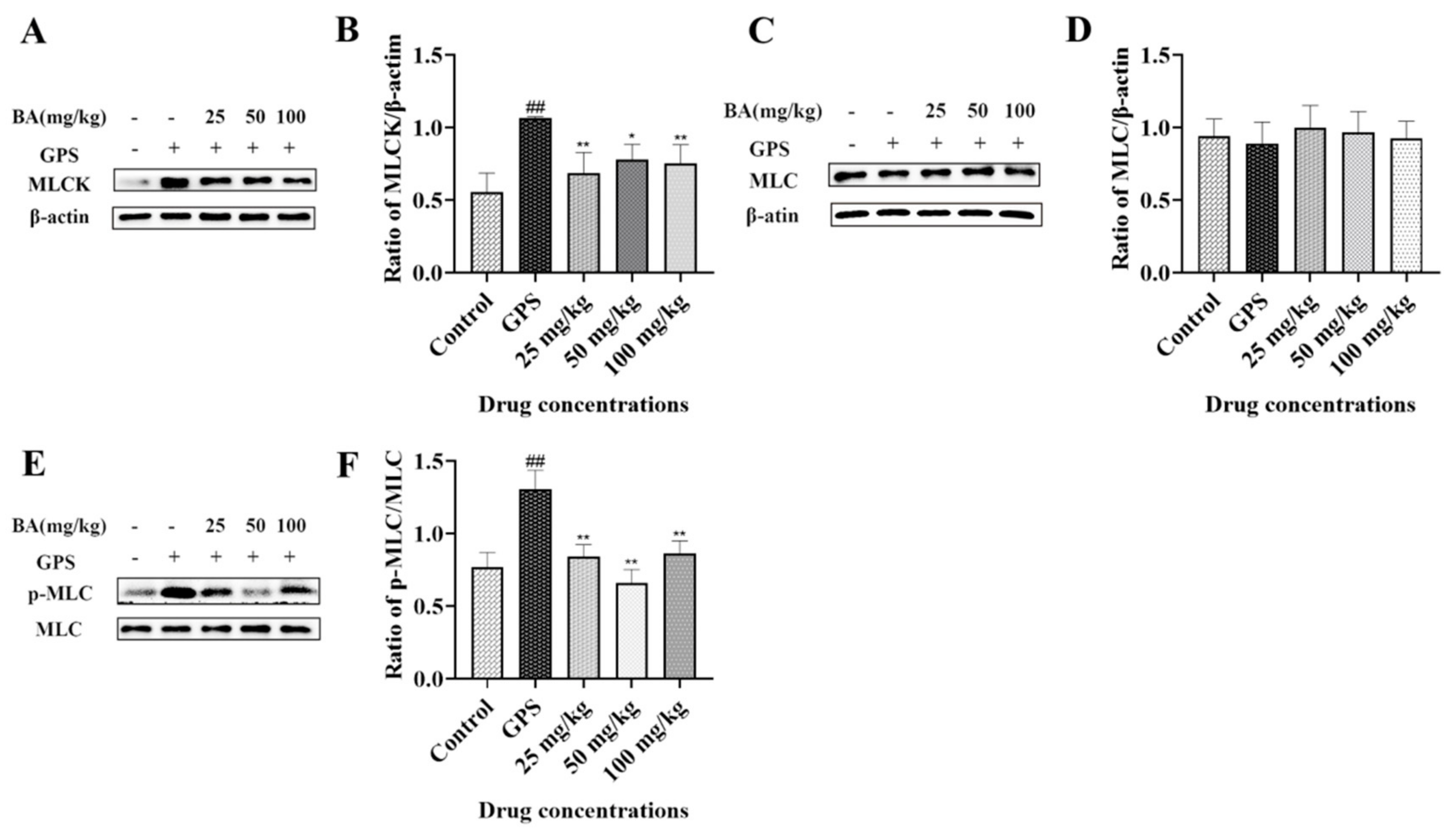
3.5. The Effects of Baicalin on Myosin-Light-Chain Kinase (MLCK) and Myosin-Light-Chain (MLC) Expression Triggered by G. parasuis in Peritoneum
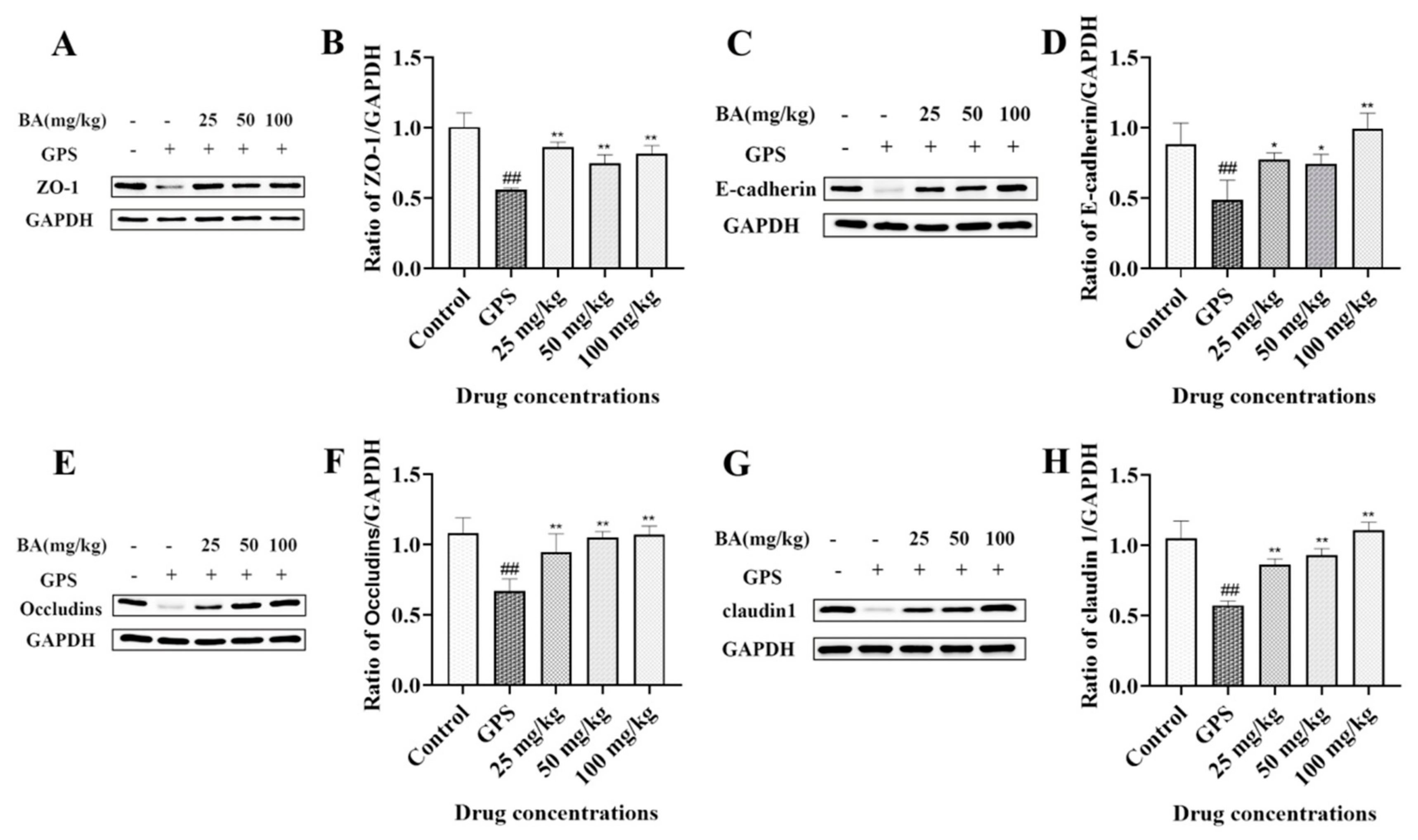
3.6. The Effects of Baicalin on ZO-1, E-Cadherin, Occludins, and Claudin 1 Expression in Peritoneum Elicited by G. parasuis
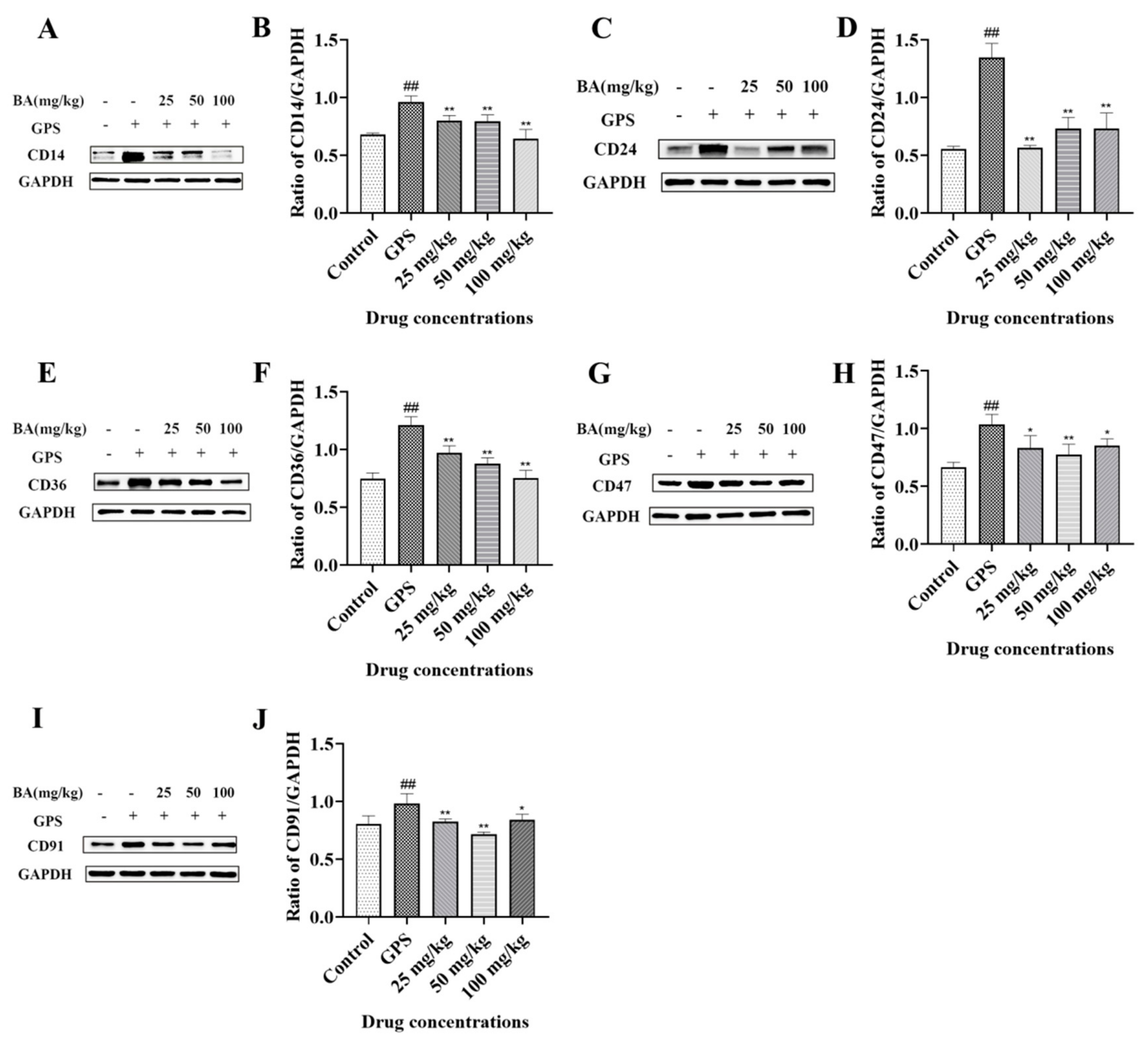
3.7. The Effects of Baicalin on Cluster of Differentiation (CD)14, CD24, CD36, CD47, and CD91 Expression Induced by G. parasuis in Peritoneum
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, M.; Wang, Q.; Wu, W.; Chen, M.; Zhang, P.; Guo, M.; Lin, H.; Ma, Z.; Zhou, H.; Fan, H. Glaesserella parasuis serotype 5 breaches the porcine respiratory epithelial barrier by inducing autophagy and blocking the cell membrane Claudin-1 replenishment. PLoS Pathog. 2022, 18, e1010912. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Jia, Y.; Huang, C.; Zhou, Y.; Chen, X.; Yin, R.; Guo, Y.; Wang, L.; Yuan, J.; Wang, J.; et al. Immunogenicity and protection against Glaesserella parasuis serotype 13 infection after vaccination with recombinant protein LolA in mice. J. Vet. Med. Sci. 2022, 84, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Ma, N.; Cao, H.; Zeng, W.; Ren, J.; Hu, Y.; Zhou, J.; Zhang, M.; Li, C.; Lang, Y.; et al. TurboID Screening of the OmpP2 Protein Reveals Host Proteins Involved in Recognition and Phagocytosis of Glaesserella parasuis by iPAM Cells. Microbiol. Spectr. 2022, 10, e0230722. [Google Scholar] [CrossRef] [PubMed]
- Kielstein, P.; Rapp-Gabrielson, V.J. Designation of 15 serovars of Haemophilus parasuis on the basis of immunodiffusion using heat-stable antigen extracts. J. Clin. Microbiol. 1992, 30, 862–865. [Google Scholar] [CrossRef]
