A Role for Secondary Metabolites in Desiccation Tolerance in Lichens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lichen Material
2.2. Acetone Rinsing
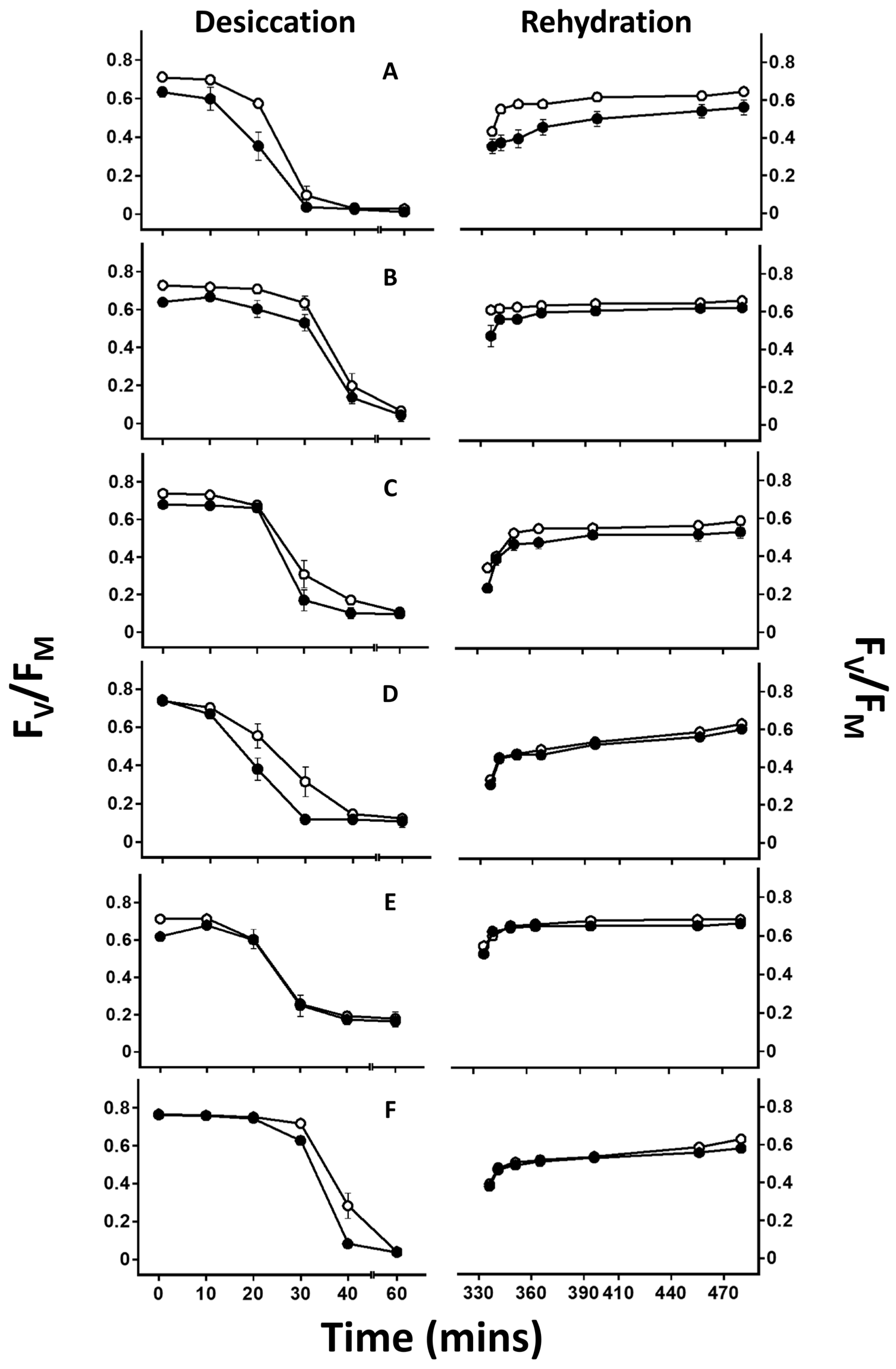
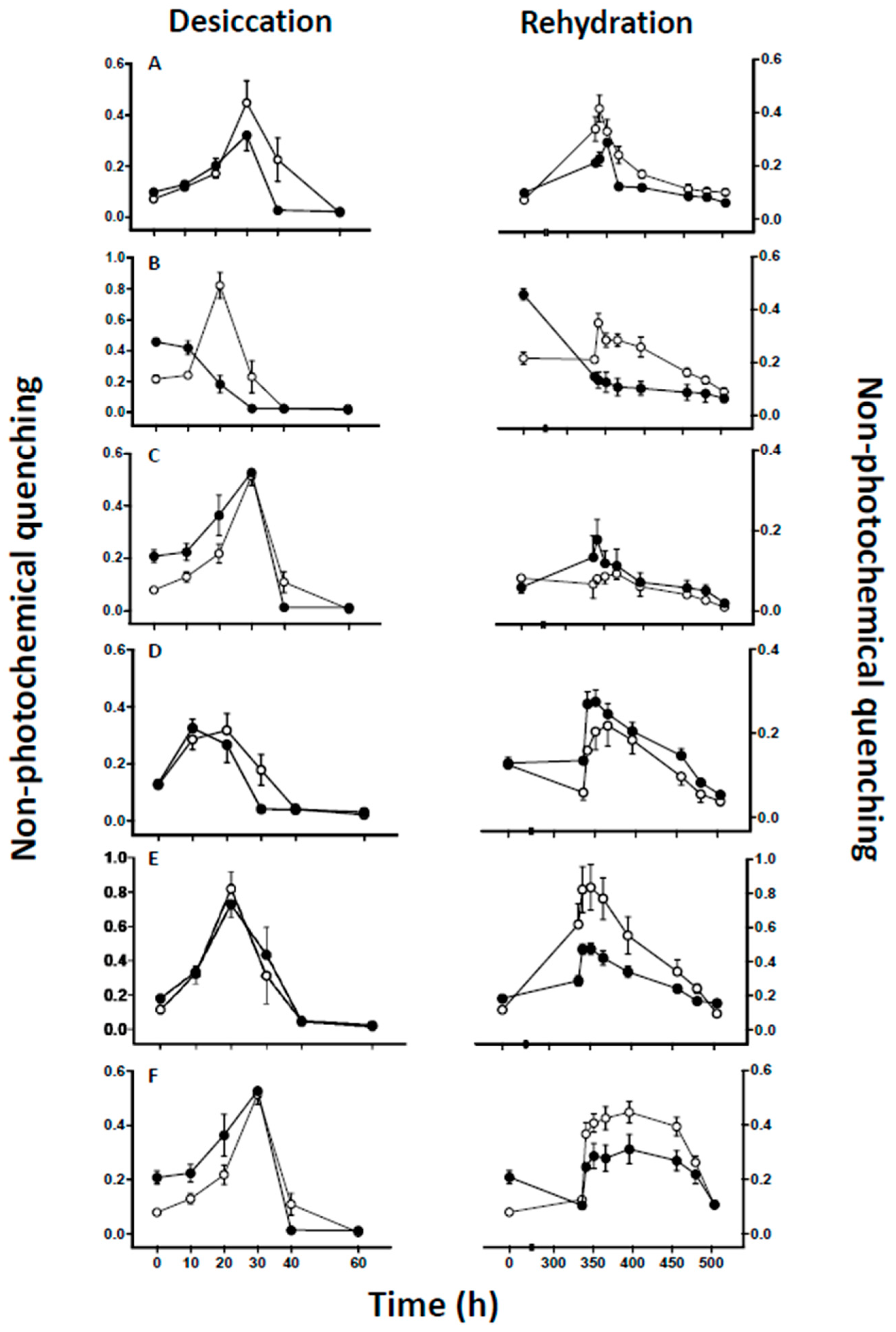
2.3. Chlorophyll Fluorescence Measurements
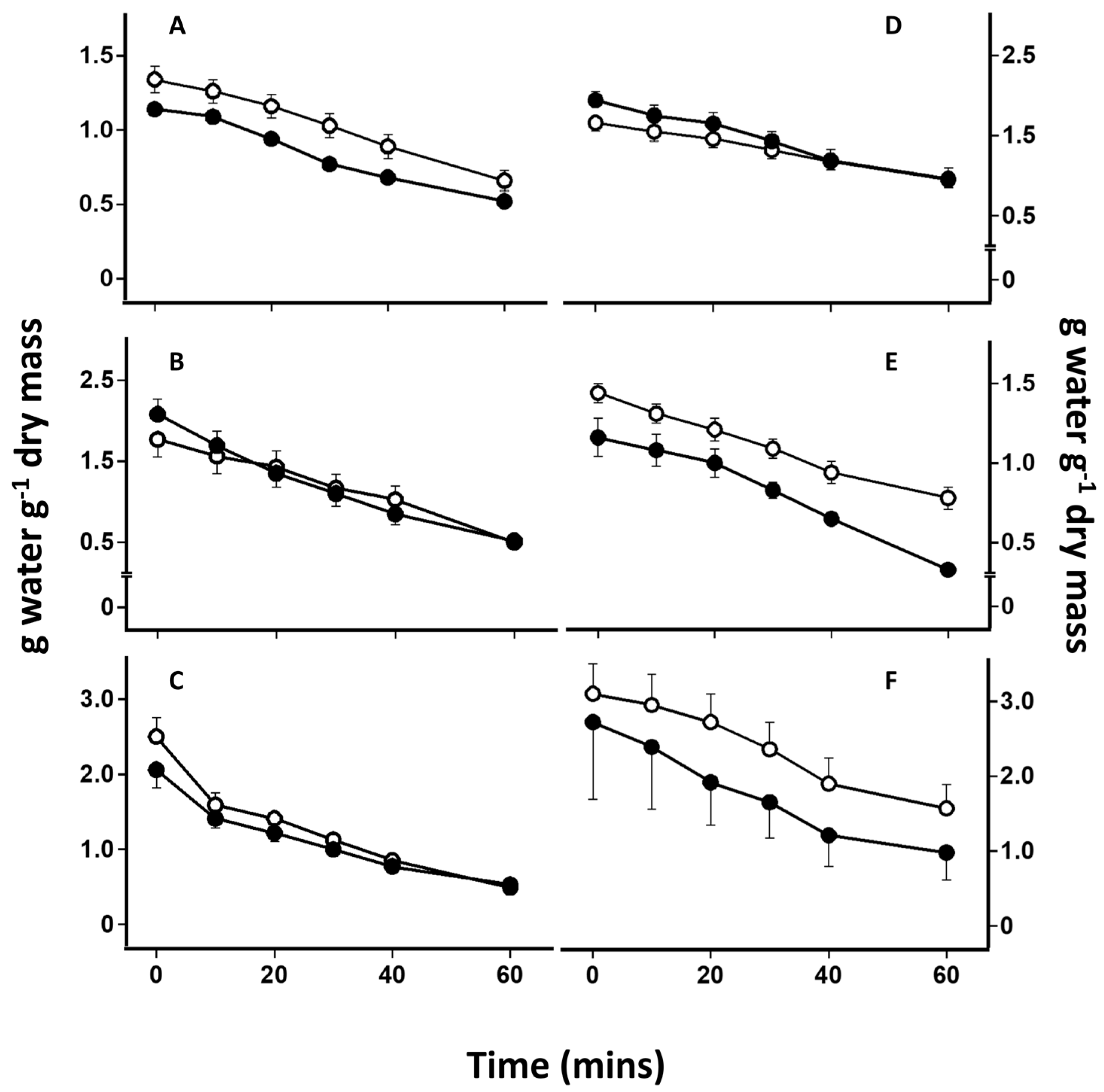
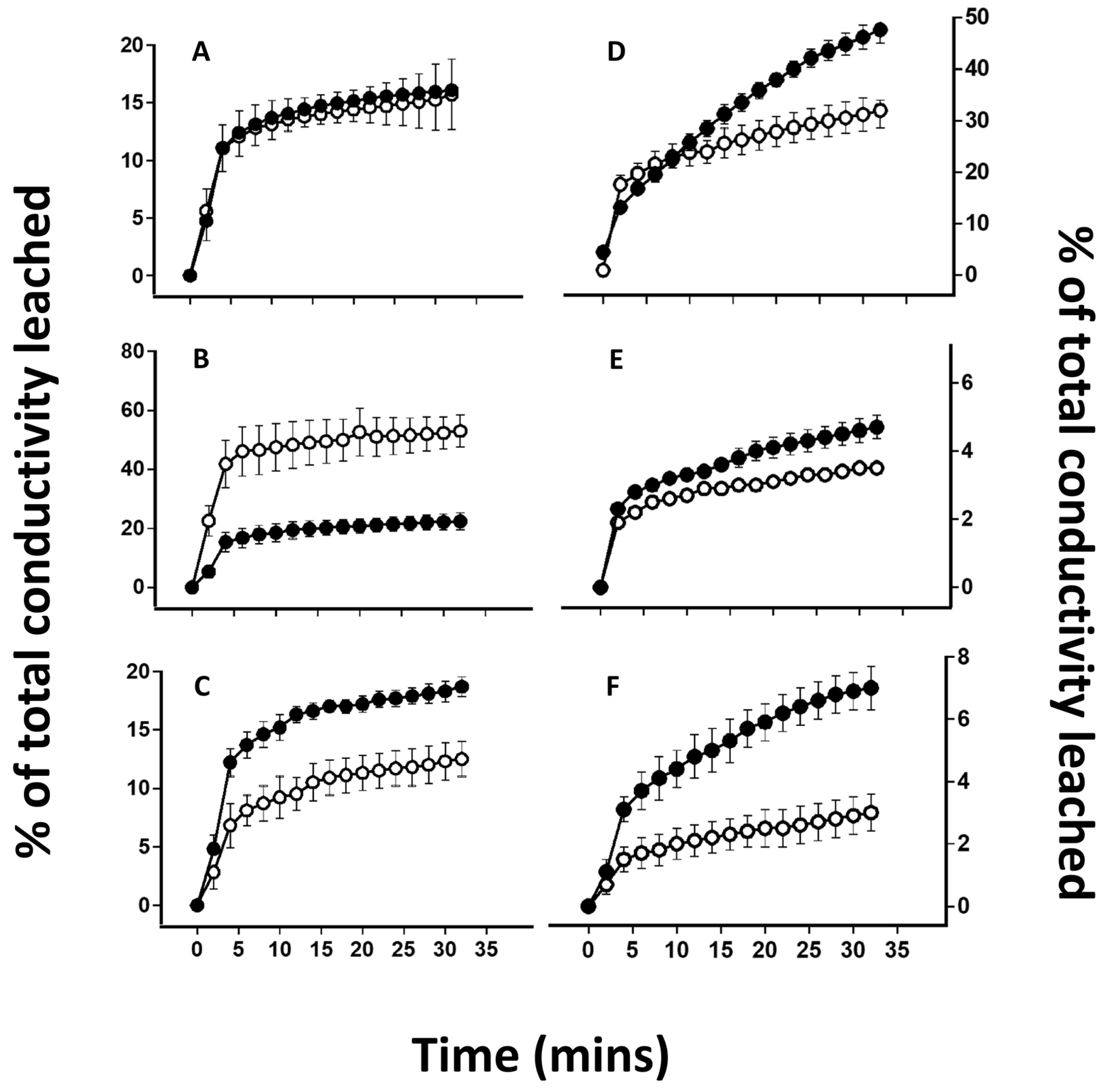
2.4. Desiccation-Induced Ion Leakage
2.5. Experimental Treatments
2.6. Statistical Analyses
3. Results
4. Discussion
4.1. Effect of Lichen Substances on Rates of Desiccation
4.2. Effect of Lichen Substance Removal on Photobiont Sensitivity to Desiccation
4.3. Effects of Lichen Substance Removal on the Tolerance of the Mycobiont to Desiccation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmadjian, V. Lichens are more important than you think. Bioscience 1995, 45, 124. [Google Scholar] [CrossRef]
- Goga, M.; Elečko, J.; Marcinčinová, M.; Ručová, D.; Bačkorová, M.; Bačkor, M. Lichen metabolites: An overview of some secondary metabolites and their biological potential. In Co-Evolution of Secondary Metabolites. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: New York, NY, USA, 2020; pp. 1–36. [Google Scholar]
- Molnár, K.; Farkas, E. Current results on biological activities of lichen secondary metabolites: A review. Z. Naturforsch. C 2010, 65, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Kosanić, M.; Ranković, B.; Vukojević, J. Antioxidant properties of some lichen species. J. Food Sci. Technol. 2011, 48, 584–590. [Google Scholar] [CrossRef]
