The Influence of Pre-Existing Immunity against Human Common Cold Coronaviruses on COVID-19 Susceptibility and Severity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Categorization of the Study Population
2.2. Determination of SARS-CoV-2 Infection
2.3. Preparation of hCCCoV Antigens
2.4. Antibody Quantification by ELISA
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Examination of hCCCoV IgG Levels in the Study Population
3.3. Evaluation of the Influence of hCCCoV IgG Levels on the Susceptibility to SARS-CoV-2 Infection
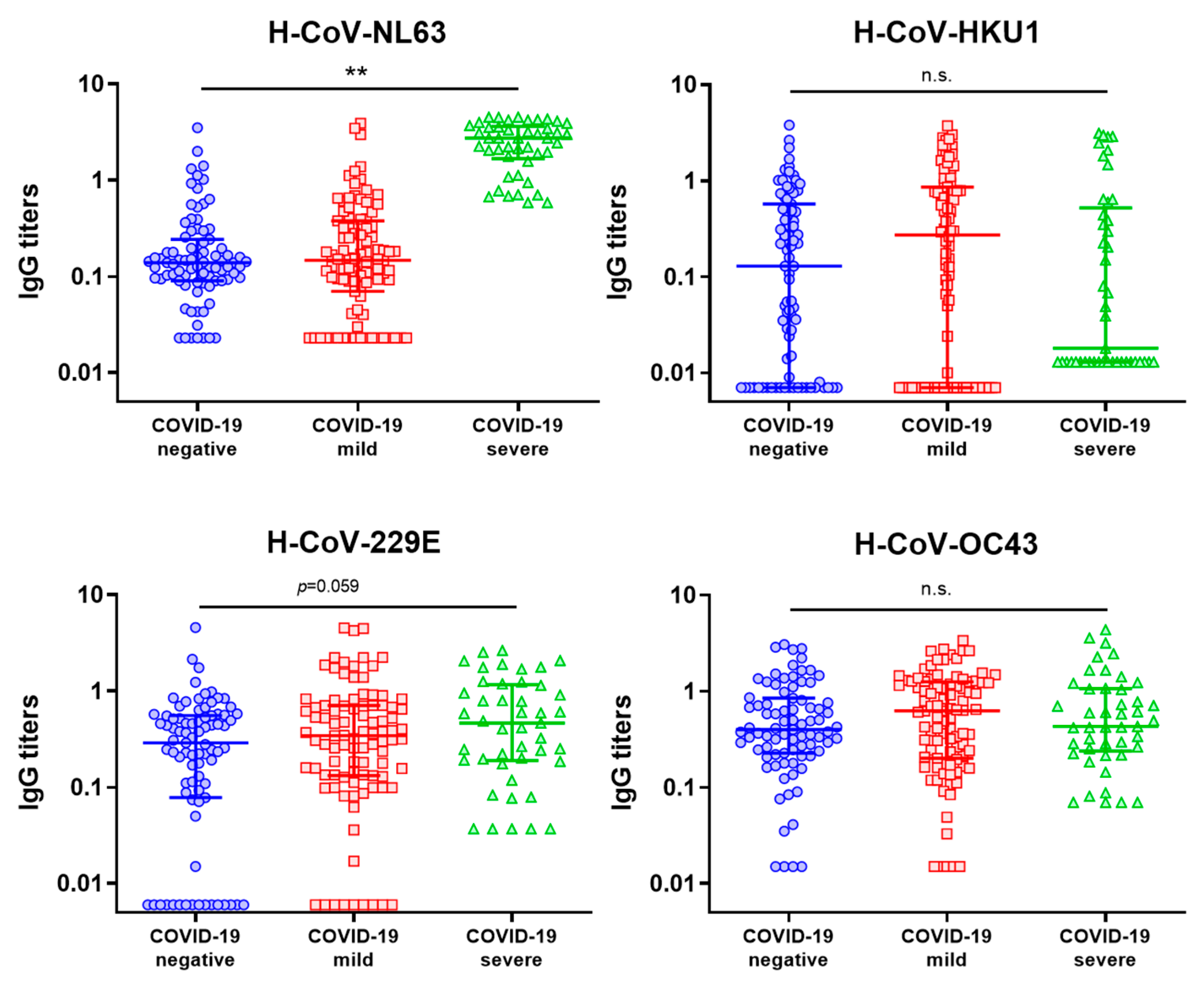
3.4. Evaluation of the Influence of hCCCoV IgG Levels on the Severity of COVID-19
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 29 June 2023).