- An, J.; Cai, J.; Zhang, B.; Li, Y. Pili Subunit PilA Contributes to the Cytoadhesion of Glaesserella Parasuis to Host Cells and Provides Immunoprotection. Appl. Environ. Microbiol. 2023, 89, e0200222. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Y.; Xu, X.; Wen, S.; Wang, Z.; Gu, J.; He, Q.; Cai, X. HtrA is involved in stress response and adhesion in Glaesserella parasuis serovar 5 strain Nagasaki. Vet. Microbiol. 2023, 282, 109748. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Xu, J.; Ye, C.; Fu, S.; Hu, C.A.; Qiu, Y.; Liu, Y. Protective Effects of Baicalin on Peritoneal Tight Junctions in Piglets Challenged with Glaesserella parasuis. Molecules 2021, 26, 1268. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Qu, H.L.; Dai, Y.J.; Wang, Q.; Ling, Z.M.; Su, W.F.; Zhao, Y.Y.; Shen, W.X.; Chen, G. Pannexin 1, a large-pore membrane channel, contributes to hypotonicity-induced ATP release in Schwann cells. Neural Regen. Res. 2021, 16, 899–904. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.Y.; Ling, Z.M.; Chen, G.; Wei, Z.Y. Inhibition of Schwann cell pannexin 1 attenuates neuropathic pain through the suppression of inflammatory responses. J. Neuroinflamm. 2022, 19, 244. [Google Scholar] [CrossRef]
- Koval, M.; Cwiek, A.; Carr, T.; Good, M.E.; Lohman, A.W.; Isakson, B.E. Pannexin 1 as a driver of inflammation and ischemia-reperfusion injury. Purinergic Signal. 2021, 17, 521–531. [Google Scholar] [CrossRef]
- Seo, J.H.; Dalal, M.S.; Contreras, J.E. Pannexin-1 Channels as Mediators of Neuroinflammation. Int. J. Mol. Sci. 2021, 22, 5189. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Li, F.; Chen, K.; Ding, W.; Xue, Y.; Wang, Y.; Wang, H.; Ding, K.; Zhao, Z. Comparison of the Glaesserella parasuis Virulence in Mice and Piglets. Front. Vet. Sci. 2021, 8, 659244. [Google Scholar] [CrossRef] [PubMed]
- Algammal, A.M.; Abo Hashem, M.E.; Alfifi, K.J.; Al-Otaibi, A.S.; Alatawy, M.; ElTarabili, R.M.; Abd El-Ghany, W.A.; Hetta, H.F.; Hamouda, A.M.; Elewa, A.A.; et al. Sequence Analysis, Antibiogram Profile, Virulence and Antibiotic Resistance Genes of XDR and MDR Gallibacterium anatis Isolated from Layer Chickens in Egypt. Infect. Drug Resist. 2022, 15, 4321–4334. [Google Scholar] [CrossRef]
