Integrated Analysis of Long Non-Coding RNA Expression Profiles in Glaesserella parasuis-Induced Meningitis: New Insight into Pathogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Bacterial and Animals
2.3. Experimental Design
2.4. RNA Extraction and Illumina Sequencing
2.5. Transcriptome Data Analysis
2.6. lncRNA Prediction
2.7. Prediction of lncRNA Targets
2.8. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis
2.9. Protein-Protein Interaction Network Construction
2.10. Co-Expression Analysis of lncRNA and mRNA
2.11. Quantitative Real-Time PCR (qRT-PCR)
3. Results
3.1. Statistics of lncRNA Sequencing Data
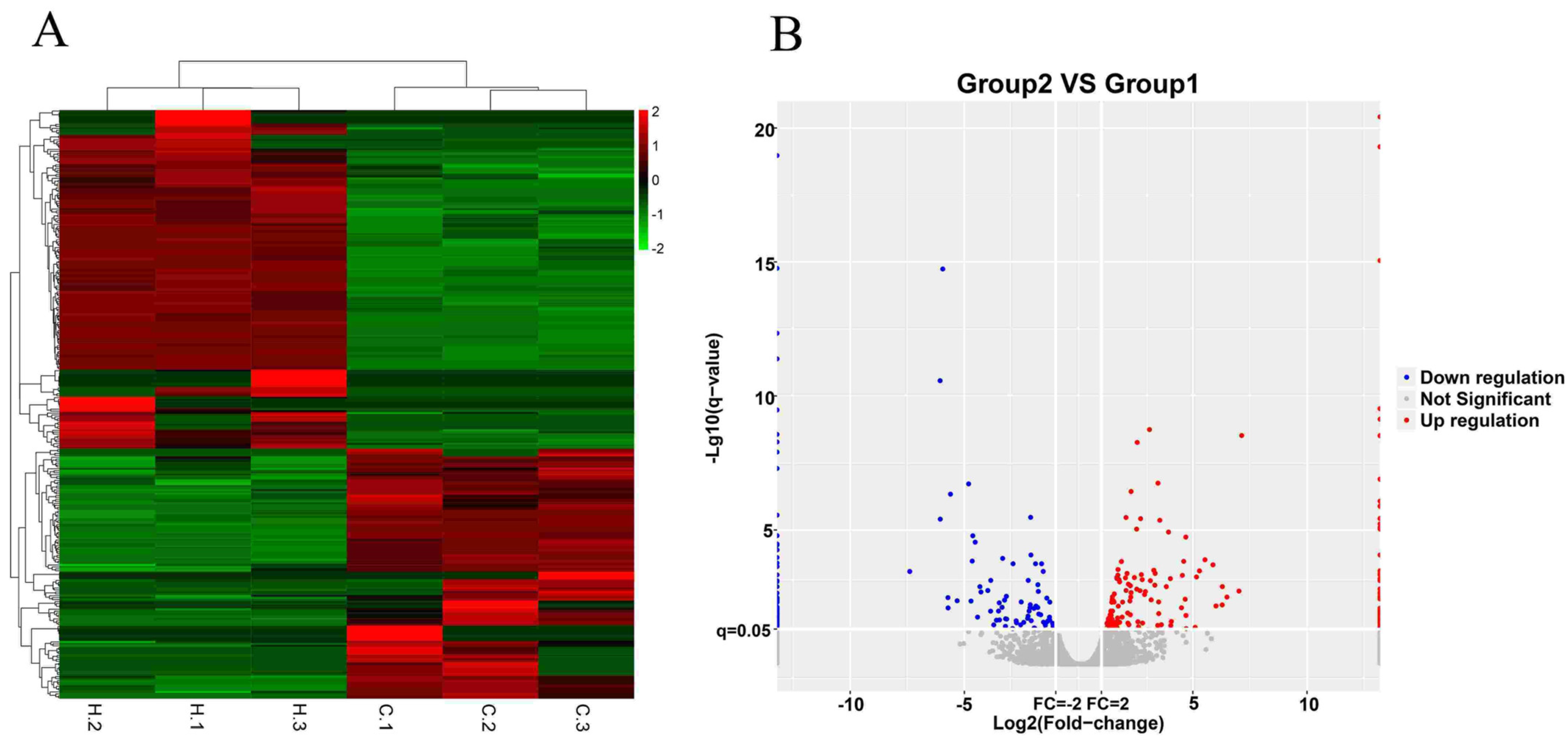
3.2. Differentially Expressed lncRNAs
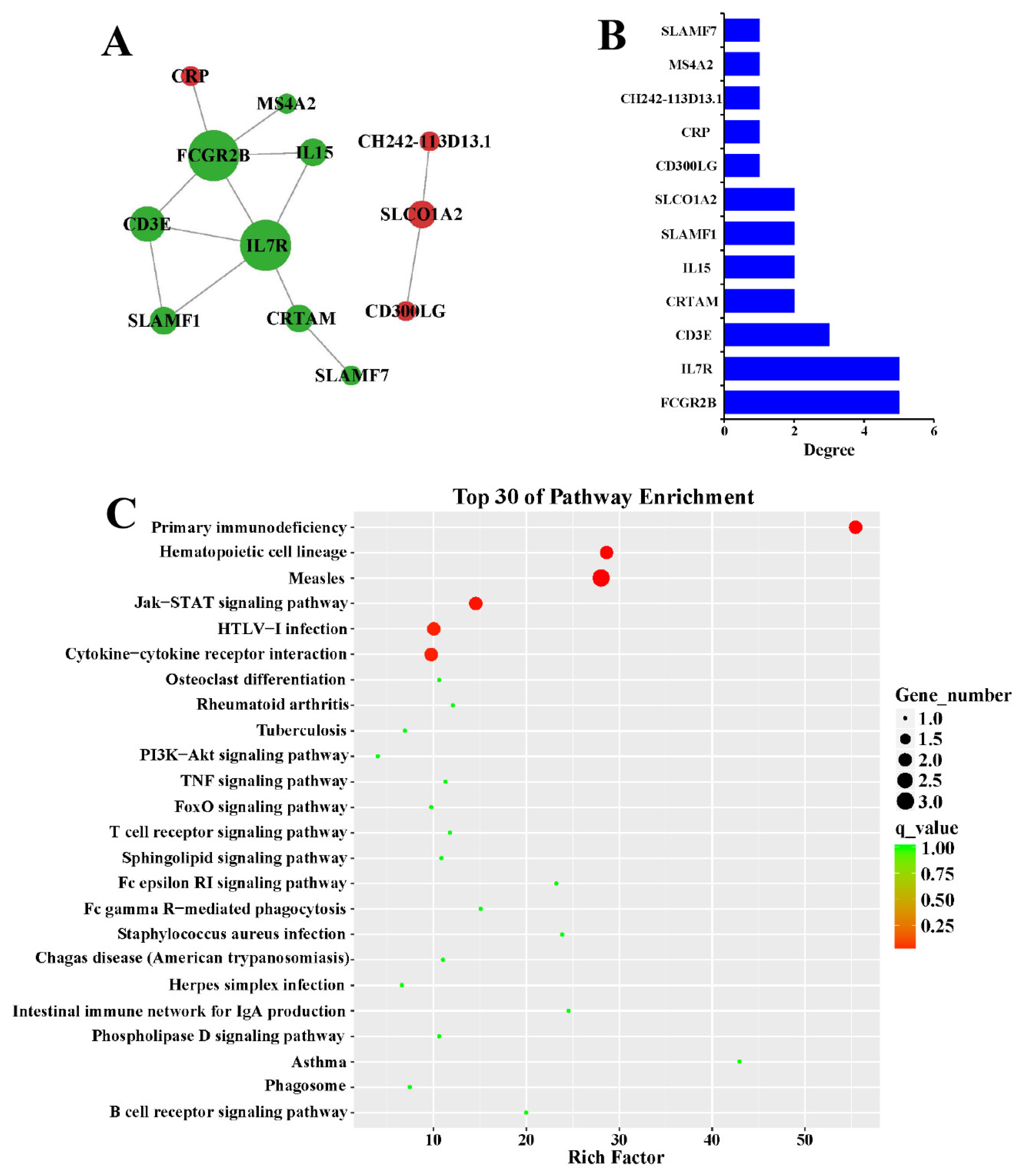
3.3. Target Prediction and Functional Enrichment
3.4. Protein-Protein Interaction Network
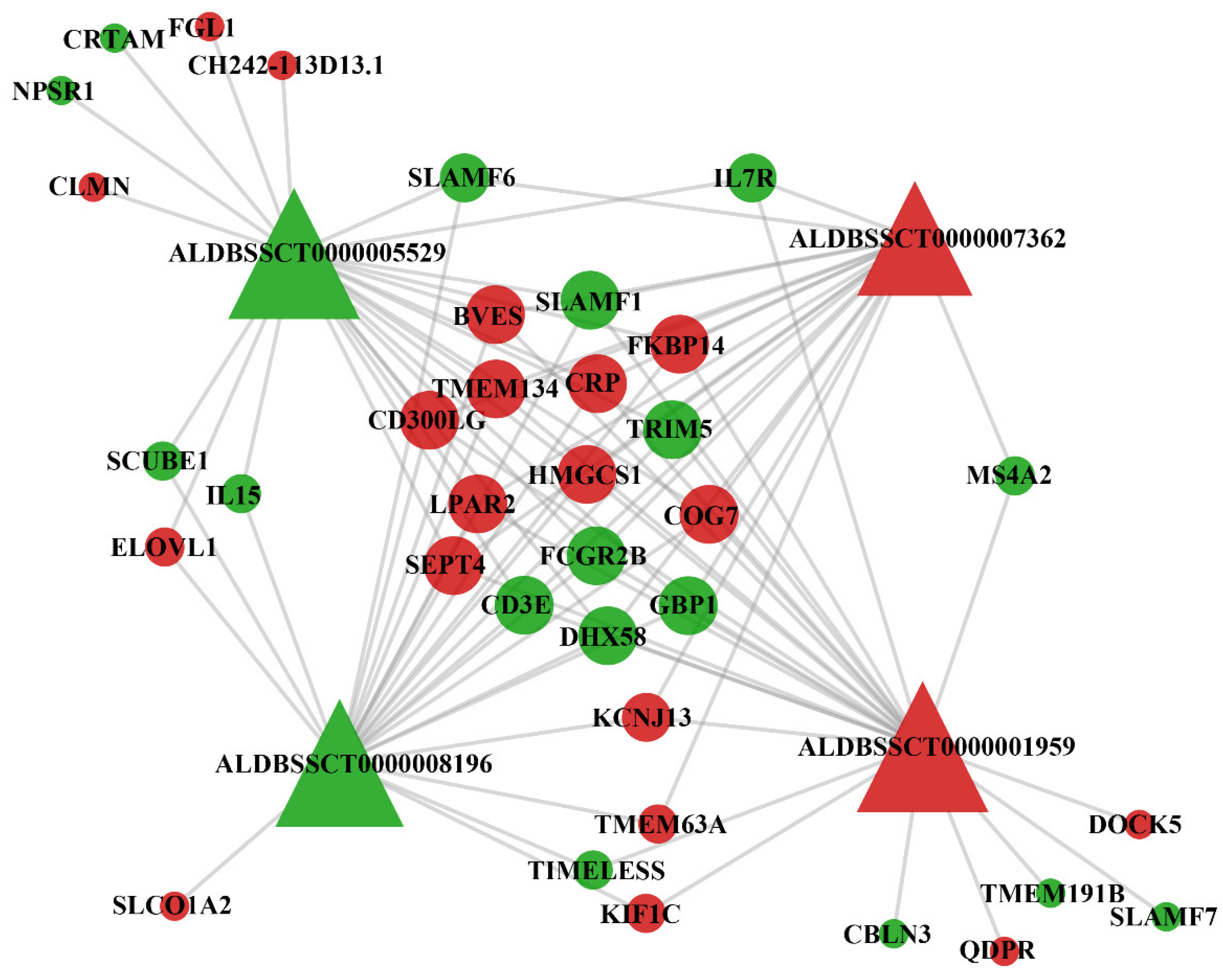
3.5. Co-Expression Analysis of DElncRNAs and DEmRNAs
3.6. Validation of the Expression of lncRNAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, L.; Cheng, H.; Fu, S.; Liu, J.; Zhang, Y.; Qiu, Y.; Chen, H. Methylome and Transcriptome-Based Integration Analysis Identified Molecular Signatures Associated with Meningitis Induced by Glaesserella parasuis. Front. Immunol. 2022, 13, 840399. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Yin, R.; Zuo, S.; Liu, J.; Zhang, Y.; Guo, L.; Qiu, Y.; Ye, C.; Liu, Y.; Wu, Z.; et al. The effects of baicalin on piglets challenged with Glaesserella parasuis. Vet. Res. 2020, 51, 102. [Google Scholar] [CrossRef]
- Rafiee, M.; Blackall, P.J. Establishment, validation and use of the Kielstein-Rapp-Gabrielson serotyping scheme for Haemophilus parasuis. Aust. Vet. J. 2000, 78, 172–174. [Google Scholar] [CrossRef]
- Qi, B.; Li, F.; Chen, K.; Ding, W.; Xue, Y.; Wang, Y.; Wang, H.; Ding, K.; Zhao, Z. Comparison of the Glaesserella parasuis Virulence in Mice and Piglets. Front. Vet. Sci. 2021, 8, 659244. [Google Scholar] [CrossRef]
- Vanier, G.; Szczotka, A.; Friedl, P.; Lacouture, S.; Jacques, M.; Gottschalk, M. Haemophilus parasuis invades porcine brain microvascular endothelial cells. Microbiology 2006, 152, 135–142. [Google Scholar] [CrossRef]