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef] [PubMed]
- Lusczek, E.R.; Ingraham, N.E.; Karam, B.S.; Proper, J.; Siegel, L.; Helgeson, E.S.; Tignanelli, C.J. Characterizing COVID-19 clinical phenotypes and associated comorbidities and complication profiles. PLoS ONE 2021, 16, e0248956. [Google Scholar] [CrossRef] [PubMed]
- Esper, F.; Weibel, C.; Ferguson, D.; Landry, M.L.; Kahn, J.S. Evidence of a novel human coronavirus that is associated with respiratory tract disease in infants and young children. J. Infect. Dis. 2005, 191, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Pyrc, K.; Berkhout, B.; van der Hoek, L. Identification of new human coronaviruses. Expert Rev. Anti Infect. Ther. 2007, 5, 245–253. [Google Scholar] [CrossRef]
- Liu, D.X.; Liang, J.Q.; Fung, T.S. Human Coronavirus-229E, -OC43, -NL63, and -HKU1 (Coronaviridae). Encycl. Virol. 2021, 2, 428–440. [Google Scholar] [CrossRef]
- Gorse, G.J.; Patel, G.B.; Vitale, J.N.; O’Connor, T.Z. Prevalence of antibodies to four human coronaviruses is lower in nasal secretions than in serum. Clin. Vaccine Immunol. 2010, 17, 1875–1880. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, H.; Wu, C.; Xiao, Y.; Ren, L.; Paranhos-Baccalà, G.; Guo, L.; Wang, J. Antibody against nucleocapsid protein predicts susceptibility to human coronavirus infection. J. Infect. 2015, 71, 599–602. [Google Scholar] [CrossRef]
- Dugas, M.; Grote-Westrick, T.; Merle, U.; Fontenay, M.; Kremer, A.E.; Hanses, F.; Vollenberg, R.; Lorentzen, E.; Tiwari-Heckler, S.; Duchemin, J.; et al. Lack of antibodies against seasonal coronavirus OC43 nucleocapsid protein identifies patients at risk of critical COVID-19. J. Clin. Virol. 2021, 139, 104847. [Google Scholar] [CrossRef]
- Loos, C.; Atyeo, C.; Fischinger, S.; Burke, J.; Slein, M.D.; Streeck, H.; Lauffenburger, D.; Ryan, E.T.; Charles, R.C.; Alter, G. Evolution of Early SARS-CoV-2 and Cross-Coronavirus Immunity. mSphere 2020, 5, e00622-20. [Google Scholar] [CrossRef]
- Anderson, E.M.; Li, S.H.; Awofolaju, M.; Eilola, T.; Goodwin, E.; Bolton, M.J.; Gouma, S.; Manzoni, T.B.; Hicks, P.; Goel, R.R.; et al. SARS-CoV-2 infections elicit higher levels of original antigenic sin antibodies compared with SARS-CoV-2 mRNA vaccinations. Cell Rep. 2022, 41, 111496. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Y.; Kang, L.; Hu, Y.; Wang, L.; Zhong, J.; Chen, H.; Ren, L.; Gu, X.; Wang, G.; et al. Cross-reactive antibody against human coronavirus OC43 spike protein correlates with disease severity in COVID-19 patients: A retrospective study. Emerg. Microbes Infect. 2021, 10, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Contant, P.; Embong, A.K.; Kanagaiah, P.; Chaves, F.A.; Yang, H.; Branche, A.R.; Topham, D.J.; Sangster, M.Y. S Protein-Reactive IgG and Memory B Cell Production after Human SARS-CoV-2 Infection Includes Broad Reactivity to the S2 Subunit. mBio 2020, 11, e01991-20. [Google Scholar] [CrossRef] [PubMed]
- Prévost, J.; Gasser, R.; Beaudoin-Bussières, G.; Richard, J.; Duerr, R.; Laumaea, A.; Anand, S.P.; Goyette, G.; Benlarbi, M.; Ding, S.; et al. Cross-Sectional Evaluation of Humoral Responses against SARS-CoV-2 Spike. Cell Rep. Med. 2020, 1, 100126. [Google Scholar] [CrossRef] [PubMed]
- Aydillo, T.; Rombauts, A.; Stadlbauer, D.; Aslam, S.; Abelenda-Alonso, G.; Escalera, A.; Amanat, F.; Jiang, K.; Krammer, F.; Carratala, J.; et al. Immunological imprinting of the antibody response in COVID-19 patients. Nat. Commun. 2021, 12, 3781. [Google Scholar] [CrossRef]