- Thadhani, V.M.; Choudhary, M.I.; Ali, S.; Omar, I.; Siddique, H.; Karunaratne, V. Antioxidant activity of some lichen metabolites. Nat. Prod. Res. 2011, 25, 1827–1837. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; González-Burgos, E.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. Evaluation of the antioxidant capacities and cytotoxic effects of ten Parmeliaceae lichen species. eCAM 2016, 2016, 3169751. [Google Scholar]
- Luo, H.; Yamamoto, Y.; Kim, J.A.; Jung, J.S.; Koh, Y.J.; Hur, J.S. Lecanoric acid, a secondary lichen substance with antioxidant properties from Umbilicaria antarctica in maritime Antarctica (King George Island). Pol. Biol. 2009, 32, 1033–1040. [Google Scholar]
- Huneck, S.; Yoshimura, I. Identification of Lichen Substances; Springer: New York, NY, USA, 1996. [Google Scholar]
- Kohlhardt-Floehr, C.; Boehm, F.; Troppens, S.; Lademann, J.; Truscott, T.G. Prooxidant and antioxidant behaviour of usnic acid from lichens under UVB-light irradiation—Studies on human cells. J. Photochem. Photobiol. B 2010, 101, 97–102. [Google Scholar] [CrossRef]
- Šeklić, D.S.; Obradović, A.D.; Stanković, M.S.; Živanović, M.N.; Mitrović, T.L.; Stamenković, S.M.; Marković, S.D. Proapoptotic and antimigratory effects of Pseudevernia furfuracea and Platismatia glauca on colon cancer cell lines. Food Technol. Biotech. 2018, 56, 421–430. [Google Scholar] [CrossRef]
