The Interaction between the Oral Microbiome and Systemic Diseases: A Narrative Review
Abstract
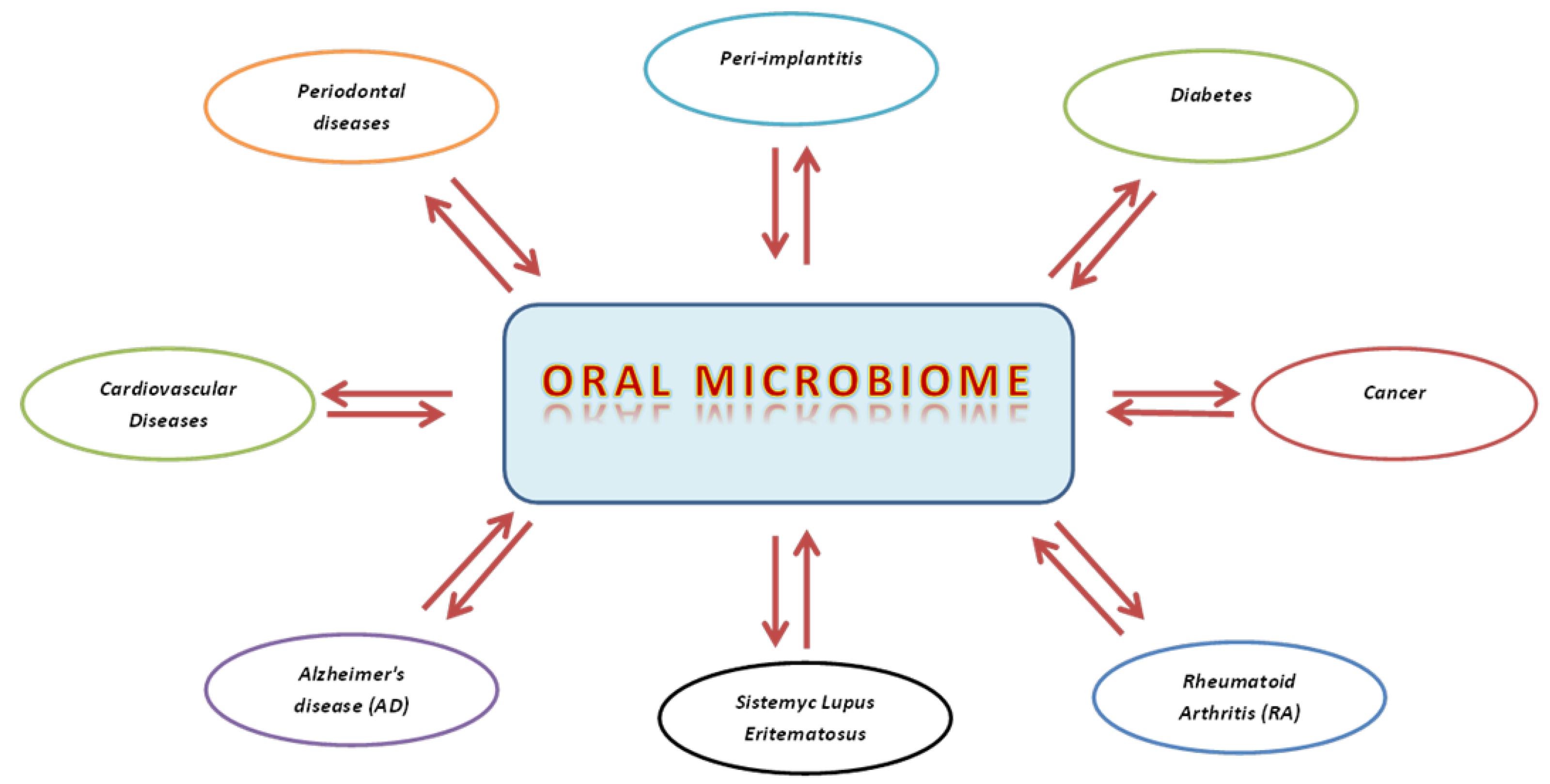
:1. Introduction
2. Relevant Sections (Methodology)
3. Discussion
3.1. Oral Microbiome in Physiologic Conditions
3.2. Oral Microbiome in Local Pathologic Conditions
3.2.1. Oral Microbiota in Periodontal Diseases
3.2.2. Oral Microbiota in Tongue
3.2.3. Oral Microbiota in Peri-Implantitis
3.3. Oral Microbiome in Systemic Pathologic Conditions
3.3.1. Oral Microbiota and Diabetes
3.3.2. Oral Microbiota and Rheumatoid Arthritis (RA)
3.3.3. Oral Microbiota and Systemic Lupus Erythematosus (SLE)
3.3.4. Oral Microbiota and Cancer
3.3.5. Oral Microbiota and Alzheimer’s Disease (AD)
3.3.6. Oral Microbiota and Cardiovascular Diseases
3.4. Clinical Considerations
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44, S12–S22. [Google Scholar] [CrossRef] [PubMed]
- Vollaard, E.J.; Clasener, H.A. Colonization resistance. Antimicrob. Agents Chemother. 1994, 38, 409–414. [Google Scholar] [CrossRef]
- Kamarajan, P.; Ateia, I.; Shin, J.M.; Fenno, J.C.; Le, C.; Zhan, L.; Chang, A.; Darveau, R.; Kapila, Y.L. Periodontal pathogens promote cancer aggressivity via tlr/myd88 triggered activation of integrin/FAK signaling that is therapeutically reversible by a probiotic bacteriocin. PLOS Pathog. 2020, 16, e1008881. [Google Scholar] [CrossRef]
- Ohki, T.; Itabashi, Y.; Kohno, T.; Yoshizawa, A.; Nishikubo, S.; Watanabe, S.; Yamane, G.; Ishihara, K. Detection of periodontal bacteria in thrombi of patients with acute myocardial infarction by polymerase chain reaction. Am. Heart J. 2012, 163, 164–167. [Google Scholar] [CrossRef]
- Di Spirito, F.; Argentino, S.; Martuscelli, R.; Sbordone, L. MRONJ incidence after multiple teeth extractions in patients taking oral bisphosphonates without “drug holiday”: A retrospective chart review. Oral Implantol. 2019, 12, 105–110. [Google Scholar]
- D’Ambrosio, F.; Amato, A.; Chiacchio, A.; Sisalli, L.; Giordano, F. Do Systemic Diseases and Medications Influence Dental Implant Osseointegration and Dental Implant Health? An Umbrella Review. Dent. J. 2023, 11, 146. [Google Scholar] [CrossRef]
- Di Spirito, F.; Sbordone, L.; Pilone, V.; D’ambrosio, F. Obesity and Periodontal Disease: A Narrative Review on Current Evidence and Putative Molecular Links. Open Dent. J. 2019, 13, 526–536. [Google Scholar] [CrossRef]
- Arweiler, N.B.; Netuschil, L. The Oral Microbiota. Adv. Exp. Med. Biol. 2016, 902, 45–60. [Google Scholar]
- Amato, M.; Di Spirito, F.; D’Ambrosio, F.; Boccia, G.; Moccia, G.; De Caro, F. Probiotics in Periodontal and Peri-Implant Health Management: Biofilm Control, Dysbiosis Reversal, And Host Modulation. Microorganisms 2022, 10, 2289. [Google Scholar] [CrossRef]
- Di Spirito, F.; Lo Giudice, R.; Amato, M.; Di Palo, M.P.; D’Ambrosio, F.; Amato, A.; Martina, S. Inflammatory, Reactive, and Hypersensitivity Lesions Potentially Due to Metal Nanoparticles from Dental Implants and Supported Restorations: An Umbrella Review. Appl. Sci. 2022, 12, 11208. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Caggiano, M.; Schiavo, L.; Savarese, G.; Carpinelli, L.; Amato, A.; Iandolo, A. Chronic Stress and Depression in Periodontitis and Peri-Implantitis: A Narrative Review on Neurobiological, Neurobehavioral and Immune–Microbiome Interplays and Clinical Management Implications. Dent. J. 2022, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Di Spirito, F.; Amato, A.; Di Palo, M.P.; Cannatà, D.; Giordano, F.; D’Ambrosio, F.; Martina, S. Periodontal Management in Periodontally Healthy Orthodontic Patients with Fixed Appliances: An Umbrella Review of Self-Care Instructions and Evidence-Based Recommendations. Dent. J. 2023, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Zijnge, V.; van Leeuwen, M.B.M.; Degener, J.E.; Abbas, F.; Thurnheer, T.; Gmür, R.; Harmsen, H.J.M. Oral Biofilm Architecture on Natural Teeth. PLoS ONE 2010, 5, e9321. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.S.O.; Freitas, A.R.; Pinheiro, M.L.L.; Nascimento, C.D.; Watanabe, E.; Albuquerque, R.F. Oral Biofilm Formation on Different Materials for Dental Implants. J. Vis. Exp. 2018, 136, 57756. [Google Scholar]
- Tiew, P.Y.; Mac Aogain, M.; Ali, N.A.B.M.; Thng, K.X.; Goh, K.; Lau, K.J.X.; Chotirmall, S.H. The Mycobiome in Health and Disease: Emerging Concepts, Methodologies and Challenges. Mycopathologia 2020, 185, 207–231. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Santella, B.; Di Palo, M.P.; Giordano, F.; Lo Giudice, R. Characterization of the Oral Microbiome in Wearers of Fixed and Removable Implant or Non-Implant-Supported Prostheses in Healthy and Pathological Oral Conditions: A Narrative Review. Microorganisms 2023, 11, 1041. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Pisano, M.; Amato, A.; Iandolo, A.; Caggiano, M.; Martina, S. Periodontal and Peri-Implant Health Status in Traditional vs. Heat-Not-Burn Tobacco and Electronic Cigarettes Smokers: A Systematic Review. Dent. J. 2022, 10, 103. [Google Scholar] [CrossRef]
- Caggiano, M.; Gasparro, R.; D’Ambrosio, F.; Pisano, M.; Di Palo, M.P.; Contaldo, M. Smoking Cessation on Periodontal and Peri-implant Health Status: A Systematic Review. Dent. J. 2022, 10, 162. [Google Scholar] [CrossRef]
- Huang, C.; Shi, G. Smoking and microbiome in oral, airway, gut and some systemic diseases. J. Transl. Med. 2019, 17, 225. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.R.; Preshaw, P.M.; Nagaraja, H.N.; Dabdoub, S.M.; Rahman, A.; Kumar, P.S. The subgingival microbiome of clinically healthy current and never smokers. ISME J. 2015, 9, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Chen, Y.C.; Chen, S.J.; Lee, C.H.; Cheng, C.M. Alcohol Addiction, Gut Microbiota, and Alcoholism Treatment: A Review. Int. J. Mol. Sci. 2020, 21, 6413. [Google Scholar] [CrossRef] [PubMed]
- Houle, M.A.; Grenier, D.; Plamondon, P.; Nakayama, K. The collagenase activity of Porphyromonas gingivalis is due to Arg-gingipain. FEMS Microbiol. Lett. 2003, 221, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Dabdoub, S.M.; Tsigarida, A.A.; Kumar, P.S. Patient-specific analysis of periodontal and peri-implant microbiomes. J. Dent. Res. 2013, 92, 168–175. [Google Scholar] [CrossRef]
- Stephen, A.S.; Dhadwal, N.; Nagala, V.; Gonzales-Marin, C.; Gillam, D.G.; Bradshaw, D.J.; Burnett, G.R.; Allaker, R.P. Interdental and subgingival microbiota may affect the tongue microbial ecology and oral malodour in health, gingivitis and periodontitis. J. Periodontal Res. 2021, 56, 1174–1184. [Google Scholar] [CrossRef]
- Diaz, P.I.; Hoare, A.; Hong, B.Y. Subgingival microbiome shifts and community dynamics in periodontal diseases. J. Calif. Dent. Assoc. 2016, 44, 421–435. [Google Scholar] [CrossRef]
- Persson, G.R.; Renvert, S. Cluster of bacteria associated with peri-implantitis. Clin. Implant. Dent. Relat. Res. 2014, 16, 783–793. [Google Scholar] [CrossRef]