- Cohen, K.W.; Linderman, S.L.; Moodie, Z.; Czartoski, J.; Lai, L.; Mantus, G.; Norwood, C.; Nyhoff, L.E.; Edara, V.V.; Floyd, K.; et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep. Med. 2021, 2, 100354. [Google Scholar] [CrossRef]
- Gouma, S.; Weirick, M.E.; Bolton, M.J.; Arevalo, C.P.; Goodwin, E.C.; Anderson, E.M.; McAllister, C.M.; Christensen, S.R.; Dunbar, D.; Fiore, D.; et al. Health care worker seromonitoring reveals complex relationships between common coronavirus antibodies and COVID-19 symptom duration. JCI Insight 2021, 6, e150449. [Google Scholar] [CrossRef]
- Aguilar-Bretones, M.; Westerhuis, B.M.; Raadsen, M.P.; de Bruin, E.; Chandler, F.D.; Okba, N.M.; Haagmans, B.L.; Langerak, T.; Endeman, H.; Akker, J.P.v.D.; et al. Seasonal coronavirus-specific B cells with limited SARS-CoV-2 cross-reactivity dominate the IgG response in severe COVID-19. J. Clin. Investig. 2021, 131, e150613. [Google Scholar] [CrossRef]
- Becker, M.; Strengert, M.; Junker, D.; Kaiser, P.D.; Kerrinnes, T.; Traenkle, B.; Dinter, H.; Häring, J.; Ghozzi, S.; Zeck, A.; et al. Exploring beyond clinical routine SARS-CoV-2 serology using MultiCoV-Ab to evaluate endemic coronavirus cross-reactivity. Nat. Commun. 2021, 12, 1152. [Google Scholar] [CrossRef]
- Ortega, N.; Ribes, M.; Vidal, M.; Rubio, R.; Aguilar, R.; Williams, S.; Barrios, D.; Alonso, S.; Hernández-Luis, P.; Mitchell, R.A.; et al. Seven-month kinetics of SARS-CoV-2 antibodies and role of pre-existing antibodies to human coronaviruses. Nat. Commun. 2021, 12, 4740. [Google Scholar] [CrossRef]
- Ng, K.W.; Faulkner, N.; Cornish, G.H.; Rosa, A.; Harvey, R.; Hussain, S.; Ulferts, R.; Earl, C.; Wrobel, A.G.; Benton, D.J.; et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 2020, 370, 1339–1343. [Google Scholar] [CrossRef]
- Shrock, E.; Fujimura, E.; Kula, T.; Timms, R.T.; Lee, I.-H.; Leng, Y.; Robinson, M.L.; Sie, B.M.; Li, M.Z.; Chen, Y.; et al. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science 2020, 370, eabd4250. [Google Scholar] [CrossRef] [PubMed]
- Sealy, R.E.; Hurwitz, J.L. Cross-Reactive Immune Responses toward the Common Cold Human Coronaviruses and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Mini-Review and a Murine Study. Microorganisms 2021, 9, 1643. [Google Scholar] [CrossRef] [PubMed]
- Gombar, S.; Bergquist, T.; Pejaver, V.; Hammarlund, N.E.; Murugesan, K.; Mooney, S.; Shah, N.; Pinsky, B.A.; Banaei, N. SARS-CoV-2 infection and COVID-19 severity in individuals with prior seasonal coronavirus infection. Diagn. Microbiol. Infect. Dis. 2021, 100, 115338. [Google Scholar] [CrossRef] [PubMed]
- Henss, L.; Scholz, T.; von Rhein, C.; Wieters, I.; Borgans, F.; Eberhardt, F.J.; Zacharowski, K.; Ciesek, S.; Rohde, G.; Vehreschild, M.; et al. Analysis of Humoral Immune Responses in Patients with Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J. Infect. Dis. 2021, 223, 56–61. [Google Scholar] [CrossRef]
- Sagar, M.; Reifler, K.; Rossi, M.; Miller, N.S.; Sinha, P.; White, L.F.; Mizgerd, J.P. Recent endemic coronavirus infection is associated with less-severe COVID-19. J. Clin. Investig. 2021, 131, e143380. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; Astudillo, M.G.; Yang, D.; Miller, T.E.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; Clayton, K.L.; Nitido, A.D.; et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell 2021, 184, 476–488.e11. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Jin, J.M.; Bai, P.; He, W.; Wu, F.; Liu, X.F.; Han, D.M.; Liu, S.; Yang, J.K. Gender Differences in Patients with COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef]