- Peralta, M.A.; da Silva, M.A.; Ortega, M.G.; Cabrera, J.L.; Paraje, M.G. Usnic acid activity on oxidative and nitrosative stress of azole-resistant Candida albicans biofilm. Planta Med. 2018, 83, 326–333. [Google Scholar] [CrossRef]
- Solhaug, K.A.; Gauslaa, Y. Acetone rinsing-a method for testing ecological and physiological roles of secondary compounds in living lichens. Symbiosis 2001, 30, 301–315. [Google Scholar]
- Beckett, R.P.; Minibayeva, F.; Solhaug, K.A.; Roach, T. Photoprotection in lichens: Adaptations of photobionts to high light. Lichenol. 2021, 53, 21–33. [Google Scholar] [CrossRef]
- Manojlović, N.T.; Rančić, A.B.; Décor, R.; Vasiljević, P.; Tomović, J. Determination of chemical composition and antimicrobial, antioxidant and cytotoxic activities of lichens Parmelia conspersa and Parmelia perlata. J. Food Meas. Charact. 2021, 15, 686–696. [Google Scholar] [CrossRef]
- Fernandes, R.F.; Porto, A.B.; Flores, L.S.; Maia, L.F.; Corrê, C.C.; Spielmann, A.S.; Edwards, H.G.M.; de Oliveira, L.F.C. Nature of light-absorbing pigments from Brazilian lichens identified by Raman spectroscopy. Vib. Spectrosc. 2018, 99, 59–66. [Google Scholar]
- Moreira, A.S.N.; Braz-Filho, R.; Mussi-Dias, V.; Vieira, I.J.C. Chemistry and biological activity of Ramalina lichenized fungi. Molecules 2015, 20, 8952–8987. [Google Scholar] [CrossRef] [PubMed]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Aschie, M.; Bucur, L.; Rambu, D.; Costache, T.; Cucolea, I.E.; Vochita, G.; Gherghel, D.; et al. Usnic acid and Usnea barbata (L.) F.H. Wigg. dry extracts promote apoptosis and DNA damage in human blood cells through enhancing ROS Levels. Antioxidants 2021, 10, 1171. [Google Scholar] [CrossRef]