- Sahrmann, P.; Gilli, F.; Wiedemeier, D.B.; Attin, T.; Schmidlin, P.R.; Karygianni, L. The Microbiome of Peri-Implantitis: A Systematic Review and Meta-Analysis. Microorganisms 2020, 8, 661. [Google Scholar] [CrossRef]
- Jepsen, S.; Caton, J.G.; Albandar, J.M.; Bissada, N.F.; Bouchard, P.; Cortellini, P.; Demirel, K.; de Sanctis, M.; Ercoli, C.; Fan, J.; et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S219–S229. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Xiao, E.; Graves, D.T. Diabetes mellitus related bone metabolism and periodontal disease. Int. J. Oral Sci. 2015, 7, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Naguib, G.; Al-Mashat, H.; Desta, T.; Graves, D.T. Diabetes prolongs the inflammatory response to a bacterial stimulus through cytokine dysregulation. J. Investig. Dermatol. 2004, 123, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Ohira, T.; Uchida, Y.; Ayilavarapu, S.; Batista, E.L.; Yagi, M.; Iwata, T.; Liu, H.; Hasturk, H.; Kantarci, A.; et al. Priming of neutrophil oxidative burst in diabetes requires preassembly of the NADPH oxidase. J. Leukoc. Biol. 2008, 84, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Lalla, E.; Lamster, I.B.; Feit, M.; Huang, L.; Spessot, A.; Qu, W.; Kislinger, T.; Lu, Y.; Stern, D.M.; Schmidt, A.M. Blockade of RAGE suppresses periodontitis-associated bone loss in diabetic mice. J. Clin. Invest. 2000, 105, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Pisano, M.; Di Spirito, F.; Martina, S.; Sangiovanni, G.; D’Ambrosio, F.; Iandolo, A. Intentional Replantation of Single-Rooted and Multi-Rooted Teeth: A Systematic Review. Healthcare 2022, 11, 11. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Genco, R. Working Group 2 of Joint EFP/AAP Workshop. Diabetes and periodontal diseases: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 40, S106–S112. [Google Scholar]
- Mashimo, P.A.; Yamamoto, Y.; Slots, J.; Park, B.H.; Genco, R.J. The periodontal microflora of juvenile diabetics: Culture, immunofluorescence, and serum antibody studies. J. Periodontol. 1983, 54, 420–430. [Google Scholar] [CrossRef]
- Campus, G.; Salem, A.; Uzzau, S.; Baldoni, E.; Tonolo, G. Diabetes and periodontal disease: A case-control study. J. Periodontol. 2005, 76, 418–425. [Google Scholar] [CrossRef]
- da Cruz, G.A.; de Toledo, S.; Sallum, E.A.; Sallum, A.W.; Ambrosano, G.M.; de Cássia Orlandi Sardi, J.; da Cruz, S.E.; Gonçalves, R.B. Clinical and laboratory evaluations of non-surgical periodontal treatment in subjects with diabetes mellitus. J. Periodontol. 2008, 79, 1150–1157. [Google Scholar] [CrossRef]
- Ganesan, S.M.; Joshi, V.; Fellows, M.; Dabdoub, S.M.; Nagaraja, H.N.; O’Donnell, B.; Deshpande, N.R.; Kumar, P.S. A tale of two risks: Smoking, diabetes and the subgingival microbiome. ISME J. 2017, 11, 2075–2089. [Google Scholar] [CrossRef]
- Casarin, R.; Barbagallo, A.; Meulman, B.; Santos, V.; Sallum, E.; Nociti, F.; Duarte, P.; Casati, M.; Gonçalves, R. Subgingival biodiversity in subjects with uncontrolled type-2 diabetes and chronic periodontitis. J. Periodontal Res. 2012, 48, 30–36. [Google Scholar] [CrossRef] [PubMed]
- de Smit, M.J.; Westra, J.; Brouwer, E.; Janssen, K.M.; Vissink, A.; van Winkelhoff, A.J. Periodontitis and rheumatoid arthritis: What do we know? J. Periodontol. 2015, 86, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, J.D.; Saraiva, A.M.; Queiroz-Junior, C.M.; Madeira, M.F.; Duarte, P.M.; Teixeira, M.M.; Souza, D.G.; da Silva, T.A. Arthritis-induced alveolar bone loss is associated with changes in the composition of oral microbiota. Anaerobe 2016, 39, 91–96. [Google Scholar] [CrossRef]