- Adams, O.; Andrée, M.; Rabl, D.; Ostermann, P.N.; Schaal, H.; Lehnert, E.; Ackerstaff, S.; Müller, L.; Fischer, J.C. Humoral response to SARS-CoV-2 and seasonal coronaviruses in COVID-19 patients. J. Med. Virol. 2022, 94, 1096–1103. [Google Scholar] [CrossRef]
- Imai, K.; Matsuoka, M.; Tabata, S.; Kitagawa, Y.; Nagura-Ikeda, M.; Kubota, K.; Fukada, A.; Takada, T.; Sato, M.; Noguchi, S.; et al. Cross-reactive humoral immune responses against seasonal human coronaviruses in COVID-19 patients with different disease severities. Int. J. Infect. Dis. 2021, 111, 68–75. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Wolf, J.; Brice, D.C.; Sun, Y.; Locke, M.; Cherry, S.; Castellaw, A.H.; Wehenkel, M.; Crawford, J.C.; Zarnitsyna, V.I.; et al. Pre-existing humoral immunity to human common cold coronaviruses negatively impacts the protective SARS-CoV-2 antibody response. Cell Host Microbe 2022, 30, 83–96.e4. [Google Scholar] [CrossRef] [PubMed]
- Ladner, J.T.; Henson, S.N.; Boyle, A.S.; Engelbrektson, A.L.; Fink, Z.W.; Rahee, F.; D’ambrozio, J.; Schaecher, K.E.; Stone, M.; Dong, W.; et al. Epitope-resolved profiling of the SARS-CoV-2 antibody response identifies cross-reactivity with endemic human coronaviruses. Cell Rep. Med. 2021, 2, 100189. [Google Scholar] [CrossRef] [PubMed]
- Wec, A.Z.; Wrapp, D.; Herbert, A.S.; Maurer, D.P.; Haslwanter, D.; Sakharkar, M.; Jangra, R.K.; Dieterle, M.E.; Lilov, A.; Huang, D.; et al. Broad neutralization of SARS-related viruses by human monoclonal antibodies. Science 2020, 369, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Saletti, G.; Gerlach, T.; Jansen, J.M.; Molle, A.; Elbahesh, H.; Ludlow, M.; Li, W.; Bosch, B.-J.; Osterhaus, A.D.M.E.; Rimmelzwaan, G.F. Older adults lack SARS CoV-2 cross-reactive T lymphocytes directed to human coronaviruses OC43 and NL63. Sci. Rep. 2020, 10, 21447. [Google Scholar] [CrossRef]
- Dobaño, C.; Santano, R.; Jiménez, A.; Vidal, M.; Chi, J.; Melero, N.R.; Popovic, M.; López-Aladid, R.; Fernández-Barat, L.; Tortajada, M.; et al. Immunogenicity and crossreactivity of antibodies to the nucleocapsid protein of SARS-CoV-2: Utility and limitations in seroprevalence and immunity studies. Transl. Res. 2021, 232, 60–74. [Google Scholar] [CrossRef]
- Miyara, M.; Saichi, M.; Sterlin, D.; Anna, F.; Marot, S.; Mathian, A.; Atif, M.; Quentric, P.; Mohr, A.; Claër, L.; et al. Pre-COVID-19 Immunity to Common Cold Human Coronaviruses Induces a Recall-Type IgG Response to SARS-CoV-2 Antigens Without Cross-Neutralisation. Front. Immunol. 2022, 13, 790334. [Google Scholar] [CrossRef]
- Premkumar, L.; Segovia-Chumbez, B.; Jadi, R.; Martinez, D.R.; Raut, R.; Markmann, A.; Cornaby, C.; Bartelt, L.; Weiss, S.; Park, Y.; et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 2020, 5, eabc8413. [Google Scholar] [CrossRef]
- Li, X.; Luk, H.K.H.; Lau, S.K.P.; Woo, P.C.Y. Human Coronaviruses: General Features. Ref. Modul. Biomed. Sci. 2019, 1–6. [Google Scholar] [CrossRef]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165878. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, S.; Teng, T.; Abdalla, A.E.; Zhu, W.; Xie, L.; Wang, Y.; Guo, X. Systematic Comparison of Two Animal-to-Human Transmitted Human Coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses 2020, 12, 244. [Google Scholar] [CrossRef]