- Algammal, A.M.; Ibrahim, R.A.; Alfifi, K.J.; Ghabban, H.; Alghamdi, S.; Kabrah, A.; Khafagy, A.R.; Abou-Elela, G.M.; Abu-Elala, N.M.; Donadu, M.G.; et al. A First Report of Molecular Typing, Virulence Traits, and Phenotypic and Genotypic Resistance Patterns of Newly Emerging XDR and MDR Aeromonas veronii in Mugil seheli. Pathogens 2022, 11, 1262. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, S.; Ma, C.; Li, T.; Yang, L. Preparation of baicalin-loaded ligand-modified nanoparticles for nose-to-brain delivery for neuroprotection in cerebral ischemia. Drug Deliv. 2022, 29, 1282–1298. [Google Scholar] [CrossRef]
- Bao, M.; Ma, Y.; Liang, M.; Sun, X.; Ju, X.; Yong, Y.; Liu, X. Research progress on pharmacological effects and new dosage forms of baicalin. Vet. Med. Sci. 2022, 8, 2773–2784. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, L.; Wang, Z.; Li, X.; Ding, L.; Qiu, Y.; Guo, P.; Ye, C.; Fu, S.; Wu, Z.; et al. Baicalin Alleviate Apoptosis via PKC-MAPK Pathway in Porcine Peritoneal Mesothelial Cells Induced by Glaesserella parasuis. Molecules 2022, 27, 5083. [Google Scholar] [CrossRef]
- Fu, S.; Zhao, W.; Xiong, C.; Guo, L.; Guo, J.; Qiu, Y.; Hu, C.A.; Ye, C.; Liu, Y.; Wu, Z.; et al. Baicalin modulates apoptosis via RAGE, MAPK, and AP-1 in vascular endothelial cells during Haemophilus parasuis invasion. Innate Immun. 2019, 25, 420–432. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.; Liu, H.; Xu, L.; Qiu, Y.; Liu, Y.; Wu, Z.; Ye, C.; Hou, Y.; Hu, C.A. Baicalin modulates NF-κB and NLRP3 inflammasome signaling in porcine aortic vascular endothelial cells Infected by Haemophilus parasuis Causing Glässer’s disease. Sci. Rep. 2018, 8, 807. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Chen, H.; Blackall, P.J.; Yin, Z.; Wang, L.; Liu, Z.; Jin, M. Serological characterization of Haemophilus parasuis isolates from China. Vet. Microbiol. 2005, 111, 231–236. [Google Scholar] [CrossRef]