- Hammer, S. A synopsis of the genus Cladonia in the Northwestern United States. Bryologist 1995, 98, 1–28. [Google Scholar] [CrossRef]
- Gudjónsdóttir, G.A.; Ingólfsdóttir, K. Quantitative determination of protolichesterinic- and fumarprotocetraric acids in Cetraria islandica by high-performance liquid chromatography. J. Chromatogr. A 1997, 757, 303–306. [Google Scholar] [CrossRef]
- Solhaug, K.A.; Larsson, P.; Gauslaa, Y. Light screening in lichen cortices can be quantified by chlorophyll fluorescence techniques for both reflecting and absorbing pigments. Planta 2010, 231, 1003–1011. [Google Scholar] [CrossRef]
- Bilger, W.; Schreiber, U.; Bock, M. Determination of the quantum efficiency of photosystem II and of non-photochemical quenching of chlorophyll fluorescence in the field. Oecologia 1995, 102, 425–432. [Google Scholar] [CrossRef]
- Munzi, S.; Pisani, T.; Loppi, S. The integrity of lichen cell membrane as a suitable parameter for monitoring biological effects of acute nitrogen pollution. Ecotox. Environ. Saf. 2009, 72, 2009–2012. [Google Scholar] [CrossRef]
- Ndhlovu, N.T.; Minibayeva, F.V.; Beckett, R.P. Unpigmented lichen substances protect lichens against photoinhibition of photosystem II in both the hydrated and desiccated states. Acta Physiol. Plant. 2022, 44, 123. [Google Scholar] [CrossRef]