- Wratil, P.R.; Schmacke, N.A.; Karakoc, B.; Dulovic, A.; Junker, D.; Becker, M.; Rothbauer, U.; Osterman, A.; Spaeth, P.M.; Ruhle, A.; et al. Evidence for increased SARS-CoV-2 susceptibility and COVID-19 severity related to pre-existing immunity to seasonal coronaviruses. Cell Rep. 2021, 37, 110169. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, T.W.; Davies, J.R.; Smith, A.J.; Miller, C.L.; Allchin, A. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ’s Hospital. Lancet 1979, 1, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Clark, J.; Carreño, J.M.; Strohmeier, S.; Yellin, T.; Meade, P.S.; Bhavsar, D.; Muramatsu, H.; Sun, W.; Coughlan, L.; et al. Immunity to Seasonal Coronavirus Spike Proteins Does Not Protect from SARS-CoV-2 Challenge in a Mouse Model but Has No Detrimental Effect on Protection Mediated by COVID-19 mRNA Vaccination. J. Virol. 2023, 97, e0166422. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, Z.; Ren, L.; Hao, Y.; Zhu, M.; Jiang, H.; Wang, S.; Li, D.; Shao, Y. Pre-existing anti-HCoV-OC43 immunity influences the durability and cross-reactivity of humoral response to SARS-CoV-2 vaccination. Front. Cell. Infect. Microbiol. 2022, 12, 978440. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Han, Z.; Lang, B.; Li, Y.; Mai, G.; Chen, H.; Feng, L.; Chen, Y.; Luo, H.; Xiong, Y.; et al. Effect of seasonal coronavirus immune imprinting on the immunogenicity of inactivated COVID-19 vaccination. Front. Immunol. 2023, 14, 1195533. [Google Scholar] [CrossRef] [PubMed]
| COVID-19-Negative | COVID-19-Mild | Logistic Regression (p Value) | |||||
|---|---|---|---|---|---|---|---|
| IgG Status a | Negative | Positive | Negative | Positive | |||
| Cut-Off (Sample/Control) | |||||||
| H-CoV-NL63 | 0.5 | 71 | 12 | 77 | 18 | 0.423 | |
| 1.0 | 77 | 6 | 90 | 5 | 0.972 | ||
| H-CoV-HKU1 | 0.5 | 61 | 22 | 56 | 39 | 0.040 | |
| 1.0 | 72 | 11 | 78 | 17 | 0.122 | ||
| H-CoV-229E | 0.5 | 58 | 25 | 59 | 36 | 0.275 | |
| 1.0 | 79 | 4 | 83 | 12 | 0.024 | ||
| H-CoV-OC43 | 0.5 | 48 | 35 | 43 | 52 | 0.094 | |
| 1.0 | 65 | 18 | 64 | 31 | 0.038 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De La Torre Tarazona, E.; Jiménez, D.; Marcos-Mencía, D.; Mendieta-Baro, A.; Rivera-Delgado, A.; Romero-Hernández, B.; Muriel, A.; Rodríguez-Domínguez, M.; Serrano-Villar, S.; Moreno, S. The Influence of Pre-Existing Immunity against Human Common Cold Coronaviruses on COVID-19 Susceptibility and Severity. Microbiol. Res. 2023, 14, 1364-1375. https://doi.org/10.3390/microbiolres14030093
De La Torre Tarazona E, Jiménez D, Marcos-Mencía D, Mendieta-Baro A, Rivera-Delgado A, Romero-Hernández B, Muriel A, Rodríguez-Domínguez M, Serrano-Villar S, Moreno S. The Influence of Pre-Existing Immunity against Human Common Cold Coronaviruses on COVID-19 Susceptibility and Severity. Microbiology Research. 2023; 14(3):1364-1375. https://doi.org/10.3390/microbiolres14030093
Chicago/Turabian StyleDe La Torre Tarazona, Erick, Daniel Jiménez, Daniel Marcos-Mencía, Alejandro Mendieta-Baro, Alejandro Rivera-Delgado, Beatriz Romero-Hernández, Alfonso Muriel, Mario Rodríguez-Domínguez, Sergio Serrano-Villar, and Santiago Moreno. 2023. "The Influence of Pre-Existing Immunity against Human Common Cold Coronaviruses on COVID-19 Susceptibility and Severity" Microbiology Research 14, no. 3: 1364-1375. https://doi.org/10.3390/microbiolres14030093
APA StyleDe La Torre Tarazona, E., Jiménez, D., Marcos-Mencía, D., Mendieta-Baro, A., Rivera-Delgado, A., Romero-Hernández, B., Muriel, A., Rodríguez-Domínguez, M., Serrano-Villar, S., & Moreno, S. (2023). The Influence of Pre-Existing Immunity against Human Common Cold Coronaviruses on COVID-19 Susceptibility and Severity. Microbiology Research, 14(3), 1364-1375. https://doi.org/10.3390/microbiolres14030093