- Fu, S.; Yin, R.; Zuo, S.; Liu, J.; Zhang, Y.; Guo, L.; Qiu, Y.; Ye, C.; Liu, Y.; Wu, Z.; et al. The effects of baicalin on piglets challenged with Glaesserella parasuis. Vet. Res. 2020, 51, 102. [Google Scholar] [CrossRef]
- Ohyama, S.; Ouchi, T.; Kimura, M.; Kurashima, R.; Yasumatsu, K.; Nishida, D.; Hitomi, S.; Ubaidus, S.; Kuroda, H.; Ito, S.; et al. Piezo1-pannexin-1-P2X(3) axis in odontoblasts and neurons mediates sensory transduction in dentinal sensitivity. Front. Physiol. 2022, 13, 891759. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, A.V.; Moura-Neto, L.I.; Martins, C.S.; Silva, P.I.M.; Lopes, M.B.S.; Leitão, R.F.C.; Coelho-Aguiar, J.M.; Moura-Neto, V.; Warren, C.A.; Costa, D.V.S.; et al. Role of Pannexin-1-P2X7R signaling on cell death and pro-inflammatory mediator expression induced by Clostridioides difficile toxins in enteric glia. Front. Immunol. 2022, 13, 956340. [Google Scholar] [CrossRef] [PubMed]
- Higashikuni, Y.; Liu, W.; Numata, G.; Tanaka, K.; Fukuda, D.; Tanaka, Y.; Hirata, Y.; Imamura, T.; Takimoto, E.; Komuro, I.; et al. NLRP3 Inflammasome Activation Through Heart-Brain Interaction Initiates Cardiac Inflammation and Hypertrophy During Pressure Overload. Circulation 2023, 147, 338–355. [Google Scholar] [CrossRef]
- Wang, Y.; Pei, Y.; Yao, Y.; Cheng, W.; Li, Y.; Duan, Y. P2Y6R: A promising new target in inflammatory diseases and the advance of its antagonists. Curr. Med. Chem. 2023, 30, 2209–2224. [Google Scholar] [PubMed]
- Wonnenberg, B.; Tschernig, T.; Voss, M.; Bischoff, M.; Meier, C.; Schirmer, S.H.; Langer, F.; Bals, R.; Beisswenger, C. Probenecid reduces infection and inflammation in acute Pseudomonas aeruginosa pneumonia. Int. J. Med. Microbiol. IJMM 2014, 304, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Onódi, Z.; Koch, S.; Rubinstein, J.; Ferdinandy, P.; Varga, Z.V. Drug repurposing for cardiovascular diseases: New targets and indications for probenecid. Br. J. Pharmacol. 