- Challabathula, D.; Zhang, Q.; Bartels, D. Protection of photosynthesis in desiccation-tolerant resurrection plants. J. Plant Physiol. 2018, 227, 84–92. [Google Scholar] [CrossRef]
- Calatayud, A.; Deltoro, V.I.; Barreno, E.; del Valle-Tascon, S. Changes in in vivo chlorophyll fluorescence quenching in lichen thalli as a function of water content and suggestion of zeaxanthin-associated photoprotection. Physiol. Plant 1997, 101, 93–102. [Google Scholar] [CrossRef]
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Ahmad, I. Lipids and proteins—Major targets of oxidative modifications in abiotic stressed plants. Environ. Sci. Poll. Res. 2015, 22, 4099–4121. [Google Scholar] [CrossRef]
- Kranner, I.; Cram, W.J.; Zorn, M.; Wornik, S.; Yoshimura, I.; Stabentheiner, E.; Pfeifhofer, H.W. Antioxidants and photoprotection in a lichen as compared with its isolated symbiotic partners. Proc. Natl. Acad. Sci. USA 2005, 102, 3141–3146. [Google Scholar] [CrossRef]
- Kranner, I.; Beckett, R.; Hochman, A.; Nash, T.H. Desiccation-tolerance in lichens: A review. Bryologist 2008, 111, 576–593. [Google Scholar] [CrossRef]
- Asta, J.; Garrec, J.P. Etude de la réparation du calcium, potassium, magnesium et phosphore dans les différentes couches anatomiques de dix lichens par analyse discrete á la microsonde électronique. Crypt. Bryol. Lichen. 1980, 1, 3–20. [Google Scholar]
- Collins, C.R.; Farrar, J.F. Structural resistance to mass transfer in the lichen Xanthoria parietina. New Phyt. 1978, 81, 71–83. [Google Scholar] [CrossRef]