- Ren, Z.; Yu, Y.; Chen, C.; Yang, D.; Ding, T.; Zhu, L.; Deng, J.; Xu, Z. The Triangle Relationship Between Long Noncoding RNA, RIG-I-like Receptor Signaling Pathway, and Glycolysis. Front. Microbiol. 2021, 12, 807737. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Q.; Yang, D.; Xie, F.; Wang, Z. The role of long non-coding RNAs in angiogenesis and anti-angiogenic therapy resistance in cancer. Mol. Ther. Nucleic Acids 2022, 28, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, X.; Zhang, Y.; Yang, J.; Li, Z.; Wu, L.; Wu, J.; Wu, N.; Liu, L.; Liu, Z.; et al. Dynamic characteristics and functional analysis provide new insights into long non-coding RNA responsive to Verticillium dahliae infection in Gossypium hirsutum. BMC Plant Biol. 2021, 21, 68. [Google Scholar] [CrossRef]
- Huang, W.; Zhong, W.; He, Q.; Xu, Y.; Lin, J.; Ding, Y.; Zhao, H.; Zheng, X.; Zheng, Y. Time-series expression profiles of mRNAs and lncRNAs during mammalian palatogenesis. Oral Dis. 2023, 29, 2163–2176. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.H.; Guo, Z.B.; Zhou, Y.Y.; Wang, C.; Yin, R.L.; Bai, W.L. LncRNA-MEG3 Regulates the Inflammatory Responses and Apoptosis in Porcine Alveolar Macrophages Infected with Haemophilus parasuis Through Modulating the miR-210/TLR4 Axis. Curr. Microbiol. 2021, 78, 3152–3164. [Google Scholar] [CrossRef]
- Dong, J.; Teng, F.; Guo, W.; Yang, J.; Ding, G.; Fu, Z. lncRNA SNHG8 Promotes the Tumorigenesis and Metastasis by Sponging miR-149-5p and Predicts Tumor Recurrence in Hepatocellular Carcinoma. Cell Physiol. Biochem. 2018, 51, 2262–2274. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yang, R.; Yang, B.; Li, L.; Chen, J.; Fu, J.; Qu, X.; Huo, D.; Tan, C.; Chen, H.; et al. Long non-coding RNA lncC11orf54-1 modulates neuroinflammatory responses by activating NF-κB signaling during meningitic Escherichia coli infection. Mol. Brain 2022, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Akavaram, S.; Bayles, D.O. Genomewide transcriptional response of Escherichia coli O157:H7 to norepinephrine. BMC Genom. 2022, 23, 107. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.M.; Zhang, Z.; Liu, J.B.; Li, N.; Yang, G.W.; Luo, D.; Zhang, Y.; Yuan, B.; Jiang, H.; Zhang, J.B. Genome-wide identification and analysis of long noncoding RNAs in longissimus muscle tissue from Kazakh cattle and Xinjiang brown cattle. Anim. Biosci. 