- Kim, D.; Lee, G.; Huh, Y.H.; Lee, S.Y.; Park, K.H.; Kim, S.; Kim, J.; Koh, J.; Ryu, J. NAMPT is an essential regulator of RA-mediated periodontal inflammation. J. Dent. Res. 2017, 96, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Queiroz-Junior, C.M.; Madeira, M.F.; Coelho, F.M.; Costa, V.V.; Bessoni, R.L.; Sousa, L.F.; Garlet, G.P.; Souza Dda, G.; Teixeira, M.M.; Silva, T.A. Experimental arthritis triggers periodontal disease in mice: Involvement of TNF-α and the oral microbiota. J. Immunol. 2011, 187, 3821–3830. [Google Scholar] [CrossRef] [PubMed]
- Golub, L.M.; Payne, J.B.; Reinhardt, R.A.; Nieman, G. Can systemic diseases co-induce (not just exacerbate) periodontitis? A hypothetical “two-hit” model. J. Dent. Res. 2006, 85, 102–105. [Google Scholar]
- Mirrielees, J.; Crofford, L.J.; Lin, Y.; Kryscio, R.J.; Dawson, D.R., III; Ebersole, J.L.; Miller, C.S. Rheumatoid arthritis and salivary biomarkers of periodontal disease. J. Clin. Periodontol. 2010, 37, 1068–1074. [Google Scholar] [CrossRef]
- Moutsopoulos, N.M.; Konkel, J.; Sarmadi, M.; Eskan, M.A.; Wild, T.; Dutzan, N.; Abusleme, L.; Zenobia, C.; Hosur, K.B.; Abe, T.; et al. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Sci. Transl. Med. 2014, 6, 229ra40. [Google Scholar] [CrossRef]
- Scher, J.U.; Ubeda, C.; Equinda, M.; Khanin, R.; Buischi, Y.; Viale, A.; Lipuma, L.; Attur, M.; Pillinger, M.; Weissmann, G.; et al. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 2012, 64, 3083–3094. [Google Scholar] [CrossRef]
- Horta-Baas, G.; Romero-Figueroa, M.D.S.; Montiel-Jarquín, A.J.; Pizano-Zárate, M.L.; García-Mena, J.; Ramírez-Durán, N. Intestinal dysbiosis and rheumatoid arthritis: A link between gut microbiota and the pathogenesis of rheumatoid arthritis. J. Immunol. Res. 2017, 2017, 4835189. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Abusleme, L.; Moutsopoulos, N.M. IL-17: Overview and role in oral immunity and microbiome. Oral Dis. 2017, 23, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Hevia, A.; Milani, C.; López, P.; Cuervo, A.; Arboleya, S.; Duranti, S.; Turroni, F.; González, S.; Suárez, A.; Gueimonde, M.; et al. Intestinal dysbiosis associated with systemic lupus erythematosus. MBio 2014, 5, 1–10. [Google Scholar] [CrossRef]
- Jensen, J.L.; Bergem, H.O.; Gilboe, I.M.; Husby, G.; Axéll, T. Oral and ocular sicca symptoms and findings are prevalent in systemic lupus erythematosus. J. Oral Pathol. Med. 1999, 28, 317–322. [Google Scholar] [CrossRef]
- Mutlu, S.; Richards, A.; Maddison, P.; Scully, C. Gingival and periodontal health in systemic lupus erythematosus. Community Dent. Oral Epidemiol. 1993, 21, 158–161. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ito, S.; Yamamoto, K.; Hasegawa, H.; Sugita, N.; Kuroda, T.; Kaneko, S.; Narita, I.; Yasuda, K.; Nakano, M.; et al. Risk of periodontitis in systemic lupus erythematosus is associated with Fcgamma receptor polymorphisms. J. Periodontol. 2003, 74, 378–384. [Google Scholar] [CrossRef]
- Rutter-Locher, Z.; Smith, T.O.; Giles, I.; Sofat, N. Association between systemic lupus erythematosus and periodontitis: A systematic review and metaanalysis. Front. Immunol. 2017, 8, 1295. [Google Scholar] [CrossRef]