2023, 180, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Andjelkovic, A.V.; Situ, M.; Citalan-Madrid, A.F.; Stamatovic, S.M.; Xiang, J.; Keep, R.F. Blood-Brain Barrier Dysfunction in Normal Aging and Neurodegeneration: Mechanisms, Impact, and Treatments. Stroke 2023, 54, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.D.; Yang, T.Q.; Zeng, J.H.; Cai, H.B.; Qi, S.H.; Jiang, J.J.; Cheng, Y.; Xu, L.S.; Bu, F. Overexpression of mitogen-activated protein kinase phosphatase-1 in endothelial cells reduces blood-brain barrier injury in a mouse model of ischemic stroke. Neural Regen. Res. 2023, 18, 1743–1749. [Google Scholar]
- Dou, Y.; Liu, P.; Ding, Z.; Zhou, Y.; Jing, H.; Ren, Y.; Heger, Z.; Adam, V.; Li, N. Orally Administrable H(2) S-Scavenging Metal-Organic Framework Prepared by Co-Flow Microfluidics for Comprehensive Restoration of Intestinal Milieu. Adv. Mater. 2023, 35, e2210047. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, H.; Tian, B.; Wang, S.; Gao, X.J. Selenium Deficiency Promotes the Expression of LncRNA-MORC3, Activating NLRP3-Caspase-1/IL-1β Signaling to Induce Inflammatory Damage and Disrupt Tight Junctions in Piglets. Biol. Trace Elem. Res. 2023, 201, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Pontejo, S.M.; Murphy, P.M. Chemokines act as phosphatidylserine-bound “find-me” signals in apoptotic cell clearance. PLoS Biol. 2021, 19, e3001259. [Google Scholar] [CrossRef] [PubMed]
- Baraniecki, Ł.; Tokarz-Deptuła, B.; Syrenicz, A.; Deptuła, W. Macrophage efferocytosis in atherosclerosis. Scand. J. Immunol. 2022, 97, e13251. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zou, B.; Gao, S.; Zhang, D.; Guo, J.; Chen, B.; Hou, H.; Zhu, X. CD14 Involvement in Third-degree Skin Burn-induced Myocardial Injury via the MAPK Signaling Pathway. Cell Biochem. Biophys. 