2021, 34, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Ueti, M.W.; Johnson, W.C.; Kappmeyer, L.S.; Herndon, D.R.; Mousel, M.R.; Reif, K.E.; Taus, N.S.; Ifeonu, O.O.; Silva, J.C.; Suarez, C.E.; et al. Transcriptome dataset of Babesia bovis life stages within vertebrate and invertebrate hosts. Data Brief 2020, 33, 106533. [Google Scholar] [CrossRef] [PubMed]
- Osabe, T.; Shimizu, K.; Kadota, K. Differential expression analysis using a model-based gene clustering algorithm for RNA-seq data. BMC Bioinform. 2021, 22, 511. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Z.; Li, J.; Bao, H.; Wu, C. Genome-Wide Association Study and Transcriptome Differential Expression Analysis of the Feather Rate in Shouguang Chickens. Front. Genet. 2020, 11, 613078. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.; Han, K.; Zhang, G.; Wang, J.; Xie, K.; Xue, Q.; Fan, X. Analysis of long noncoding RNA and mRNA using RNA sequencing during the differentiation of intramuscular preadipocytes in chicken. PLoS ONE 2017, 12, e0172389. [Google Scholar] [CrossRef]
- Zhang, S.; Tong, Y.; Li, Y.; Cheng, Z.M.; Zhong, Y. Genome-wide identification of the HKT genes in five Rosaceae species and expression analysis of HKT genes in response to salt-stress in Fragaria vesca. Genes Genom. 2019, 41, 325–336. [Google Scholar] [CrossRef]
- Liu, X.Q.; Li, B.X.; Zeng, G.R.; Liu, Q.Y.; Ai, D.M. Prediction of Long Non-Coding RNAs Based on Deep Learning. Genes 2019, 10, 273. [Google Scholar] [CrossRef]
- Guo, J.C.; Fang, S.S.; Wu, Y.; Zhang, J.H.; Chen, Y.; Liu, J.; Wu, B.; Wu, J.R.; Li, E.M.; Xu, L.Y.; et al. CNIT: A fast and accurate web tool for identifying protein-coding and long non-coding transcripts based on intrinsic sequence composition. Nucleic Acids Res. 2019, 47, W516–W522. [Google Scholar] [CrossRef]
- Feng, X.; Han, H.; Guo, Y.; Feng, X.; Guo, S.; Zhou, W. LncRNA ENST869 Targeting Nestin Transcriptional Region to Affect the Pharmacological Effects of Chidamide in Breast Cancer Cells. Front. Oncol. 2022, 12, 874343. [Google Scholar] [CrossRef]