- Marques, C.P.; Victor, E.C.; Franco, M.M.; Fernandes, J.M.; Maor, Y.; de Andrade, M.S.; Rodrigues, V.P.; Benatti, B.B. Salivary levels of inflammatory cytokines and their association to periodontal disease in systemic lupus erythematosus patients: A case-control study. Cytokine 2016, 85, 165–170. [Google Scholar] [CrossRef]
- Xiao, E.; Mattos, M.; Vieira, G.H.A.; Chen, S.; Corrêa, J.D.; Wu, Y.; Albiero, M.L.; Bittinger, K.; Graves, D.T. Diabetes enhances IL-17 expression and alters the oral microbiome to increase its pathogenicity. Cell Host Microb. 2017, 22, 120–128.e4. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, C.; Fuller, R.; Bonfá, E.; Guedes, L.K.; D’Alleva, P.S.; Borba, E.F. Periodontitis treatment improves systemic lupus erythematosus response to immunosuppressive therapy. Clin. Rheumatol. 2014, 33, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. The inflammophilic character of the periodontitis-associated microbiota. Mol. Oral Microbiol. 2014, 29, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Caggiano, M.; Di Spirito, F.; Acerra, A.; Galdi, M.; Sisalli, L. Multiple-Drugs-Related Osteonecrosis of the Jaw in a Patient Affected by Multiple Myeloma: A Case Report. Dent. J. 2023, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Edlund, A. Exploiting the Oral Microbiome to Prevent Tooth Decay: Has Evolution Already Provided the Best Tools? Front. Microbiol. 2019, 9, 3323. [Google Scholar] [CrossRef]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The Oral Microbiome: Role of Key Organisms and Complex Networks in Oral Health and Disease. Periodontol. 2000 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The Oral Microbiome—An Update for Oral Healthcare Professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef]
- Kleinstein, S.E.; Nelson, K.E.; Freire, M. Inflammatory Networks Linking Oral Microbiome with Systemic Health and Disease. J. Dent. Res. 2020, 99, 1131–1139. [Google Scholar] [CrossRef]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal Disease, Tooth Loss, and Cancer Risk. Epidemiol. Rev. 2017, 39, 49–58. [Google Scholar] [CrossRef]
- Peters, B.A.; Wu, J.; Pei, Z.; Yang, L.; Purdue, M.P.; Freedman, N.D.; Jacobs, E.J.; Gapstur, S.M.; Hayes, R.B.; Ahn, J. Oral Microbiome Composition Reflects Prospective Risk for Esophageal Cancers. Cancer Res. 2017, 77, 6777–6787. [Google Scholar] [CrossRef]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human Oral Microbiome and Prospective Risk for Pancreatic Cancer: A Population Based, Nested Case Control Study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Sinha, R.; Pei, Z.; Dominianni, C.; Wu, J.; Shi, J.; Goedert, J.J.; Hayes, R.B.; Yang, L. Human Gut Microbiome and Risk for Colorectal Cancer. J. Natl. Cancer Inst. 2013, 105, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Bulgart, H.R.; Neczypor, E.W.; Wold, L.E.; Mackos, A.R. Microbial Involvement in Alzheimer Disease Development and Progression. Mol. Neurodegener. 2020, 15, 42. [Google Scholar] [CrossRef]
- Bonfili, L.; Cecarini, V.; Gogoi, O.; Gong, C.; Cuccioloni, M.; Angeletti, M.; Rossi, G.; Eleuteri, A.M. Microbiota Modulation as Preventative and Therapeutic Approach in Alzheimer’s Disease. FEBS J. 2021, 288, 2836–2855. [Google Scholar] [CrossRef]
- Allen, H.B. Alzheimer’s Disease: Assessing the Role of Spirochetes, Biofilms, the Immune System, and Amyloid with Regard to Potential Treatment and Prevention. J. Alzheimer’s Dis. 2016, 53, 1271–1276. [Google Scholar] [CrossRef]