2022, 80, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.S.; Gao, F.H. Molecular Mechanism of Tumor Cell Immune Escape Mediated by CD24/Siglec-10. Front. Immunol. 2020, 11, 1324. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Sun, X.; Liu, Y.; Tang, M.; Wei, Y.; Shi, Y.; Li, R.; Xiao, G.; Kang, J.; Wang, F.; et al. Palmitic acid-induced ferroptosis via CD36 activates ER stress to break calcium-iron balance in colon cancer cells. FEBS J. 2023, 290, 3664–3687. [Google Scholar] [CrossRef]
- Zhao, W.; Shen, B.; Cheng, Q.; Zhou, Y.; Chen, K. Roles of TSP1-CD47 signaling pathway in senescence of endothelial cells: Cell cycle, inflammation and metabolism. Mol. Biol. Rep. 2023, 50, 4579–4585. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Xu, Y.; Wu, D.; Gao, Z.; Zhou, J.; Qian, H.; He, B.; Wang, G. Extracellular HMGB1 Impairs Macrophage-Mediated Efferocytosis by Suppressing the Rab43-Controlled Cell Surface Transport of CD91. Front. Immunol. 2022, 13, 767630. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, S.; Tian, X.; Li, J.; Yuan, Y.; Li, X.; Ren, M.; Guo, L.; Ye, C.; Zong, B.; Liu, Y.; et al. Baicalin Attenuated PANX-1/P2X7 Axis, P2Y6, and NLRP3/Caspase-1 Signaling Pathways in Peritonitis Induced by Glaesserella parasuis. Microbiol. Res. 2023, 14, 1114-1123. https://doi.org/10.3390/microbiolres14030074
Fu S, Tian X, Li J, Yuan Y, Li X, Ren M, Guo L, Ye C, Zong B, Liu Y, et al. Baicalin Attenuated PANX-1/P2X7 Axis, P2Y6, and NLRP3/Caspase-1 Signaling Pathways in Peritonitis Induced by Glaesserella parasuis. Microbiology Research. 2023; 14(3):1114-1123. https://doi.org/10.3390/microbiolres14030074
Chicago/Turabian StyleFu, Shulin, Xinyue Tian, Jingyang Li, Yuzhen Yuan, Xiaoyi Li, Mingxing Ren, Ling Guo, Chun Ye, Bingbing Zong, Yu Liu, and et al. 2023. "Baicalin Attenuated PANX-1/P2X7 Axis, P2Y6, and NLRP3/Caspase-1 Signaling Pathways in Peritonitis Induced by Glaesserella parasuis" Microbiology Research 14, no. 3: 1114-1123. https://doi.org/10.3390/microbiolres14030074