- He, L.; Yan, X.; Dai, K.; Wen, X.; Cao, S.; Huang, X.; Wu, R.; Zhao, Q.; Huang, Y.; Yan, Q.; et al. Comparative transcriptome analysis reveals that deletion of CheY influences gene expressions of ABC transports and metabolism in Haemophilus parasuis. Funct. Integr. Genom. 2021, 21, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Iannello, A.; Ciarrocchi, A.; Fragliasso, V.; Vaisitti, T. Lift the curtain on long non-coding RNAs in hematological malignancies: Pathogenic elements and potential targets. Cancer Lett. 2022, 536, 215645. [Google Scholar] [CrossRef] [PubMed]
- Espinal, E.R.; Matthews, T.; Holder, B.M.; Bee, O.B.; Humber, G.M.; Brook, C.E.; Divyapicigil, M.; Sharp, J.; Kim, B.J. Group B Streptococcus-Induced Macropinocytosis Contributes to Bacterial Invasion of Brain Endothelial Cells. Pathogens 2022, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, P.; Satchell, S.C.; Ramnath, R. Cerebral microvascular endothelial glycocalyx damage, its implications on the blood-brain barrier and a possible contributor to cognitive impairment. Brain Res. 2022, 1780, 147804. [Google Scholar] [CrossRef]
- Ghahramani Almanghadim, H.; Ghorbian, S.; Khademi, N.S.; Soleymani Sadrabadi, M.; Jarrahi, E.; Nourollahzadeh, Z.; Dastani, M.; Shirvaliloo, M.; Sheervalilou, R.; Sargazi, S. New Insights into the Importance of Long Non-Coding RNAs in Lung Cancer: Future Clinical Approaches. DNA Cell Biol. 2021, 40, 1476–1494. [Google Scholar] [CrossRef]
- Sadrkhanloo, M.; Entezari, M.; Orouei, S.; Zabolian, A.; Mirzaie, A.; Maghsoudloo, A.; Raesi, R.; Asadi, N.; Hashemi, M.; Zarrabi, A.; et al. Targeting Nrf2 in ischemia-reperfusion alleviation: From signaling networks to therapeutic targeting. Life Sci. 2022, 300, 120561. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xu, B.; Yang, R.; Fu, J.; Li, L.; Huo, D.; Chen, J.; Yang, X.; Tan, C.; Chen, H.; et al. Long Non-coding Antisense RNA DDIT4-AS1 Regulates Meningitic Escherichia coli-Induced Neuroinflammation by Promoting DDIT4 mRNA Stability. Mol. Neurobiol. 2022, 59, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yang, R.; Fu, J.; Yang, B.; Chen, J.; Tan, C.; Chen, H.; Wang, X. LncRSPH9-4 Facilitates Meningitic Escherichia coli-Caused Blood-Brain Barrier Disruption via miR-17-5p/MMP3 Axis. Int. J. Mol. Sci. 2021, 22, 6343. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Xie, X.; Zhou, J.; Fang, X.; Wang, F.; Wang, M. Identification of TAF1, SAT1, and ARHGEF9 as DNA methylation biomarkers for hepatocellular carcinoma. J. Cell Physiol. 2020, 235, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.E.; Martin-Ramirez, J.; Boross, P.; Mangsbo, S.M.; Reynolds, J.; Moss, J.; Pusey, C.D.; Cook, H.T.; Tarzi, R.M.; Verbeek, J.S. Increased incidence of anti-GBM disease in Fcgamma receptor 2b deficient mice, but not mice with conditional deletion of Fcgr2b on either B cells or myeloid cells alone. Mol. Immunol. 2012, 50, 49–56. [Google Scholar] [CrossRef]
- Li, Q.; Zhong, J.; Luo, H.; Urbonaviciute, V.; Xu, Z.; He, C.; Holmdahl, R. Two major genes associated with autoimmune arthritis, Ncf1 and Fcgr2b, additively protect mice by strengthening T cell tolerance. Cell Mol. Life Sci. 2022, 79, 482. [Google Scholar] [CrossRef] [PubMed]
- Petty, A.; Glass, L.J.; Rothmond, D.A.; Purves-Tyson, T.; Sweeney, A.; Kondo, Y.; Kubo, S.; Matsumoto, M.; Weickert, C.S. Increased levels of a pro-inflammatory IgG receptor in the midbrain of people with schizophrenia. J. Neuroinflamm. 2022, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, J.; Li, Y.; Zhao, R.; Du, S.; Lv, C.; Wu, W.; Liu, R.; Sheng, X.; Song, Y.; et al. MicroRNA-31 Reduces Inflammatory Signaling and Promotes Regeneration in Colon Epithelium, and Delivery of Mimics in Microspheres Reduces Colitis in Mice. Gastroenterology 2019, 156, 2281–2296.e2286. [Google Scholar] [CrossRef] [PubMed]
- Beristain-Covarrubias, N.; Canche-Pool, E.B.; Ramirez-Velazquez, C.; Barragan-Galvez, J.C.; Gomez-Diaz, R.A.; Ortiz-Navarrete, V. Class I-Restricted T Cell-Associated Molecule Is a Marker for IFN-γ-Producing iNKT Cells in Healthy Subjects and Patients with Type 1 Diabetes. J. Interferon Cytokine Res. 2017, 37, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lopez, A.; Nuccio, S.P.; Ushach, I.; Edwards, R.A.; Pahu, R.; Silva, S.; Zlotnik, A.; Raffatellu, M. CRTAM Shapes the Gut Microbiota and Enhances the Severity of Infection. J. Immunol. 2019, 203, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, M.; Skjesol, A.; Ryan, L.; Richard, G.M.; Kandasamy, R.K.; Wang, N.; Terhorst, C.; Husebye, H.; Espevik, T. SLAMF1 is required for TLR4-mediated TRAM-TRIF-dependent signaling in human macrophages. J. Cell Biol. 2018, 217, 1411–1429. [Google Scholar] [CrossRef]