- Kato, T.; Yamazaki, K.; Nakajima, M.; Date, Y.; Kikuchi, J.; Hase, K.; Ohno, H.; Yamazaki, K. Oral Administration of Porphyromonas Gingivalis Alters the Gut Microbiome and Serum Metabolome. mSphere 2018, 3, e00460-18. [Google Scholar] [CrossRef]
- Miklossy, J.; Kis, A.; Radenovic, A.; Miller, L.; Forro, L.; Martins, R.; Reiss, K.; Darbinian, N.; Darekar, P.; Mihaly, L. Beta-Amyloid Deposition and Alzheimer’s Type Changes Induced by Borrelia Spirochetes. Neurobiol. Aging 2006, 27, 228–236. [Google Scholar] [CrossRef]
- Narengaowa; Kong, W.; Lan, F.; Awan, U.F.; Qing, H.; Ni, J. The Oral-Gut-Brain AXIS: The Influence of Microbes in Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 633735. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas Gingivalis in Alzheimer’s Disease Brains: Evidence for Disease Causation and Treatment with Small-Molecule Inhibitors. Sci. Adv. 2019, 5, e0204941. [Google Scholar] [CrossRef]
- Leblhuber, F.; Huemer, J.; Steiner, K.; Gostner, J.M.; Fuchs, D. Knock-on Effect of Periodontitis to the Pathogenesis of Alzheimer’s Disease? Wien. Klin. Wochenschr. 2020, 132, 493–498. [Google Scholar] [CrossRef]
- Chi, L.; Cheng, X.; Lin, L.; Yang, T.; Sun, J.; Feng, Y.; Liang, F.; Pei, Z.; Teng, W. Porphyromonas Gingivalis-Induced Cognitive Impairment Is AssociatedWith Gut Dysbiosis, Neuroinflammation, and Glymphatic Dysfunction. Front. Cell. Infect. Microbiol. 2021, 11, 977. [Google Scholar] [CrossRef]
- Borsa, L.; Dubois, M.; Sacco, G.; Lupi, L. Analysis the Link between Periodontal Diseases and Alzheimer’s Disease: A Systematic Review. Int. J. Environ. Res. Public. Health 2021, 18, 9312. [Google Scholar] [CrossRef]
- Leblhuber, F.; Ehrlich, D.; Steiner, K.; Geisler, S.; Fuchs, D.; Lanser, L.; Kurz, K. The Immunopathogenesis of Alzheimer’s Disease Is Related to the Composition of Gut Microbiota. Nutrients 2021, 13, 361. [Google Scholar] [CrossRef]
- Olsen, I. Can Porphyromonas Gingivalis Contribute to Alzheimer’s Disease Already at the Stage of Gingivitis? J. Alzheimer’s Dis. Rep. 2021, 5, 237–241. [Google Scholar] [CrossRef]
- Leszek, J.; Mikhaylenko, E.V.; Belousov, D.M.; Koutsouraki, E.; Szczechowiak, K.; Kobusiak-Prokopowicz, M.; Mysiak, A.; Diniz, B.S.; Somasundaram, S.G.; Kirkland, C.E.; et al. The Links between Cardiovascular Diseases and Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 19, 152–169. [Google Scholar] [CrossRef]
- Dibello, V.; Lozupone, M.; Manfredini, D.; Dibello, A.; Zupo, R.; Sardone, R.; Daniele, A.; Lobbezoo, F.; Panza, F. Oral Frailty and Neurodegeneration in Alzheimer’s Disease. Neural Regen. Res. 2021, 16, 2149–2153. [Google Scholar]
- Simas, A.M.; Kramer, C.D.; Weinberg, E.O.; Genco, C.A. Oral Infection with a Periodontal Pathogen Alters Oral and Gut Microbiomes. Anaerobe 2021, 71, 102399. [Google Scholar] [CrossRef]
- Boeri, L.; Perottoni, S.; Izzo, L.; Giordano, C.; Albani, D. Microbiota-Host Immunity Communication in Neurodegenerative Disorders: Bioengineering Challenges for In Vitro Modeling. Adv. Healthc. Mater. 2021, 10, 2002043. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of Fish and N-3 Fatty Acids and Risk of Incident Alzheimer Disease. Arch. Neurol. 2003, 60, 940–946. [Google Scholar] [CrossRef]