- Buck, G.W.; Brown, D.H. Effect of desiccation on cation location in lichens. Ann. Bot. 1979, 44, 265–277. [Google Scholar] [CrossRef]
| Species | Water | FV/FM | NPQ | Ion | ||
|---|---|---|---|---|---|---|
| Content | Desiccation | Rehydration | Desiccation | Rehydration | Leakage | |
| Cetraria islandica | *** | ** | *** | 0.083 | *** | 0.237 |
| Parmotrema perlata | *** | *** | *** | *** | *** | *** |
| Ramalina celastri | 0.190 | 0.552 | *** | 0.686 | 0.132 | *** |
| Usnea undulata | * | *** | 0.852 | 0.388 | *** | *** |
| Cladonia coniocraea | 0.112 | 0.533 | * | 0.981 | *** | *** |
| Crocodia aurata | 0.204 | *** | 0.136 | * | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndhlovu, N.T.; Minibayeva, F.; Beckett, R.P. A Role for Secondary Metabolites in Desiccation Tolerance in Lichens. Microbiol. Res. 2024, 15, 225-235. https://doi.org/10.3390/microbiolres15010016
Ndhlovu NT, Minibayeva F, Beckett RP. A Role for Secondary Metabolites in Desiccation Tolerance in Lichens. Microbiology Research. 2024; 15(1):225-235. https://doi.org/10.3390/microbiolres15010016
Chicago/Turabian StyleNdhlovu, Nqobile Truelove, Farida Minibayeva, and Richard Peter Beckett. 2024. "A Role for Secondary Metabolites in Desiccation Tolerance in Lichens" Microbiology Research 15, no. 1: 225-235. https://doi.org/10.3390/microbiolres15010016
APA StyleNdhlovu, N. T., Minibayeva, F., & Beckett, R. P. (2024). A Role for Secondary Metabolites in Desiccation Tolerance in Lichens. Microbiology Research, 15(1), 225-235. https://doi.org/10.3390/microbiolres15010016