- Bergemalm, D.; Andersson, E.; Hultdin, J.; Eriksson, C.; Rush, S.T.; Kalla, R.; Adams, A.T.; Keita, Å.V.; D’Amato, M.; Gomollon, F.; et al. Systemic Inflammation in Preclinical Ulcerative Colitis. Gastroenterology 2021, 161, 1526–1539.e1529. [Google Scholar] [CrossRef]
- Abraham, C.; Abreu, M.T.; Turner, J.R. Pattern Recognition Receptor Signaling and Cytokine Networks in Microbial Defenses and Regulation of Intestinal Barriers: Implications for Inflammatory Bowel Disease. Gastroenterology 2022, 162, 1602–1616.e1606. [Google Scholar] [CrossRef]
- Gong, P.; Zhang, Z.; Zou, Y.; Tian, Q.; Han, S.; Xu, Z.; Liao, J.; Gao, L.; Chen, Q.; Li, M. Tetramethylpyrazine attenuates blood-brain barrier disruption in ischemia/reperfusion injury through the JAK/STAT signaling pathway. Eur. J. Pharmacol. 2019, 854, 289–297. [Google Scholar] [CrossRef] [PubMed]
- King-Robson, J.; Hampton, T.; Rosadas, C.; Taylor, G.P.; Stanton, B. HTLV-1 encephalitis. Pract. Neurol. 2022, 22, 60–63. [Google Scholar] [CrossRef]
- Moles, R.; Sarkis, S.; Galli, V.; Omsland, M.; Artesi, M.; Bissa, M.; McKinnon, K.; Brown, S.; Hahaut, V.; Washington-Parks, R.; et al. NK cells and monocytes modulate primary HTLV-1 infection. PLoS Pathog. 2022, 18, e1010416. [Google Scholar] [CrossRef] [PubMed]
- Oppegaard, K.; Harris, C.S.; Shin, J.; Paul, S.M.; Cooper, B.A.; Chan, A.; Anguera, J.A.; Levine, J.; Conley, Y.; Hammer, M.; et al. Cancer-related cognitive impairment is associated with perturbations in inflammatory pathways. Cytokine 2021, 148, 155653. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lu, Y.; Sun, W.; Han, M.; Zhang, Y.; Zhang, J. Changing expression profiles of lncRNAs, circRNAs and mRNAs in esophageal squamous carcinoma. Oncol. Lett. 2019, 18, 5363–5373. [Google Scholar] [CrossRef] [PubMed]