- Yurko-Mauro, K.; McCarthy, D.; Rom, D.; Nelson, E.B.; Ryan, A.S.; Blackwell, A.; Salem, N.; Stedman, M.; MIDAS Investigators. Beneficial Effects of Docosahexaenoic Acid on Cognition in Age-Related Cognitive Decline. Alzheimer’s Dement. 2010, 6, 456–464. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral Diseases: A Global Public Health Challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Ribeiro-Vidal, H.; Sánchez, M.C.; Alonso-Español, A.; Figuero, E.; Ciudad, M.J.; Collado, L.; Herrera, D.; Sanz, M. Antimicrobial Activity of Epa and Dha against Oral Pathogenic Bacteria Using an in Vitro Multi-Species Subgingival Biofilm Model. Nutrients 2020, 12, 2812. [Google Scholar] [CrossRef]
- Khalifa, K.; Bergland, A.K.; Soennesyn, H.; Oppedal, K.; Oesterhus, R.; Dalen, I.; Larsen, A.I.; Fladby, T.; Brooker, H.; Wesnes, K.A.; et al. Effects of Purified Anthocyanins in People at Risk for Dementia: Study Protocol for a Phase II Randomized Controlled Trial. Front. Neurol. 2020, 11, 916. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, M.; Shah, S.A.; Saeed, K.; Kim, M.O. Natural Antioxidant Anthocyanins—A Hidden Therapeutic Candidate in Metabolic Disorders with Major Focus in Neurodegeneration. Nutrients 2019, 11, 1195. [Google Scholar] [CrossRef]
- Khan, M.S.; Ikram, M.; Park, J.S.; Park, T.J.; Kim, M.O. Gut Microbiota, Its Role in Induction of Alzheimer’s Disease Pathology, and Possible Therapeutic Interventions: Special Focus on Anthocyanins. Cells 2020, 9, 853. [Google Scholar] [CrossRef]
- Sukprasansap, M.; Chanvorachote, P.; Tencomnao, T. Cyanidin-3-Glucoside Activates Nrf2-Antioxidant Response Element and Protects against Glutamate-Induced Oxidative and Endoplasmic Reticulum Stress in HT22 Hippocampal Neuronal Cells. BMC Complement. Med. Ther. 2020, 20, 46. [Google Scholar] [CrossRef]
- Benahmed, A.G.; Gasmi, A.; Do¸sa, A.; Chirumbolo, S.; Mujawdiya, P.K.; Aaseth, J.; Dadar, M.; Bjørklund, G. Association between the Gut and Oral Microbiome with Obesity. Anaerobe 2020, 70, 102248. [Google Scholar] [CrossRef]
- Giordano-Kelhoffer, B.; Lorca, C.; March Llanes, J.; Rábano, A.; del Ser, T.; Serra, A.; Gallart-Palau, X. Oral Microbiota, Its Equilibrium and Implications in the Pathophysiology of Human Diseases: A Systematic Review. Biomedicines 2022, 10, 1803. [Google Scholar] [CrossRef]
- Lee, H.; Jun, H.; Kim, H.; Lee, S.; Choi, B. Fusobacterium Nucleatum GroEL Induces Risk Factors of Atherosclerosis in Human Microvascular Endothelial Cells and ApoE(−/−) Mice. Mol. Oral Microbiol. 2012, 27, 109–123. [Google Scholar] [CrossRef]
- He, J.; Li, Y.; Cao, Y.; Xue, J.; Zhou, X. The Oral Microbiome Diversity and Its Relation to Human Diseases. Folia Microbiol. 2014, 60, 69–80. [Google Scholar] [CrossRef]
- Amato, M.; Zingone, F.; Caggiano, M.; Iovino, P.; Bucci, C.; Ciacci, C. Tooth Wear Is Frequent in Adult Patients with Celiac Disease. Nutrients 2017, 9, 1321. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Di Spirito, F.; De Caro, F.; Lanza, A.; Passarella, D.; Sbordone, L. Adherence to Antibiotic Prescription of Dental Patients: The Other Side of the Antimicrobial Resistance. Healthcare 2022, 10, 1636. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Di Spirito, F.; Amato, A.; Caggiano, M.; Lo Giudice, R.; Martina, S. Attitudes towards Antibiotic Prescription and Antimicrobial Resistance Awareness among Italian Dentists: What Are the Milestones? Healthcare 2022, 10, 1585. [Google Scholar] [CrossRef]
- Boccia, G.; Di Spirito, F.; D’Ambrosio, F.; Di Palo, M.P.; Giordano, F.; Amato, M. Local and Systemic Antibiotics in Peri-Implantitis Management: An Umbrella Review. Antibiotics 2023, 12, 114. [Google Scholar] [CrossRef]