- Barlev, A.N.; Malkiel, S.; Kurata-Sato, I.; Dorjée, A.L.; Suurmond, J.; Diamond, B. FcγRIIB regulates autoantibody responses by limiting marginal zone B cell activation. J. Clin. Investig. 2022, 132, e157250. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.P.; Roghanian, A.; Oldham, R.J.; Chan, H.T.C.; Penfold, C.A.; Kim, H.J.; Inzhelevskaya, T.; Mockridge, C.I.; Cox, K.L.; Bogdanov, Y.D.; et al. FcγRIIB controls antibody-mediated target cell depletion by ITIM-independent mechanisms. Cell Rep. 2022, 40, 111099. [Google Scholar] [CrossRef]
- Pellegrini, J.M.; Sabbione, F.; Morelli, M.P.; Tateosian, N.L.; Castello, F.A.; Amiano, N.O.; Palmero, D.; Levi, A.; Ciallella, L.; Colombo, M.I.; et al. Neutrophil autophagy during human active tuberculosis is modulated by SLAMF1. Autophagy 2021, 17, 2629–2638. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, Y.; Tian, Y. SLAMF1 promotes methotrexate resistance via activating autophagy in choriocarcinoma cells. Cancer Manag. Res. 2021, 12, 13427–13436. [Google Scholar] [CrossRef]
- Nieves, D.J.; Pandzic, E.; Gunasinghe, S.D.; Goyette, J.; Owen, D.M.; Justin Gooding, J.; Gaus, K. The T cell receptor displays lateral signal propagation involving non-engaged receptors. Nanoscale 2022, 14, 3513–3526. [Google Scholar] [CrossRef]
- Pathak, A.; Agrawal, A. Evolution of C-reactive protein. Front. Immunol. 2019, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, B.; Zhang, Z.; Huang, Y.; Xu, Z.; Chen, X.; Cai, J.; Huang, Y.; Jian, J. CRP involved in Nile tilapia (Oreochromis niloticus) against bacterial infection. Biology 2022, 11, 1149. [Google Scholar] [CrossRef] [PubMed]



| Gene | Nucleotide Sequence (5′-3′) | Tm (℃) | Length (bp) | |
|---|---|---|---|---|
| ALDBSSCT0000001959 | Forward | GGGAGGTTGTTCAGTCGCTAT | 62 | 120 |
| Reverse | GCCCTGGCTTGTTTCTTGC | |||
| ALDBSSCT0000007362 | Forward | GTTGGAATTAGCCGCTTCG | 58 | 152 |
| Reverse | GTTGTCTGCTCGTCTCCTGTT | |||
| ALDBSSCT0000005529 | Forward | TCAGGGGTAAACGGCAACAA | 60 | 138 |
| Reverse | ACCAGGTGACAACGAAGCAA | |||
| MSTRG.2939.1 | Forward | CTCTGGTAGGTCCTCGGTCA | 60 | 182 |
| Reverse | ATTGTGCCAAGTGCGGTCT | |||
| MSTRG.32374.1 | Forward | CCTGTCCTGTCCTTGAGAGC | 62 | 137 |
| Reverse | TCCCCTCAGAGTGACTGCTT | |||
| β-actin | Forward | TGCGGGACATCAAGGAGAAG | 59 | 216 |
| Reverse | AGTTGAAGGTGGTCTCGTGG | |||
| Sample | Raw Reads | Clean Reads | Clean Ratio | No rRNA Pair | Mapped Reads | Mapping Ratio | Mapped Unique Reads |
|---|---|---|---|---|---|---|---|
| C-1 | 64,124,710 | 61,586,207 | 96.04% | 60,412,722 | 52,262,851 | 86.51% | 51,951,853 |
| C-2 | 69,686,926 | 66,887,581 | 95.98% | 65,559,666 | 56,684,179 | 86.46% | 56,346,602 |
| C-3 | 61,066,694 | 58,592,815 | 95.95% | 57,427,572 | 49,605,377 | 86.38% | 49,306,096 |
| H-1 | 63,440,584 | 61,501,011 | 96.94% | 60,510,314 | 52,479,035 | 86.73% | 52,159,338 |
| H-2 | 74,152,268 | 71,256,636 | 96.10% | 69,862,342 | 60,585,217 | 86.72% | 60,211,534 |
| H-3 | 71,567,326 | 68,667,614 | 95.95% | 67,215,096 | 58,278,436 | 86.70% | 57,911,410 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, P.; Yang, Y.; Cheng, H.; Fu, S.; Liu, Y.; Qiu, Y.; Chen, H.; Zhang, J.; Zhou, H.; Shi, L.; et al. Integrated Analysis of Long Non-Coding RNA Expression Profiles in Glaesserella parasuis-Induced Meningitis: New Insight into Pathogenesis. Microbiol. Res. 2023, 14, 1427-1441. https://doi.org/10.3390/microbiolres14030097
Sun P, Yang Y, Cheng H, Fu S, Liu Y, Qiu Y, Chen H, Zhang J, Zhou H, Shi L, et al. Integrated Analysis of Long Non-Coding RNA Expression Profiles in Glaesserella parasuis-Induced Meningitis: New Insight into Pathogenesis. Microbiology Research. 2023; 14(3):1427-1441. https://doi.org/10.3390/microbiolres14030097
Chicago/Turabian StyleSun, Peiyan, Yaqiong Yang, Hongxing Cheng, Shulin Fu, Yulan Liu, Yinsheng Qiu, Hongbo Chen, Jing Zhang, Huanhuan Zhou, Liangyu Shi, and et al. 2023. "Integrated Analysis of Long Non-Coding RNA Expression Profiles in Glaesserella parasuis-Induced Meningitis: New Insight into Pathogenesis" Microbiology Research 14, no. 3: 1427-1441. https://doi.org/10.3390/microbiolres14030097
APA StyleSun, P., Yang, Y., Cheng, H., Fu, S., Liu, Y., Qiu, Y., Chen, H., Zhang, J., Zhou, H., Shi, L., Ren, H., Chao, Z., & Guo, L. (2023). Integrated Analysis of Long Non-Coding RNA Expression Profiles in Glaesserella parasuis-Induced Meningitis: New Insight into Pathogenesis. Microbiology Research, 14(3), 1427-1441. https://doi.org/10.3390/microbiolres14030097