- Pisano, M.; Sangiovanni, G.; D’Ambrosio, F.; Romano, A.; Di Spirito, F. Oral Care in a Patient with Long Arm Deletion Syndrome of Chromosome 18: A Narrative Review and Case Presentation. Am. J. Case Rep. 2022, 23, e936142. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Ronsivalle, V.; Shapira, I.; Cicciù, M. Prevalence of Temporomandibular Disorders in subjects affected by Parkinson Disease: A systematic review and metanalysis. J. Oral Rehabil. 2023, 13, 496. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Caggiano, M.; Acerra, A.; Pisano, M.; Giordano, F. Is Ozone a Valid Adjuvant Therapy for Periodontitis and Peri-Implantitis? A Systematic Review. J. Pers. Med. 2023, 13, 646. [Google Scholar] [CrossRef]
- Pantaleo, G.; Acerra, A.; Giordano, F.; D’Ambrosio, F.; Langone, M.; Caggiano, M. Immediate loading of fixed prostheses in fully edentulous jaws—7-years follow-up from a single-cohort retrospective study. Appl. Sci. 2022, 12, 12427. [Google Scholar] [CrossRef]
- Di Spirito, F.; Amato, A.; Di Palo, M.P.; Contaldo, M.; D’Ambrosio, F.; Lo Giudice, R.; Amato, M. Oral Lesions Following Anti-Sars-Cov-2 Vaccination: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 10228. [Google Scholar] [CrossRef]
- Pisano, M.; Romano, A.; Di Palo, M.P.; Baroni, A.; Serpico, R.; Contaldo, M. Oral Candidiasis in Adult and Pediatric Patients with COVID-19. Biomedicines 2023, 11, 846. [Google Scholar] [CrossRef]
- Di Spirito, F.; Iandolo, A.; Amato, A.; Caggiano, M.; Raimondo, A.; Lembo, S.; Martina, S. Prevalence, Features and Degree of Association of Oral Lesions in COVID-19: A Systematic Review of Systematic Reviews. Int. J. Environ. Res. Public Health 2022, 19, 7486. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Iandolo, A.; Scelza, G.; Spirito, F.; Martina, S. COVID-19: The Patients’ Perceived Impact on Dental Care. Eur. J. Dent. 2022, 16, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Martina, S.; Amato, A.; Faccioni, P.; Iandolo, A.; Amato, M.; Rongo, R. The perception of COVID-19 among Italian dental patients: An orthodontic point of view. Prog. Orthod. 2021, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Caggiano, M.; Acerra, A.; Martina, S.; Galdi, M.; D’Ambrosio, F. Infection Control in Dental Practice during the COVID-19 Pandemic: What Is Changed? Int. J. Environ. Res. Public Health 2023, 20, 3903. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisano, M.; Giordano, F.; Sangiovanni, G.; Capuano, N.; Acerra, A.; D’Ambrosio, F. The Interaction between the Oral Microbiome and Systemic Diseases: A Narrative Review. Microbiol. Res. 2023, 14, 1862-1878. https://doi.org/10.3390/microbiolres14040127
Pisano M, Giordano F, Sangiovanni G, Capuano N, Acerra A, D’Ambrosio F. The Interaction between the Oral Microbiome and Systemic Diseases: A Narrative Review. Microbiology Research. 2023; 14(4):1862-1878. https://doi.org/10.3390/microbiolres14040127
Chicago/Turabian StylePisano, Massimo, Francesco Giordano, Giuseppe Sangiovanni, Nicoletta Capuano, Alfonso Acerra, and Francesco D’Ambrosio. 2023. "The Interaction between the Oral Microbiome and Systemic Diseases: A Narrative Review" Microbiology Research 14, no. 4: 1862-1878. https://doi.org/10.3390/microbiolres14040127
APA StylePisano, M., Giordano, F., Sangiovanni, G., Capuano, N., Acerra, A., & D’Ambrosio, F. (2023). The Interaction between the Oral Microbiome and Systemic Diseases: A Narrative Review. Microbiology Research, 14(4), 1862-1878. https://doi.org/10.3390/microbiolres14040127









